Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
β-Ti alloys have long been investigated and applied in the biomedical field due to their exceptional mechanical properties, ductility, and corrosion resistance. Metastable β-Ti alloys have garnered interest in the realm of biomaterials owing to their notably low elastic modulus. Nevertheless, the inherent correlation between a low elastic modulus and relatively reduced strength persists, even in the case of metastable β-Ti alloys. Enhancing the strength of alloys contributes to improving their fatigue resistance, thereby preventing an implant material from failure in clinical usage.
- biomedical alloy
- high-entropy alloys
- medium-entropy alloy
- metastable
- β-Ti alloys
1. Introduction
In the field of biomedical materials, the quest for ideal implant materials that simultaneously possess excellent mechanical properties, corrosion resistance, and biocompatibility remains a paramount challenge. Conventional 316L stainless steel, Co-based alloys, and Ti-based alloys have long served as the industry standards for hip and knee joint replacements [1]. However, the ever-increasing demand for these materials, driven by an aging population and expanding medical applications, has exposed their limitations.
316L stainless steel, while widely used, exhibits insufficient corrosion resistance and wear resistance, leading to issues like stress corrosion and the release of allergenic Ni and toxic Cr ions [2]. Similarly, the Co–Cr–Mo alloy exhibits excellent corrosion resistance attributed to the formation of a passive Cr2O3 passivation layer on its surface. However, when this passivation layer wears off, its corrosion resistance significantly decreases [3]. The corrosion products of Co–Cr–Mo alloy are also more biologically toxic than those of 316L stainless steel [4]. Ti–6Al–4V ELI alloy exhibits excellent mechanical strength, lower elastic modulus, and good biocompatibility. However, the Al ions in the alloy are suspected to be associated with symptoms such as peripheral neuropathy, osteomalacia, and Alzheimer’s disease [5]. Additionally, V ions can induce cytotoxicity [5]. Moreover, when the elastic modulus of implant materials significantly exceeds that of human cortical bone, it can lead to stress shielding, causing bone tissue atrophy and implant failure [6]. The elastic moduli of 316L stainless steel (210 GPa), Co–Cr–Mo (240 GPa), and Ti–6Al–4V ELI (110 GPa) are much higher than that of the human cortical bone (about 30 GPa) [6], making them less suitable as a biomedical implant.
In recent years, metastable β-Ti alloys have gained attention for their low elastic moduli and outstanding properties, which encompass high specific strength, low elastic modulus, excellent fatigue resistance, high toughness, exceptional corrosion resistance, and remarkable biocompatibility [7]. These alloys present a promising alternative to traditional implant materials, addressing the issue of stress shielding effectively. Their exceptional blend of characteristics renders them as excellent candidates for orthopedic and dental implant applications. However, metastable β-Ti alloys with low elastic moduli often exhibit concomitantly reduced mechanical strength [7]. Furthermore, the advent of High-Entropy Alloys (HEAs) has revolutionized alloy design by incorporating multiple primary elements, offering new possibilities for orthopedic and dental implant applications [8]. However, challenges, such as compositional segregation and a high elastic modulus, still persist.
2. Metastable β-Phase Biomedical Ti Alloys
2.1. Phase Stability Calculations
Ti alloy systems are primarily categorized into α (Hexagonal), α + β (hexagonal + body-centered cubic), and β (body-centered cubic) types, with the phase structure depending on the types of solute elements present in the alloy. Alloys with approximately 1–2 wt% of β stabilizers and around 5–10 wt% of the β phase are classified as near-α alloys. Alloys containing higher levels of β stabilizers, resulting in 10–30 wt% of the β phase in the microstructure, are categorized as α + β alloys. Alloys with even higher β stabilizers, allowing the β phase retention through rapid cooling, are termed as metastable β-Ti alloys [9]. Commonly used β-stabilizing elements include Nb, Ta, Mo, and W, etc. Additionally, Zr, Sn, and Si are neutral elements. It is important to note that Zr and Sn are identified as β-stabilizing elements in the Ti–Nb alloy system [10,11].
The commonly used theories for the phase stability of Ti alloys are: (1) bond order (Bo) and d-orbital energy level (Md), (2) valence electron concentration (VEC), (3) Mo equivalent ([Mo]eq), and (4) martensite start temperature (Ms). The Bo-Md theory is based on DV-Xα-Cluster molecular orbital calculations, utilizing Bo and Md to predict the stability and phase transformation of Ti alloys. The calculation formulas for the Bo and Md values of Ti alloys are as follows [7]: Bo = ΣXi(Bo)i, Md = ΣXi(Md)i, where Xi is the atomic fraction of each element in the alloy, and (Bo)i and (Md)i are the Bo and Md values of alloy element i in β-type Ti alloy, respectively.
Ikehata et al. [12] used first-principles calculations to study the effect of VEC in binary Ti alloy systems on elastic modulus, suggesting that the VEC value of the alloy predicts its crystal structure. When the VEC value of the alloy is approximately 4.2–4.24, the alloy can reach the BCC metastable state and obtain a lower elastic modulus.
[Mo]eq is an empirical formula derived from experimental results [13] that quantifies the contribution of each alloying element to the β-phase stability of Ti alloys. When the [Mo]eq value is less than 10 wt% and close to 10 wt%, the alloy achieves a metastable β-phase, resulting in a lower elastic modulus [14]. The formula for calculating [Mo]eq is as follows [13]: [Mo]eq = 1.0 [Mo] + 0.74 [V] + 0.50 [W] + 0.39 [Nb] + 0.28 [Ta] + 2.2 [Fe] + 1.69 [Cr] + 0.85 [Cu] + 1.22 [Ni] + 1.57 [Co] + 1.69 [Mn] + 0 [Sn] + 0 [Zr] − 1.0 [Al] (wt%).
Furthermore, within the design process, adjusting the compositions of Ti alloys to shift the Ms closer to room temperature, a Ti alloy with a metastable β phase at room temperature can be obtained. The Ms temperature formula for binary Ti alloy systems is [15]: Ms (°C) = 1156 − 150[Fe] − 96[Cr] − 49[Mo] − 37[V] − 17[Nb] − 7[Zr] + 15[Al] (wt%).
2.2. Advantages of Metastable β-Ti Alloys
Among the developed biomedical Ti alloys, metastable β-Ti alloys typically exhibit lower elastic moduli [16]. Figure 1 presents a comparison of the elastic moduli of conventional biomedical metals (Co–Cr–Mo, 316L, and Ti–6Al–4V), metastable β-Ti alloys, and the human cortical bone [17,18,19,20,21,22,23,24,25,26,27]. Metastable β-Ti alloys with a low elastic modulus include Ti–12Mo–6Zr–2Fe [20] and Ti–15Mo [28], which have been included in ASTM standards.
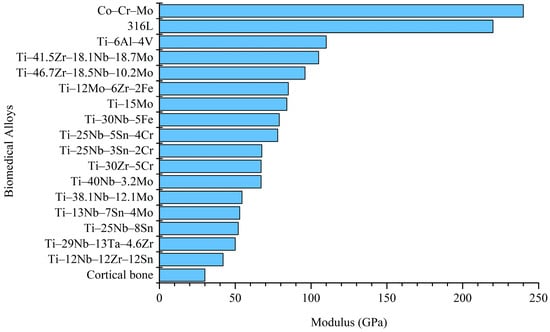
In 1998, Niinomi [16] and Kuroda et al. [26] developed the Ti–29Nb–13Ta–4.6Zr (TNTZ) metastable β-Ti alloy with an elastic modulus of 65 GPa using the d-electron design method, with Bo and Md values of 2.878 and 2.462, respectively. Additionally, Ho et al.’s [14] study on Ti–Mo alloys found that when [Mo]eq is below 10 wt% and close to 10 wt%, the alloy achieves a metastable β phase. Subsequently, in 2005, Hao et al. [29] developed the Ti–24Nb–4Zr–7.9Sn (TNZS) metastable β-Ti alloy with an elastic modulus of 42 GPa using the VEC theory, with a VEC value of 4.15. Hao et al. [30] reported that when the VEC of β-Ti alloys is within the range of 4.1 to 4.25, it may induce the formation of metastable α″ and ω phases. Suppression of these metastable phases (α″ and ω) can lead to obtaining a metastable β phase with an exceptionally low modulus.
In addition to mechanical performance, biomedical metals need to demonstrate excellent corrosion resistance and biocompatibility. Common biomedical β-Ti alloys are composed of toxic-free elements such as Ti, Zr, Nb, Ta, and Mo. A substantial body of literature has confirmed that the excellent corrosion resistance of β-type biomedical Ti alloys is attributed to the high stability of their oxidation passivation layer [31]. Taking Ti–Zr–Nb alloy as an example, the surface oxide layer is composed of oxides such as TiO2, ZrO2, Nb2O3, and Ta2O3 [31]. These oxides contribute significantly to the formation of a continuous and stable oxidation passivation layer on the alloy’s surface [31]. On the other hand, the biocompatibility of elements such as Ti, Zr, Nb, Ta, and Mo has been confirmed through in vitro cell culture tests [32,33]. Moreover, the Ti and Zr elements further promote the growth of fibrillar adhesions of osteoblasts [32].
The design of Ti alloys in the metastable β-phase achieves an ultra-low elastic modulus while maintaining good biocompatibility. However, in metastable β-Ti alloys, there is a trade-off between a low elastic modulus and high yield strength, resulting in low yield strength while maintaining a low elastic modulus [34]. Increasing the strength of the implant material not only contributes to applications in various hard tissues in the human body but also enhances the material’s fatigue resistance, reducing the likelihood of clinical failure and extending the life of the implant material [35]. Furthermore, increasing hardness helps overcome the poor wear resistance of conventional Ti alloys [36]. Therefore, the inadequate mechanical properties of conventional biomedical alloys have created an opportunity for the development of high-entropy alloys in the field of biomedical metal implants.
This entry is adapted from the peer-reviewed paper 10.3390/ma16217046
This entry is offline, you can click here to edit this entry!
