Natural cyclodextrins (CDs) can be formed by 6, 7, or 8 glucose molecules (α-, β-, and γ-, respectively) linked in a ring, creating a cone shape. Its interior has an affinity for hydrophobic molecules, while the exterior is hydrophilic and can interact with water molecules. This feature has been used to develop active packaging applied to food, interacting with the product or its environment to improve one or more aspects of its quality or safety. It also provides monitoring information when food is optimal for consumption, as intelligent packaging is essential for the consumer and the merchant.
1. Introduction
For some years now, and even faster with the passing of the pandemic (COVID-19), consumers have chosen to have food stored in their homes for easy access and, in case of an emergency, as a future food supply. Therefore, the time frame in which these packaged foods are no longer suitable for consumption is a concern [
1,
2,
3]. Food packaging was developed to serve several purposes, such as limiting food loss and preserving food quality for extended periods. Its main functions can be summarized as protection against possible contamination (acting as a barrier), containment, communication of package information about brands and nutritional content, and convenience to adapt to the fast-paced customer lifestyle [
4].
It is necessary to be aware of the conditions in which the food is stored, considering the appropriate temperature range and the suggested consumption date, to be fully informed of their quality concerning time [
5]. This is where smart packaging (SP) comes in, which is a technology applied in the packaging system to extend shelf life and reduce food waste [
6]. SP can be divided into active packaging (AP) and intelligent packaging (IP). AP interacts directly with the food, focusing on incorporating food additives to prevent or delay deterioration. It considers food factors such as respiration rate, humidity, and microbial attack to maintain a remarkable quality by reacting against unfavorable components. On the contrary, IP does not directly interact with the food; it only provides real-time information on the state of the food, indicating whether it is still optimal for consumption, always working in the distribution chain, from the industrial plant to the consumer’s home [
5].
Therefore, researchers and industries have focused on finding efficient ways to achieve this, designing them based on polymers, improving the characteristics of foods without affecting their sensory properties, synthesizing molecules with complex structures capable of stopping catalytic reactions or even slowing them down, and assembling with the ‘problem molecules’ by trapping them inside their cavity. This can be achieved, for example, with inclusion complexes, with a ‘host’ and a ‘guest’ molecule [
7,
8,
9].
Inclusion complexes are aggregates of molecules stabilized via non-covalent bonds (for example, van der Waals, hydrogen bonds, and hydrophobic interactions) [
10]. Host molecules are characterized by having an inner cavity where another molecule, usually referred to as a “guest molecule”, can be incorporated [
11]. Therefore, hosts will act as receptors and guests as substrates, inhibitors, or cofactors [
12]. The resulting molecular inclusion complex can easily break down in determined physiological environments [
13]. These inclusion systems can improve the physicochemical properties of the molecules hosted in the cavity, such as solubility, dissolution, absorption, bioavailability, and biological activity [
14,
15]. Various inclusion complexes have been synthesized, including cyclodextrins, both modified and native, highlighting the latter as the most used [
16].
Our article includes the most recent studies on food packaging using the CD, which have presented essential applications. Over the last decades, critical reviews such as Pereira et al. have developed [
17], reviewing the latest advances in the application of CDs and their current legislation. The main difference between their study and ours is that they focus on the general sensory qualities of all varieties, both native and modified, without indicating their direct (active packaging) or indirect (smart packaging) interaction with food and not highlighting the disadvantages of their application. More recent studies, such as Zhou et al. [
18], do not highlight the organoleptic qualities enhanced by this packaging method. This study sheds light on the improved properties of CD and the differences in the applications of the two packaging methods (active and intelligent), discerning their application disadvantages.
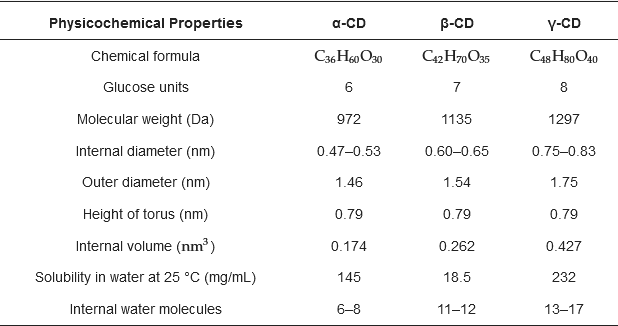
2. Cyclodextrins
Cyclodextrins (CDs) have piqued the scientific community’s interest due to their structural characteristics, for which various applications have been found. CDs are cyclic oligosaccharide structures composed of α-1,4 D-glucopyranoside bonds with a hydrophobic core inside and a hydrophilic outer surface due to the position of their hydroxyl groups [
19,
20,
21]. They can be differentiated by the number of glucoses that present their α, β, and γ structures. The main differences are found in
Table 1. The main characteristic that distinguishes CDs is their ability to easily interact with water due to their hydrophilic surface, which presents good solubility, and their interior hydrophobic cavity, which forms inclusion complexes with lipophilic molecules [
22,
23,
24,
25].
To know which CD is most likely to encapsulate a guest inside its cavity, the size of the hole must first be evaluated to see if host-guest interaction is possible. A-CD has the most minor cavity. Therefore, it cannot accept some large molecules. Γ-CD presents the largest cavity, being able to encapsulate larger molecules. A-CD and γ-CD present the highest solubilities in contrast to β-CD, which has an intermediate-size cavity and poor solubility compared to the first two (
Table 1). However, the most widely used due to its encapsulation performance is β-CD, added to the fact that it can encapsulate molecules such as lipids, vitamins, and other hydrophobic compounds [
26,
27]. Furthermore, it has the most outstanding strength due to hydrogen bond interactions, which provide greater interaction strength with the host molecule, avoiding its early release upon application [
28]. Β-CD consists of 7 glucopyranose units in the shape of a truncated cone [
29,
30,
31,
32]. It has been widely used as an encapsulant or carrier of food additives because it improves the water solubility of the guest component, as well as its permeability, and provides stability to lipophilic compounds thanks to the hydroxyl groups that allow it to form hydrogen bonds (weak bonds), resulting in its nonpolar character [
33,
34,
35,
36,
37].
Table 1. Main properties of the three native cyclodextrins. Created based on information from [
38,
39,
40].
3. Solubility and Toxicological Considerations
CDs have high solubility in water because their hydrophilic outer part is polar, possessing the ability to form stable emulsions due to the difference in polarity with their cavity [
22]. Solubility is a quality that can be attributed to CDs, making it a significant application option within packaging systems since it can release hydrophobic host molecules found in its cavity in the aqueous phase.
CDs have numerous uses in different industrial areas, such as chemistry, pharmaceuticals, food, etc. In the pharmaceutical field, native and modified CDs have been utilized as a drug release system due to their high solubility, easy dilution, and ability to improve the physicochemical properties of the guest molecule while maintaining the conditions specified on the package, such as effectiveness and purity, for established periods [
41,
42,
43,
44,
45]. Considerable improvements in active pharmaceutical ingredients (APIs) have been observed, presenting greater solubility in water, effectiveness, and physical and chemical stability [
46]. CDs have been used for some years in the chemical industry to increase the solubility of hydrophobic molecules; such examples are essential oils in perfumes [
47]. Specifically for food, CDs have been employed to take care of the organoleptic properties, increasing the shelf life of products and wholly or partially eliminating odors, flavors, and unwanted compounds. They are also applied as aroma stabilizers and increase the solubility of vitamins and lipids in aqueous systems [
22,
48,
49,
50,
51,
52,
53,
54,
55].
These processes of entrapment of molecules with a hydrophobic character are reversible when the inclusion complex encounters a solvent. In response to this interaction, the molecules are released into the medium in which this solution is found [
17].
Consequently, the CDs have obtained some recognition, namely their entry to the GRAS list (a list of food additives by the Food and Drug Administration that recognizes them as safe) and their approval by the European Medicines Agency (EMA), which allows their commercialization with a certain degree of purity [
56]. The World Health Organization (WHO) recommends a maximum of 5 mg/kg per day as a food additive. It is essential to consider this when devising an active container that will be in contact with the food and could be ingested by the consumer. The Environmental Protection Agency (EPA) also ruled out the need to have maximum permissible levels for the residues of the three main native CDs (α, β, and γ). Therefore, it is concluded that, according to all the available studies, CD presents an almost insignificant toxicity [
57,
58,
59].
4. Formation of Inclusion Complexes
The formation of inclusion complexes has been studied using analytical techniques to determine the possible structures that encapsulation can form [
60]. Inclusion complexes are formed when the host molecule, with the correct size for absorption, is positioned within the CD cavity. It should be mentioned that CD has the possibility of including both hydrophobic and hydrophilic molecules in its structure [
40,
54,
61]. Once encapsulated, the hydrophobic molecule can increase its water solubility and bioavailability [
62].
There are some parameters to consider before carrying out the encapsulation or packaging of the molecule inside the cavity of the CDs. The size of the cavity is essential to knowing if the host molecule could habituate in it.
Table 1 shows the respective measures for each native CD. In addition, it should also be considered that CDs crystallize by two mechanisms, which depend on the guest molecule and the CDs that form the complex. This will result in one of the categories of the crystal packing phenomenon resulting in channel or cage structures [
40].
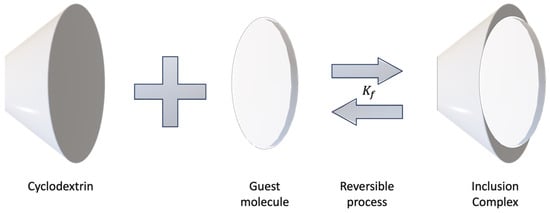
Inclusion complex formation is governed by the equilibrium association/dissociation constant for the host molecule, CDs, and inclusion complex [
63]. Because weak interactions govern CDs, the encapsulated host can break free of its environment without much effort [
40]. The higher the formation constant (
𝐾𝑓) for this reversible process (
Figure 1), the more stable the inclusion complex will be and the less in favor of dissociation [
35,
38].
Figure 1. Schematic illustration of the formation of an inclusion complex.
In the same way, the formation of inclusion complexes is also strongly affected by interaction forces such as hydrogen bonds, van der Waals forces, and electrostatic and hydrophobic interactions [
10,
35,
36]. In other words, to increase the entropy of the system, the water molecules located in the CD cavity are displaced by the hydrophobic molecules to form an apolar–apolar association, achieving better energetic stability for the complex [
34].
5. Cyclodextrins in Food Packaging
There has been an unceasing interest for a long time in preventing food spoilage by increasing its shelf life and controlling spoilage reactions such as the action of polyphenol oxidase (PPO) and enzymatic browning reactions. This is why microbial inhibition mechanisms, among others, have been studied to increase the shelf life of food [
70,
95,
96,
97,
98]. Many of the food packaging commonly sold in stores contains compound polymers derived from the petrochemical industry (low-density polyethylene, polyethylene terephthalate, and polypropylene, among others) that have the possibility of migrating into the food, altering its quality. These polymers also impact the environment due to their difficulty deleting [
99]. Therefore, the development of more environmentally friendly packaging has become a necessity.
Consumers are looking for more natural and organic foods that meet specific characteristics, including their nutritional needs and being regarded as healthy. That is why scientists and researchers have focused on developing new technologies that meet these requirements, including additives, treatments with modified atmospheres, and intelligent and active packaging techniques to preserve their nutritional value. Encapsulation is presented as an advantage to meet the needs requested by consumers, stabilizing the compounds present in food that cause their degradation, oxidation, and unpleasant flavors and odors, as well as improving sensory quality. It is a technology for packaging solids, liquids, and gases to be released under specific conditions, considering that they can also be affected by external factors such as temperature, light, humidity, etc. [
81].
Intelligent and active packaging are emerging technologies that are presented as a great potential option to erase the problem or somehow delay the deterioration of natural and everyday foods consumed, further satisfying the needs constantly changing within the international market. The benefit of taking advantage of these packaging systems lies in prolonging the shelf life of food products, proving them safe to consume, and simultaneously reducing food waste and negative environmental impacts [
100]. Active packaging that comes into contact with food releases, emits, absorbs, or scavenges substances directly or indirectly into the food to maintain quality or delay degradation. Intelligent packaging acts as an indicator that provides information on the quality of the product without coming into direct contact with the food. It provides information to the consumer about the condition of packaged food using materials that monitor and interact with the package’s environment through an internal or external indicator.
It has been observed that when molecules are encapsulated in β-CD, they increase their stability when subjected to light, temperature, and oxygen. Still, they can also be used to hide the unpleasant flavors of some biomolecules [
101,
102]. Likewise, multicomponent encapsulations are sometimes carried out using a third molecule that can affect the stability and interaction between the CD and the guest molecule [
103,
104].
This entry is adapted from the peer-reviewed paper 10.3390/polym15214317