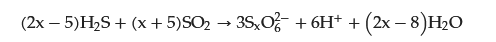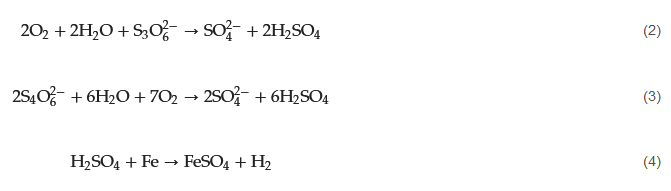Polythionic acid (PTA) corrosion is a significant challenge in the refinery industry, leading to equipment degradation, safety risks, and costly maintenance.
1. Introduction
Corrosion poses a significant challenge in today’s society, particularly in the industrial sector, where its impact on equipment longevity must be noticed. Recent industrial disasters have resulted in substantial financial losses, highlighting the urgent need to address corrosion-related issues. This concern has prompted petroleum, chemical, and mechanical engineers and chemists to consider the possibility of corrosion occurring in industrial plants thoroughly. It is now recognized that corrosion can influence the chemistry of processes and impact reaction efficiency and product quality. Furthermore, the detrimental effects of corrosion extend beyond financial losses, encompassing large-scale ecological damage [
1].
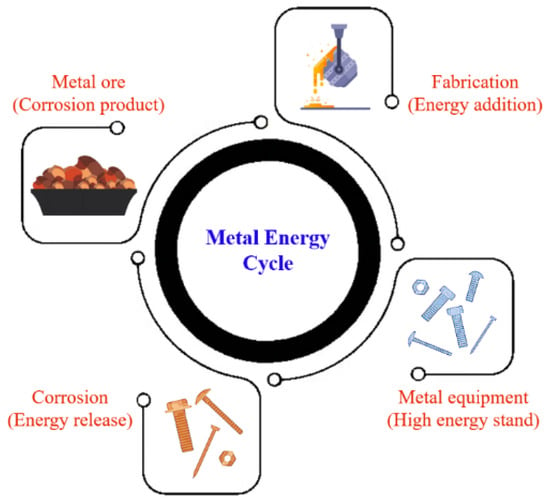
Metal corrosion is an irreversible process driven by the inherent tendency of metals, such as oxides, sulfides, or metal hydroxides, to transition to more stable and lower energy states. Most metals are naturally found in compound form in ores, except for noble metals like gold and platinum, as compounds provide greater thermodynamic stability than their elemental counterparts [
2]. The extraction of metals from ores demands a substantial amount of energy to convert them into their pure form. This energy input contributes to the eventual corrosion of the metal when exposed to external elements such as moisture and oxygen (O
2) (
Figure 1). The susceptibility of a metal to corrosion increases with the amount of energy required for its production [
3]. Although complete corrosion prevention is impossible, it can be mitigated by slowing down the process. Any factors that promote energy loss from the metal will accelerate corrosion. In industries like oil and gas, corrosion is influenced by various factors, including the presence of water (H
2O), carbon dioxide (CO
2), hydrogen sulfide (H
2S), high temperatures, pressures, and mechanical stresses [
4].
Figure 1. Energy cycle in metal corrosion.
Corrosion results from chemical reactions in a corrosive environment, often called electrolytes, which facilitate the transfer of electrons and ions (cations and anions) [
5]. These reactions can be categorized into two types: anodic and cathodic. During the anodic reaction, corrosion takes place at the anode. The metal at the anode combines with O
2 and releases free electrons through oxidation. Typically, the metal with a higher reduction potential is more susceptible to corrosion and is designated as the anode.
On the other hand, reduction occurs at the cathode as the metal accepts electrons from the anode [
6]. The corrosion of iron in contact with an electrolyte such as SOO is an example to illustrate the electrochemical reactions. When the electrolyte is slightly acidic, it dissociates into positive hydrogen ions (
H+) and negative hydroxide ions (
OH−). Upon immersing iron in the electrolyte, ionization occurs, causing the iron to dissolve as ferrous ions (Fe
2⁺). This ionization results from the difference in electric charge at the interface between the solid (iron) and the liquid (electrolyte). The Fe
2⁺, moving away from the metal surface, undergoes further oxidation, transforming into ferric ions (Fe
3⁺). These positively charged Fe
3⁺ are attracted to the negatively charged
OH− present in the electrolyte, leading to the formation of the corrosion product ferric hydroxide (Fe(OH)
3) [
7].
In the oil and gas industries, corrosion remains a paramount concern due to its profound impact on safety and the substantial economic burdens it imposes on the sector. Aggressive substances, specific flow conditions, and operating temperatures prevalent in oil refineries make corrosion pervasive across numerous processing units [
8]. The fluids transported within these industries, often characterized by high sulfur content, contain significant quantities of H
2S at concentrations ranging from 5 to 5000 ppm [
9]. In the refinery where equipment comes into contact with H
2S-containing fluids, the presence of H
2O and O
2 further exacerbates the corrosion potential, creating an environment ripe for corrosive attacks.
In the oil and gas industry, where challenges often arise from processing and handling sulfur-containing materials, understanding PTA corrosion is of paramount importance. This form of corrosion can significantly impact infrastructure and equipment, leading to the thinning of critical components and an elevated risk of structural failure. This, in turn, may result in leaks, ruptures, and, potentially, accidents. Additionally, PTA corrosion diminishes the operational lifespan of equipment, calling for expensive maintenance or replacement. It can also hamper the efficiency of essential processes, leading to reduced output and higher energy consumption. Understanding and reducing the corrosion of PTA in the oil and gas sector has significant practical benefits. It significantly enhances safety by preventing failures and maintaining equipment, reducing the risk of accidents and environmental harm. This approach also extends equipment lifetime, minimizing costly replacements and downtime for repairs. Moreover, it optimizes operational efficiency by ensuring consistent and productive operations, reducing energy consumption, and enhancing flow assurance. Addressing PTA corrosion not only ensures regulatory compliance but also protects the environment, mitigating risks of contamination. Overall, these measures lead to substantial cost savings, improved effectiveness, and a more sustainable and secure operation within the oil and gas industry. An examination of PTA corrosion issues in the oil and gas sectors uncovers several crucial areas warranting further investigation. One such area is the development of advanced coating technologies that provide greater resistance to PTA corrosion. Exploring new materials and adapting them to aggressive environments represents another crucial possibility for investigation. Additionally, a deeper understanding of the electrochemical processes underlying PTA corrosion and the factors influencing its initiation and propagation is essential. Furthermore, emerging technologies such as predictive modeling and real-time corrosion monitoring systems hold great promise in enhancing preventative measures.
2. Polythionic Acids (PTAs)
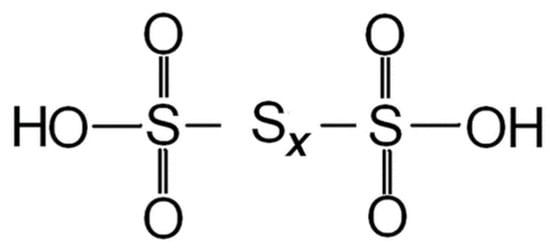
Thionic acids, including PTA, have gained significant attention in the study of crude oil composition and its corrosive properties. Crude oil is a complex mixture comprising a vast array of components, and, among these constituents, thionic acids stand out due to their sulfur and oxygen content. Thionic acids are characterized by sulfonic acid groups (-SO
2OH), which are directly connected or linked through sulfur atoms (S). The sulfur atoms within the thionic acid compound are solely attached to other S, distinguishing them from other sulfur-containing compounds [
12]. As a specific type of thionic acid, PTA exhibits a unique molecular structure characterized by multiple (more than two) sulfur atoms. It is classified as an oxoacid and features an un-branched and linear sulfur chain (-S-S-) attached to an end sulfonic acid group (-SO
3H), as shown in
Figure 2.
Figure 2. Structure of polythionic acid.
The chemical formula H
2S
xO
6 represents PTA, where x signifies the number of sulfur atoms in the chain. While PTA can potentially have an extensive range of x values, exceeding 50 for some compounds, the most commonly encountered PTA falls within the x range of 3 to 6 [
13].
PTA can be synthesized by reacting H₂S and sulfur dioxide (SO
2) in an aqueous solution. This reaction occurs under typical operating conditions, including ambient pressure, temperature, and a pH range of 3 to 2. When H
2S is oxidized in the liquid phase, it forms polythionate ions (
SxO2−6) (Equation (1)). The ratio of H
2S to SO
2 in the reactants strongly influences the type and distribution of the resulting products [
12]. In the presence of an excess of H
2S, short-chain polythionates, mainly
S4O2−6, are preferentially produced. Conversely, when SO
2 is the dominant reagent, the primary products are longer-chain polythionates with x values ranging from 4 to 8. The pH of the reaction environment also plays a significant role, with higher pH values leading to the formation of shorter polythionates and, ultimately, the generation of thiosulfate (
S2O2−3) at pH > 8 [
13].
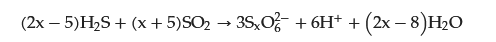
PTA exhibits stability only in aqueous solutions and rapidly decomposes at higher concentrations, releasing elemental S, SO
2, and occasionally sulfuric acid (H
2SO
4). Polythionate ions are significantly more stable than their corresponding acids, with PTA containing fewer S in the chain (x = 3, 4, 5, 6) being the most durable. Among these, H
2S
3O
6 is the least stable [
14]. The free acid form of PTA slowly decomposes in aqueous solutions, even at room temperature, resulting in the formation of S and sulfates (
SO2−4) as end products. The free acid exists solely as a colorless and odorless aqueous solution. In contrast, H
2S
4O
6 is the most stable of the PTAs and exhibits properties similar to H
2S
2O
6 in terms of its heat of neutralization with dilute sodium hydroxide and electrical conductivity [
15]. Both H
2S
5O
6 and H
2S
6O
6 acids are relatively stable in acidic solutions but decompose in nearly neutral or alkaline solutions, producing elemental S and lower polythionates (
S4O2−6 for pentathionic acid and
S5O2−6 for H
2S
6O
6) [
16].
2.1. Factors Affecting PTA
Several factors can affect PTA preparation, including (a) the type, concentration, and purity of sulfur-containing compounds used in PTA synthesis and (b) the reaction conditions, such as temperature, pH, and reaction time. Sulfur-containing compounds play a crucial role in preparing PTA as they can impact the final product’s properties, reactivity, and quality. Different sulfur-containing compounds used in PTA development can significantly affect the properties of the prepared PTA, including its concentration, stability, and corrosivity. For example, H
2S is a highly reactive and corrosive compound that readily reacts with oxygen and water, forming a highly reactive and corrosive PTA solution. PTA can also be synthesized using SO
2. However, due to the lower corrosivity of SO
2 compared to H
2S, the resulting PTA solutions exhibit lower reactivity [
17].
Temperature is crucial in PTA synthesis, affecting the reaction rate and product stability. The temperature must be carefully controlled within a generally suitable range of 20 °C to 30 °C to ensure the efficient conversion of the initial materials into PTA [
12]. However, at excessively high temperatures, the thermal instability of the compound (e.g., H
2S and SO
2) causes the molecular bonds in PTA to become increasingly unstable and weaker, leading to the breakdown of the chemical structure and thermal degradation of the resulting product [
19]. The reaction time significantly impacts the synthesis of PTA.
In the synthesis of PTA, the pH of the reaction medium plays a crucial role. The sulfur-rich compounds (e.g., H2S and SO2) used in the preparation of PTA are soluble in H₂O and stable at acidic pH levels. Consequently, PTA can exist and demonstrate improved stability under acidic conditions, typically ranging from pH 2 to 3, where its structure and properties are maintained.
Polythionate anions exhibit a remarkable property: they tend to form hydrophilic solutions in water, even at high S concentrations. In contrast, elemental sulfur is inherently hydrophobic, and the same applies to sulfur-rich compounds (R-Sx-R) with hydrophobic organic terminal groups. However, strongly hydrophilic groups like SO
3 can transform these hydrophobic substances into hydrophilic materials. When polythionate anions are dissolved in water, they demonstrate amphiphilic behavior [
20]. In the case of higher polythionates, the ions form colloidal structures and aggregate to create ion micelles.
2.2. Polythionates Measurement
Polythionates are challenging to analyze accurately due to their decomposing tendency, particularly in solution. Furthermore, individual polythionate species exhibit similar chemical and physical properties, making their characterization complex. A spectrophotometric method was proposed by Nietzel and De Sesa [
21] for determining low concentrations of tetrathionate (
S4O2−6) ions. This method involves the stoichiometric conversion of
S4O2−6 to thiocyanate through a reaction with cyanide in an alkaline medium. The excess ferric chloride then forms a red ferric thiocyanate complex. However, this method is unsuitable for measuring higher polythionate concentrations (mainly pentathionate and hexathionate) due to the decomposition experienced by these species.
3. PTA in the Refinery
PTA formation in refineries is commonly observed in units exposed to sulfur-containing compounds like H
2S and SO
2, particularly under corrosive conditions involving the presence of O
2, H
2O, high temperatures, and low pH [
25]. Units such as crude distillation, amine systems, and sour water strippers are particularly susceptible to PTA formation due to the high concentrations of sulfur compounds [
26]. The desulfurization processes employed in refineries, including oxidation-extraction desulfurization (OEDS), oxidative desulfurization (ODS), hydrodesulfurization (HDS), adsorptive desulfurization, and bio-desulfurization (BDS), can also contribute to the generation of polythionates as sulfur compounds are converted and transformed during these processes [
26].
Operating at high temperatures and pressures, refinery processes can lead to sensitization and reduced ductility in construction materials due to the presence of S and other impurities. The reactions of sulfur impurities with H
2O and O
2 result in the formation of H
2S and SO
2, which further react to form complex compounds such as
S4O2−6
, polythionates, and polythionic acid [
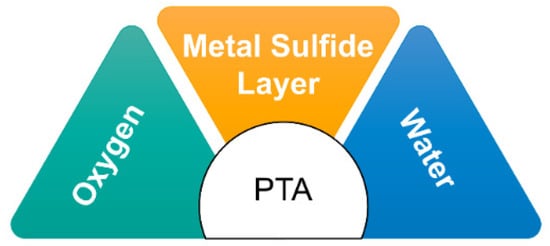
36]. PTA formation primarily occurs in refinery equipment through the reaction of O
2 and H
2O with sulfide corrosion products that accumulate on the internal surfaces of the equipment (
Figure 4). Moisture, often present from vessel washing or steaming during shutdowns, and oxygen from the air that enters when the vessel is opened contribute to the PTA formation process [
37].
Figure 4. PTA preparation factors.
While the high operational temperature may cause sensitization of stainless steel (SS), the actual formation of PTA occurs when the material is exposed to air at ambient temperature during shutdown periods [
37]. PTA can also be generated by the reaction of H
2O and O
2 with oxidizable sulfur species during the combustion of H
2S in refinery flares. Flare tips, typically composed of austenitic stainless steels (ASS) or high-nickel alloys, are particularly prone to attack by PTA. These acids act as cathodic depolarizers, facilitating metal dissolution at chromium-depleted grain boundaries through cathodic reduction [
9].
3.1. PTA Corrosion in Refiney
In the field of oil and gas operations, the selection of materials for equipment and infrastructure is a fundamental consideration as their susceptibility to corrosion greatly impacts the lifetime and safety of equipment and infrastructure. Different metals and alloys exhibit varying degrees of vulnerability to corrosion, which significantly impacts the lifetime and safety of equipment and infrastructure. For example, carbon steel, which is widely used in desalination plants, atmospheric desalination, vacuum distillation, catalytic cracking, visebreakers, cokers, and sour water separators, shows corrosion rates between 16 and 315 mpy, depending on the specific environment. It is particularly susceptible to localized pitting corrosion as well as high-temperature oxidation and sulfidation. Cr-Mo steels, present in desalters, atmospheric desalination, and hydrotreating units, have a corrosion rate of around 137 mpy. They may be prone to localized pitting corrosion, hydrogen-induced cracking (hydrogen flaking), and PTA corrosion during the hydrotreating process. Stainless steels of varying grades—316L, 321, and 304L—serve diverse functions in different units, with corrosion rates ranging from 16 to 383 mpy [
38]. Their susceptibility to localized pitting corrosion, intergranular cracking, and erosion–corrosion hinges on the specific alloy and environmental conditions. Alloys like Inconel, Monel, and Alloy 800, used in units like catalytic cracking and hydrotreating, exhibit corrosion rates that vary based on the specific alloy composition and prevailing conditions. Copper/nickel alloys, used in atmospheric desalination, record a corrosion rate of about 70 mpy. They are subject to localized pitting corrosion and flow-induced localized corrosion, primarily influenced by factors like naphthenic acid and sulfur.
Among the diverse range of metals applied to the oil and gas sector, carbon steel is used as a widely utilized material. However, due to its absence of protective alloying elements like chromium and nickel, it is particularly susceptible to PTA corrosion, especially in environments characterized by high-temperature sulfur compounds. In contrast, stainless steel, endowed with these protective elements, naturally becomes more susceptible to aggressive corrosive agents like PTA [
39]. However, some austenitic stainless steels like 304 and 316 can be prone to PTA corrosion due to their higher nickel content and potential sensitization along grain boundaries [
40].
Components in the oil and gas industry, especially those exposed to elevated temperatures and sulfur compounds, are primarily affected by PTA corrosion. Welded joints, commonly found in pipelines and structural elements, are particularly vulnerable due to alterations in their metallurgical structure during welding. This renders them more prone to corrosion, potentially leading to intergranular or transgranular cracking and compromising structural integrity [
43]. Heat exchangers are also notably susceptible to PTA corrosion. This susceptibility arises from the combination of high operating temperatures and potential exposure to sulfur compounds. The manifestation of PTA corrosion in heat exchangers is typically observed as pitting and localized corrosion. These corrosive attacks on the surface of the heat exchanger can significantly damage its heat transfer efficiency, resulting in decreased performance, increased energy consumption, and ultimately necessitating costly maintenance or replacement [
44]. Boilers, pressure vessels, and piping systems, experiencing both high temperatures and pressures, are at risk. PTA corrosion in these components leads to localized thinning, which poses safety hazards [
45]. Downhole tubing and casings, subjected to harsh downhole conditions, are susceptible to pitting that can weaken their load-bearing capacity. Valves and fittings, especially those in contact with corrosive fluids, face localized corrosion, potentially leading to leaks and reduced operational efficiency.
3.2. PTA Corrosion Mechanism
PTA corrosion in refineries occurs in environments containing sulfur-containing compounds such as H2S and SO2. The mechanism of PTA corrosion involves several stages, including the formation of PTA and polythionates, the attack on metal surfaces, and the acceleration of corrosion. When sulfur-containing compounds in refining processes come into contact with H2O, they undergo a series of chemical reactions and form H2SO4, which can further be oxidized (in the presence of O2) to form PTA.
The presence of PTA can lead to localized corrosion of metal surfaces by attacking the protective oxide layers on metal surfaces and initiating corrosion. When carbon steel is exposed to SxO2−6, it undergoes immediate attack, releasing hydrogen gas (H2) and forming Fe2⁺. These ferrous ions react with polythionates to create a protective layer of ferrous sulfate (FeSO4) on the metal surface (Equations (2)–(4)). This protective layer is a barrier, safeguarding the metal from further attack.
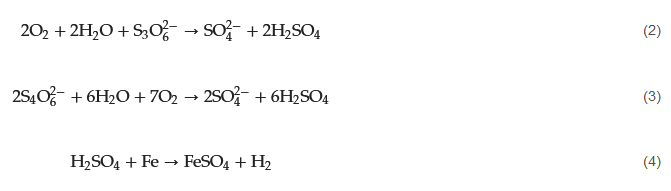
3.3. PTA Stress Corrosion Cracking (PTASCC)
Austenitic stainless alloys are commonly chosen as structural materials due to their favorable mechanical properties and corrosion resistance. However, under certain circumstances, these alloys can experience stress corrosion cracking (SCC) if they have not undergone appropriate fabrication treatments or are exposed to aggressive solution chemistries. SCC refers to the cracking of a material caused by the combined influence of tensile stress and a specific environment. The initiation and propagation of this type of corrosion form are influenced by factors such as sensitized materials (e.g., stainless steel with high carbon content, copper alloys, carbon steels, etc.), the presence of tensile stress (applied, thermal, or residual stress), and specific environmental conditions (e.g., aqueous solutions, moisture, chloride or caustic solutions, high-temperature water, PTA, etc.) [
47]. Aggressive ions are required to promote SCC for alloys that develop a protective film. In the case of austenitic stainless steel, PTA and other caustic substances and chlorides can disrupt the protective layer.
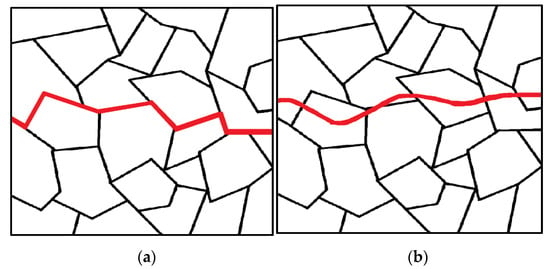
Cracks often originate from corrosion pits and surface imperfections and propagate in a brittle manner. The fracture behavior is not purely mechanical as it is strongly influenced by the corrosive nature of the environment [
48]. Once a crack initiates in the metal, it can propagate within the individual grains (transgranular) or along the boundaries between grains (intergranular) (
Figure 6). The change in fracture direction occurs when the crack encounters a new grain as the different orientations of atoms within each grain make it easier for the crack to change its path rather than continue tearing through the material [
49].
Figure 6. Intergranular fracture along (a) and transgranular fracture through (b) grain boundaries.
PTA is known to cause stress corrosion cracking (PTASCC) in ASS. PTASCC is a type of material failure typically occurring in areas with high stress or near welds. The challenge posed by PTASCC is prevalent in the oil refining sector, notably in desulfurizer, hydrocracker, and reformer processes. Typically, PTASCC is an issue that occurs internally, affecting the process-exposed side of heater tubes, vessels, or piping. Practically monitoring PTASCC is challenging since the cracking may not manifest until well into a turnaround [
45]. The cracking damage is localized and may not be apparent until a leak occurs. PTASCC is particularly severe, with the potential to lead to equipment failures within a day at room temperature. The propagation velocity of PTASCC cracks is faster than that of other forms of stress corrosion cracking. These cracks can propagate rapidly in minutes or hours, leading to containment loss and environmental damage [
50]. PTASCC usually happens in austenitic stainless steels and some nickel alloy steels that have been sensitized and develop a sulfide scale on their surfaces when exposed to air and moisture.
PTASCC is influenced by several critical factors, including the surrounding environment, material condition, and applied tensile stress. One essential requirement for PTASCC is the presence of a sensitized microstructure in the alloy characterized by chromium depletion near the grain boundaries. Sensitization typically happens when the metal is exposed to high temperatures ranging from approximately 400 to 800 °C, forming chromium-rich carbides along the grain boundaries. Even austenitic stainless steel grades with low carbon content and stabilization can undergo sensitization. PTASCC primarily manifests as intergranular cracking, propagating along the grain boundaries [
52].
3.4. PTASCC Mechanism
Understanding the mechanism of PTASCC is essential for applying effective preventive methods. PTASCC is a specific corrosion mechanism in at-risk materials under the combined influence of PTA and tensile stress. PTA concentration, alloy structure, stress level, and environmental conditions can impact the PTASCC mechanism. The PTASCC mechanism involves several steps: (a) formation of PTA through the reaction of O2, H2O, and sulfur-containing compounds; (b) initiation of the corrosion process by the adsorption of PTA molecules onto the metal surface; (c) application of tensile stress to the metal; (d) initiation of cracking due to the combination of PTA exposure and tensile stress; (e) propagation of cracks; and (f) formation of corrosion products. By understanding these steps, appropriate preventive measures can be implemented to mitigate the risk of PTASCC.
PTASCC primarily occurs in ASS. The presence of chromium (>10.5 wt%) is crucial for the stainless and austenitic properties of ASS as it enables the formation of a protective surface oxide layer [
54]. However, sensitization happens when carbon in the alloy reacts with chromium, leading to the formation of chromium carbides at the grain boundaries. This results in chromium depletion near the grain boundaries, making them vulnerable to corrosive environments, particularly acidic conditions, and causing rapid intergranular cracking.
3.5. Detection of PTASCC in Refinery
Detecting PTASCC is most important in preventing risky incidents and ensuring effective maintenance within refinery operations. Regular inspection, non-destructive testing (NDT), and risk-based inspection (RBI) are widely adopted in refineries to identify PTASCC and implement effective prevention and mitigation measures on time. However, each refinery may have specific inspection and detection protocols tailored to industry best practices and regulatory requirements. These practices are essential for safeguarding the integrity of refinery equipment and promoting the safe and efficient operation of the facility. It is worth noting that PTASCC detection is an ongoing process, and a combination of these methods can be employed to ensure comprehensive inspection and continuous monitoring of refinery equipment.
Regular inspection and evaluation of equipment and high-risk areas (e.g., piping, vessels, and tanks) by trained inspectors are crucial for reducing corrosion risks and extending the equipment’s lifespan. This proactive approach involves identifying signs of corrosion, thinning, cracking, and pitting, enabling early detection of damage and preventing equipment shutdowns or disruptions to production processes. Visual inspection, the most widely utilized technique, offers several advantages. It is cost-effective, can be conducted while work is in progress, and allows for the early correction of faults.
NDT techniques are commonly utilized in refineries for the timely detection of PTASCC, ensuring its prevention and mitigation. These methods offer a cost-effective approach to corrosion detection without causing significant operational disruption. Refineries can detect PTASCC without significantly impacting their operations by utilizing these NDT methods, effectively addressing corrosion-related concerns. NDT techniques commonly include infrared thermography, radiography examination, ultrasonic inspection, and eddy current [
58].
4. Prevention of PTA Corrosion
4.1. Material Selection
Highly alloyed materials are required for effective resistance to different types of corrosion, such as general and PTA corrosion. These materials must have high chromium and nickel content to resist corrosion. Additionally, stabilization with titanium or niobium is necessary to resist intergranular sensitivity and reduce PTA corrosion. Austenitic stainless steel (ASS) is an excellent choice for PTA corrosion. ASS contains high levels of nickel and chromium, which allows the formation of a very thin (1–3 nm) chromium oxide/hydroxide-rich passive film, giving it excellent corrosion resistance [
61]. Thus, selecting the appropriate grade of ASS prevents PTA corrosion as the material’s microstructure significantly influences its susceptibility to corrosion. Notably, types 321, 347, and 347LN have shown high resistance against PTA corrosion [
62].
On the other hand, materials that are not resistant to PTASCC include some sensitized alloys that are susceptible to the corrosive effects of PTA. This proneness can occur when certain alloys are exposed to specific environmental conditions. The materials composed of austenitic stainless steels, high-nickel alloys, and iron–nickel–chromium alloys are open to attack by PTA. These acids act as cathodic depolarizers, facilitating metal dissolution at chromium-depleted grain boundaries through cathodic reduction [
9].
It was observed that Undeformed AISI 304, sensitized at 500 °C for 24 h, exhibited a ductile fracture in the PTA solution due to its limited chromium-depleted zone, reducing PTASCC susceptibility [
63]. Cold rolling at 20% and 40% before sensitization (at 500 °C for 24 h) made stainless steel prone to PTASCC, which is attributed to severe chromium depletion. Deformation beyond 40% prevented PTASCC despite higher sensitization levels. Only 20% and 40% deformation induced sufficient chromium depletion along grain boundaries for crack propagation. Deformation greater than 40% did not induce this effect, even with a higher degree of sensitization (at 60% deformation).
4.2. Nitrogen Purging
This method involves purging the equipment by displacing the oxygen present in the environment with nitrogen, leading to the generation of an inert environment during shutdown and preventing PTA formation. Moreover, this method can eliminate existing PTA from the metal surface, decreasing maintenance requirements. Additionally, nitrogen’s non-toxicity and non-flammability certify the operational safety and environmental friendliness of this approach. The method is applicable during start-ups, shutdowns, and maintenance processes, proving particularly beneficial for preserving catalysts. Ensuring that the nitrogen used is dry and free of O
2 is crucial as commercial nitrogen often contains around 1000 ppm of O
2. When steam is employed for purging, steam injection should be halted before the metal temperature drops to 72 °C (130 °F).
4.3. Alkaline Washing
The standard method for protecting sensitized stainless steel involves either preventing the formation of PTAs or neutralizing them. To neutralize PTA, washing the equipment with a weak soda ash solution (1–5%) before exposing it to air is recommended. It is essential to soak the equipment for at least 2 h to ensure effective neutralization. Simply spraying the equipment with a soda ash solution is insufficient to prevent PTA formation. If deposits or sludge are present, the solution should be circulated vigorously for at least 2 h [
66]. Using a soda ash solution for neutralizing acids should consider the formation of a Na
2CO
3 film that can further neutralize acids. It is advisable to assess the influence of alkaline chemicals on catalysts before employing a soda ash wash. Equipment should be hydrojetted with a soda ash solution and reinstalled with the residual soda ash film on surfaces.
4.4. Amide Solutions
An alternative approach to washing and neutralizing with an aqueous alkali solution addresses the challenges posed by stress-corrosion cracking due to repulsion by sulfide-containing fluids on the equipment’s surface. Additionally, residual aqueous alkali solution in certain areas can lead to corrosion, making the procedure complex. Instead, washing the equipment with amide solutions prevents the formation of PTA when iron sulfide contacts mineral oil, effectively safeguarding against stress-corrosion cracking of austenitic stainless steel. This technique ensures adequate protection of metal surfaces from PTA-induced corrosion, providing increased durability and dependability for metal equipment exposed to sulfide-containing fluids by leveraging the unique properties of amides and their derivatives to prevent stress-corrosion cracking [
67].
4.5. Dry Air
Eliminating moisture is vital for suppressing corrosion rates in atmospheric conditions. The dry air method effectively limits the risk of polythionic acid (PTA) corrosion by preventing free water formation, a crucial component in PTA production. The dry air method effectively mitigates the risk of PTA corrosion by reducing moisture in the environment to a level where surface wetness cannot form. This preventative technique is essential when metal surfaces are susceptible to corrosion or exposed to sulfur-containing components. Utilizing dry (dehumidified) air offers a cost-effective means to prevent free water formation and reduce the risk of PTASCC. When handling non-regenerable catalysts, which may be pyrophoric, it is essential to keep them moist or isolated from oxygen.
5. Conclusions
Future investigation in PTA corrosion prevention could explore several promising directions. One avenue is the development of smart coatings and protective materials tailored to resist PTA corrosion under specific environmental conditions. Investigating advanced monitoring technologies, such as sensors and non-destructive testing methods, could enable real-time corrosion detection and intervention. Exploring innovative alloy compositions with enhanced resistance to PTA corrosion is another area of interest. Additionally, delving into eco-friendly inhibitors and coatings aligns with sustainability goals in the industry. These avenues collectively offer exciting opportunities to enhance PTA corrosion prevention measures in the oil and gas sector.
This entry is adapted from the peer-reviewed paper 10.3390/ma16217043