Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Physical exercise has emerged as a promising non-pharmacological intervention for Alzheimer’s disease (AD), with demonstrated effects on promoting neurogenesis, activating neurotrophic factors, reducing Aβ aggregates, minimizing the formation of neurofibrillary tangles (NFTs), dampening inflammatory processes, mitigating oxidative stress, and improving the functionality of the neurovascular unit (NVU). Overall, the neuroprotective effects of exercise are not singular, but are multi-targets.
- physical exercise
- Alzheimer’s disease
1. Introduction
Numerous studies conducted on mouse AD models have consistently highlighted the positive effects of exercise and physical activity on memory-related tasks. This correlation extends to individuals diagnosed with AD, where engaging in exercise and physical activity has demonstrated tangible enhancements in mental well-being, cognitive progress, and brain functionality (Figure 1) [1][2][3][4].
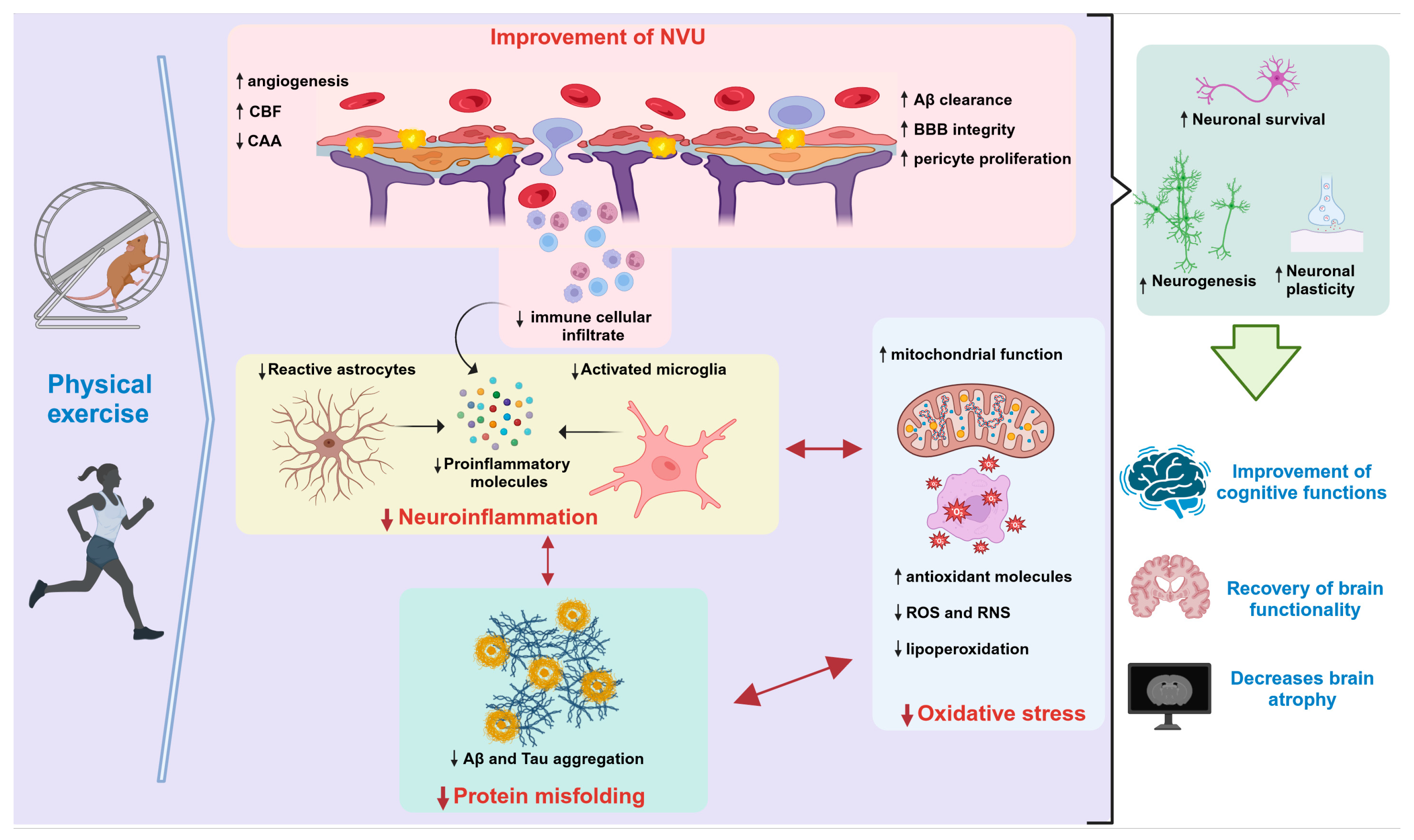
Figure 1. Neuroprotective effects of physical exercise in AD. Abbreviations: Aβ: amyloid beta; BBB: blood–brain barrier; CAA: cerebral amyloid angiopathy; CBF: cerebral blood flow; NVU: neurovascular unit; RNS: reactive nitrogen species; ROS: reactive oxygen species. ↑ (Increase) ↓ (Decrease). This figure was created with BioRender.com (accessed on 13 October 2023).
Exercise triggers a cascade of cellular and molecular transformations within the mouse brain. These cascades facilitate a range of physiological phenomena, including the promotion of neurogenesis and the activation of neurotrophic factors. These factors, such as those contributing to long-term potentiation (LTP), play pivotal roles in bolstering learning, memory, and neural plasticity. Moreover, exercise contributes to a reduction in Aβ peptides, minimizes the formation of NFTs, dampens inflammatory processes, and mitigates oxidative stress (as depicted in Figure 1 and Figure 1) [5][6]. Therefore, physical exercise exerts neuroprotective effects by acting on multiple targets, rather than relying on a single mechanism.
2. Beneficial Mechanisms of Physical Exercise on Neuropathological Hallmarks of AD
The core neuropathological characteristics of AD encompass Aβ peptide and the hyperphosphorylated tau protein. Consequently, most of the research pertaining to the influence of exercise has been centered on assessing its impact on these defining attributes. In multiple investigations employing murine disease models, interventions involving both mandatory and voluntary exercise have consistently demonstrated a dampening effect on these traits [7][8][9][10]. Several potential mechanisms have emerged, encompassing diminished Aβ production, augmented Aβ clearance, and reduced NFTs (Figure 1) [11][12].
Patient studies employ specialized tools like positron emission tomography (PET) radiotracers, including the Pittsburgh B (PiB) compound, and magnetic resonance imaging (MRI) to effectively identify Aβ peptide deposits and their neurological consequences. These techniques also allow for a precise assessment of how exercise impacts these pathological markers. By using 11C-PiB PET, researchers obtain images of Aβ aggregates and measure the cerebral quantity of Aβ [13][14][15]. Notably, these studies highlight that engaging in physical activity could potentially enhance the clearance of Aβ 1–42 or reduce its deposition [16][17]. However, to thoroughly understand the effects of exercise on both Aβ and tau imaging, rigorous clinical trials are essential. These trials would bridge the gap between experimental findings and potential applications for AD diagnosis and treatment.
Animal studies have demonstrated that interventions involving forced and voluntary exercise can attenuate neuropathological signs [7][8][9][10]. Concerning Aβ peptide levels, physical exercise appears to influence volume modulation, particularly for Aβ 1–42, in brain regions like the hippocampus and neocortex [18]. In models of tauopathy, exercise has been shown to reduce brain tau phosphorylation [19]. This cumulative body of evidence suggests that exercise could hold promise as a therapeutic strategy to address the neuropathological aspects of AD.
3. Impact of Physical Exercise on Neurotrophins
Neurotrophic factors (NTFs) and their receptors play a crucial role in neural cell maturation and proliferation, regulate the development and survival of neurons, and appear to be involved in the endogenous neuroprotection of different neurons [20]. NTFs support neuronal survival and function in the adult central nervous system (CNS), generating broad interest in the use of these factors to intervene in neurodegenerative diseases [21].
Most of the favorable effects of exercise in AD have been ascribed to signaling enhancement and the release of NTFs, notably brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), glia-derived neurotrophic factor (GDNF), and type 1 insulin-like growth factor (IGF-1). These factors are essential in maintaining neuronal functionality and fostering neuroplasticity (Figure 1) [22]. Decreased levels of BDNF in blood plasma have been linked to neurodegeneration, a decline in hippocampal volume, and cognitive impairment associated with AD [22]. Nonetheless, BDNF activates an array of signaling pathways that are integral to neural function, and its levels have a positive impact on memory and a reduction in cognitive decline, as evidenced through exercise interventions [22][23][24][25].
On the other hand, NGF plays a pivotal role in ensuring the survival of cholinergic neurons and shielding them against chemical stressors. In the context of AD, a reduction in NGF has been linked to cognitive deterioration, while exercise in AD mouse models has demonstrated its potential to enhance the expression of this factor. Nevertheless, exercise has inconsistent effects on NGF levels in patients [22]. Conversely, diminished levels of IGF-1 have been associated with both aging and AD [26][27]. However, a rat model of AD exhibited elevated serum IGF-1 levels and improved working memory following aerobic and resistance exercise interventions, suggesting a positive impact on cognition [27]. Similarly, in patients with AD, exercise has the capacity to elevate IGF-1 levels, thereby potentially yielding enduring effects [26]. This collective understanding underscores exercise’s multifaceted impact on essential NTFs, offering a promising avenue for addressing cognitive impairments in AD.
4. Influence of Physical Exercise on Neuroinflammation
Neuroinflammation is an inflammatory reaction in the CNS that includes immune cell infiltration, microglial activation, and pro-inflammatory cytokine release (Figure 1) [28]. In postmortem AD brains, inflammatory markers have been identified surrounding the extracellular Aβ deposits [29]. In murine models of AD, exercise has a downregulating effect on proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, contributing to a less inflammatory environment [30][31]. Moreover, exercise triggers notable morphological and functional transformations in astrocytes by promoting their growth and attenuating astrogliosis. This suggests that physical activity can potentially modulate inflammatory responses within the brain, particularly in the hippocampus [32][33]. Cognitive improvements induced by exercise in mice are associated with the modulation of neuroinflammation [34].
Likewise, in individuals with AD, elevated levels of cytokine profiles, such as IL-6, TNF-α, and IL-1β, have been seen in plasma and cerebrospinal fluid samples [35][36]. These cytokines are known to stem from reactive astrocytes and microglia, as observed in postmortem AD patient brains [37][38][39], and impact gliotransmission and neurotransmitter uptake, which are closely associated with cognitive dysfunction [40]. Notably, aerobic exercise can reduce elevated cytokine levels [41][42], suggesting that it can potentially counteract the inflammation-related cognitive decline observed in AD.
5. Effects of Exercise on Oxidative Stress
In AD, an elevation in ROS and reactive nitrogen species (RNS) production leads to oxidative stress, exacerbating damage linked to inflammatory responses and instigating neurodegeneration through apoptosis [43]. Interestingly, physical exercise emerges as a significant contributor to neuronal activation in the hippocampal region, as it requires a heightened mitochondrial capacity to generate ATP via the oxidative phosphorylation of glucose. Paradoxically, this heightened oxidative activity also triggers ROS accumulation, posing a threat to neurons [44]. However, exercise initiates a cascade of counteractive mechanisms that enhance mitochondrial function and attenuate the impact of ROS (Figure 1) [45]. Enzymes like superoxide dismutase 1 and 2 (SOD1 and SOD2) and increased catalase levels, elevated through exercise, augment brain antioxidant capacity [8][9][27]. Notably, chronic exercise in AD mouse models boosts glutathione (GSH), the primary antioxidant enzyme, along with the essential tripeptide (glutamate, cysteine, and glycine) [27][46].
In AD, elevated lipid peroxidation contributes to neuronal membrane damage. Malondialdehyde is a significant marker of this deterioration, but aerobic exercise, resistance exercise, and forced swimming interventions have demonstrated the ability to diminish it [27]. Patient studies substantiate the efficacy of exercise in decreasing pro-oxidant parameters and bolstering antioxidant capacity, irrespective of exercise type or intensity. Exercise has also been linked to reduced ROS levels and catalase serum activity, accompanied by heightened nitrite levels and interleukin 4 (IL4), affirming its potency in curbing oxidative stress in AD [47].
Collectively, the interplay between oxidative stress and physical exercise in AD underscores the potential of exercise interventions to strategically counteract the detrimental effects of ROS and RNS production. These insights not only shed light on the multifaceted nature of AD pathogenesis but also present a compelling case for the inclusion of exercise as a promising adjunctive therapeutic strategy for attenuating the oxidative burden and potentially slowing the progression of AD.
6. Effects of Exercise on Neurotransmitters
Neuropsychiatric symptoms during AD-like aggression, agitation, and depression are attributed to an alteration in neurotransmission systems, including the serotoninergic, dopaminergic, noradrenergic, and cholinergic systems [48]. Cholinergic system alterations are present in the early stages of the disease and are exacerbated with degeneration [49][50]. In the Veronese study, it is proposed that exercise modulates neurotransmitter production, thereby decreasing depressive behavior and aggression [48]. It has been found that voluntary and forced physical exercise prevent the loss of cholinergic innervation to the hippocampus as well as the decrease in cholinergic fibers, resulting in improved cognition and motor skills [51].
Serotonergic system activation through physical exercise benefits cognitive performance and the emotional component. Animal studies using physical exercise have reported an increase in the activity of serotonergic neurons. This effect has been seen with both acute and chronic exercise [51].
Another neurotransmission system that benefits from the practice of exercise is the dopaminergic system. Dopamine levels rise in the hippocampus after exercise. Moreover, in an animal study with an aerobic exercise intervention, such as swimming, researchers found increased dopamine levels that were associated with memory improvement [51].
Regarding norepinephrine (NE), studies have shown that long-term exercise may lead to increased levels of NE [51]. Voluntary exercise in mouse models increases NE levels in the hippocampus and amygdala, which is proposed to contribute to the control of neuropsychiatric symptoms [48].
In patients, exercise intervention can increase the availability of tryptophan, a serotonin precursor that can decrease depressive symptoms and improve cognition [48][51]. Concerning dopamine, receptor affinity is enhanced, and dopamine levels increase in the hippocampus [51][52]. In patients with MCI, aerobic exercise has been shown to increase NE levels and improve memory [53]. However, there are no data regarding the effects on acetylcholine levels in patients with this type of intervention.
The relationship between exercise and neurotransmission systems presents a hopeful avenue for enhancing cognitive function and managing neuropsychiatric symptoms in AD. Further exploration through clinical trials and comprehensive studies will be essential to fully understand the potential of exercise as a therapeutic strategy for alleviating the cognitive and emotional burdens associated with the disease.
7. Effects of Exercise on Neurogenic and Anatomical Aspects
In their seminal studies, Van Praag and colleagues (1999) [54] established the existence of neurogenic zones in the hippocampus of adult mice. These zones are situated in the subventricular and subgranular regions of the dentate gyrus [55]. In both regions, voluntary wheel running has been linked to an augmentation in the maturation of new neurons, complementing their proliferation, survival, and differentiation into new entities [56][57][58][59].
Aerobic exercise and physical activity also attenuate brain atrophy, which is correlated with gray matter volume and white matter diffusion tensor imaging, as well as with cognitive severity [60][61][62]. This effect is rooted in the capacity of exercise to induce plasticity and functional changes in the brain. Similar structural modifications have been demonstrated in mouse models of AD, where both enriched environments and physical exercise interventions induced distinct structural changes within regions like the cerebellum, cerebral cortex, and hippocampus when compared to sedentary groups [63][64].
Human studies involving histological markers and cellular division assessments via BrDU or Carbon 14 have further validated hippocampal neurogenesis in adults [65][66]. Additionally, the concept of separation patterns, denoting the differentiation of similar experiences via distinct activity patterns, has been associated with the observation of neurogenesis in the hippocampus. This correlation is supported by studies of young adult participants who underwent light physical exercise interventions. These studies revealed memory enhancement through the mediation of separation patterns within the dentate gyrus [65][67].
The integration of these insights into clinical practice holds tremendous potential. Incorporating exercise interventions as a proactive approach to stimulate neurogenesis and combat brain atrophy could pave the way for novel strategies in treating neurodegenerative conditions, including AD (Figure 1). By harnessing the power of exercise and leveraging these findings to promote neuroplasticity and structural changes, scientists can substantially improve cognitive health and enhance quality of life.
8. Effects of Exercise on Cognition
Considering that AD predominantly manifests with cognitive symptoms rather than motor deficits, it is imperative to comprehensively assess the impact of exercise on cognition. Thus, a strong body of evidence has emerged from animal models [59]. Studies in AD mouse models have evaluated a range of parameters, including learning, spatial memory, working memory, exploratory behavior, and affective behavior. Although some results have been inconsistent [68][69], possibly due to the diversity of the utilized models, variations in exercise protocols, and the spectrum of behaviors assessed, most studies agree that physical exercise has a positive impact and no detrimental effects on cognitive function have been observed [70][71].
In studies involving human patients, the findings remain heterogeneous. A systematic review covering 13 studies with a total of 869 AD patients unveiled significant effects in eight studies, while five reported no discernible changes following interventions [72]. Another comprehensive report encompassing 1145 patients with MCI and AD revealed favorable outcomes attributed to aerobic exercise [73]. Subsequently, a study by Demurtas et al. (2020), encompassing 14,209 patients with MCI and dementia, highlighted positive exercise effects on global cognition, although no disparities were noted in specific evaluations of attention, executive function, and memory [74].
Consequently, discerning the precise impact of exercise on cognition is complex due to the dearth of specific exercise guidelines for brain health and AD. This complexity arises from the diverse array of protocols applied across different disease stages as well as the heterogeneous assessment methods and scales employed, all of which contribute to the variances in outcomes. As such, substantiating the benefits of exercise on cognitive functions in the context of AD requires a more robust body of evidence.
9. Effects of Exercise on the Neurovascular Unit
Aβ peptide deposition is not exclusive to neuritic plaques within the brain parenchyma. It also accumulates in cerebral blood vessels, leading to cerebral amyloid angiopathy (CAA) [75]. The histopathological transformations observed in CAA are intimately interconnected with the neurovascular unit (NVU) and blood–brain barrier (BBB) dysfunction. In AD, the integrity of both the BBB and cerebral blood flow is compromised due to NVU components, such as pericyte degeneration, endothelial cell alterations, astrocytic foot dysfunction, and basal membrane deterioration, which collectively exacerbate disease progression [76]. Although specific treatments for vascular dysfunctions are lacking, exercise emerges as a potential therapeutic measure.
Evidence from the TgCRND8 model of vascular amyloidosis indicates that exercise intervention yields reductions in the CAA along with hippocampal vasculature normalization [77]. Similarly, researchers' working group demonstrated CAA reduction after three months of voluntary exercise, coupled with beneficial effects on vascular morphology and NVU components in the 3xTg-AD model (Figure 1) [78]. Another study employing the 5xFAD model reported that exercise can ameliorate BBB dysregulation, fostering pericyte proliferation and elevating levels of ZO-1 and claudin-5 proteins over a four-month treatment period [79].
While human research into the effects of exercise on these alterations remains limited, increased cerebral blood flow, reduced cardiovascular risk factors, and enhanced angiogenic factors following interventions have been linked to improved memory [80][81][82]. These findings collectively underscore the pivotal role of physical exercise in addressing vascular disorders, presenting a promising avenue for comprehensive treatment strategies.
10. Effects of Exercise on Metabolism
AD is closely interlinked with metabolic diseases and their alterations. An evident association is observed in the intricate connection between type 2 diabetes mellitus (T2DM) and cognitive impairment. Furthermore, AD is distinguished by a reduction in glucose consumption, directly impacting learning and memory capacities. Perturbations in glucose metabolism reverberate through neurotransmission maintenance and neuronal function, prompting considerable investigation into the link between AD and metabolic pathways, including enzymes associated with glycolysis [83][84].
Lifestyle habits play a pivotal role in the context of AD. Obesity stands as a prominent risk factor due to its potential to induce insulin resistance, intricately intertwined with AD. Notably, dietary habits come into play; individuals consuming diets that are rich in cholesterol, saturated fats, and hypercaloric components exhibit an elevated AD risk, while those favoring fiber, vegetables, and fruits demonstrate lower susceptibility [83][84].
In this landscape, regular exercise emerges as a potent tool for overall health, capable of preventing and managing various health conditions. Exercise serves as a preventive measure and treatment approach for obesity and metabolic dysregulation. Its impact extends to positively influencing metabolic syndrome, obesity, insulin resistance/T2DM, dyslipidemia, and hypertension [85][86].
Moreover, physical exercise fosters glucose uptake by facilitating crucial processes such as efficient glucose delivery, transportation across muscle membranes, and heightened intracellular flux through metabolic pathways involved in glycolysis and glucose oxidation [87][88]. Consequently, exercise assumes a pivotal role in the prevention and management of overweight and obesity. The WHO recommends 150 to 250 min of moderate-intensity physical exercise per week to stave off weight gain. A plethora of studies underscore exercise’s capacity to enhance cardiometabolic health, insulin sensitivity, and lipolysis [85].
Looking ahead, integrating exercise interventions as a comprehensive strategy for managing and potentially preventing AD holds promise. However, challenges remain, including the need for tailored exercise guidelines and understanding the optimal exercise regimens for various stages of the disease. Long-term studies examining the sustained impact of exercise on metabolic and cognitive health in AD patients are essential for providing solid evidence for its effectiveness.
This entry is adapted from the peer-reviewed paper 10.3390/cells12212531
References
- Silveira-Rodrigues, J.G.; Pires, W.; Gomes, P.F.; Ogando, P.H.M.; Melo, B.P.; Aleixo, I.M.S.; Soares, D.D. Combined exercise training improves specific domains of cognitive functions and metabolic markers in middle-aged and older adults with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2021, 173, 108700.
- Gomes-Osman, J.; Cabral, D.F.; Morris, T.P.; McInerney, K.; Cahalin, L.P.; Rundek, T.; Oliveira, A.; Pascual-Leone, A. Exercise for cognitive brain health in aging: A systematic review for an evaluation of dose. Neurol. Clin. Pract. 2018, 8, 257–265.
- Gomes-Osman, J.; Cabral, D.F.; Hinchman, C.; Jannati, A.; Morris, T.P.; Pascual-Leone, A. The effects of exercise on cognitive function and brain plasticity—A feasibility trial. Restor. Neurol. Neurosci. 2017, 35, 547–556.
- Brown, B.M.; Peiffer, J.J.; Martins, R.N. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry 2013, 18, 864–874.
- Heyn, P.; Abreu, B.C.; Ottenbacher, K.J. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch. Phys. Med. Rehabil. 2004, 85, 1694–1704.
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130.
- Ohia-Nwoko, O.; Montazari, S.; Lau, Y.S.; Eriksen, J.L. Long-term treadmill exercise attenuates tau pathology in P301S tau transgenic mice. Mol. Neurodegener. 2014, 9, 54.
- Leem, Y.H.; Lim, H.J.; Shim, S.B.; Cho, J.Y.; Kim, B.S.; Han, P.L. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J. Neurosci. Res. 2009, 87, 2561–2570.
- Um, H.S.; Kang, E.B.; Leem, Y.H.; Cho, I.H.; Yang, C.H.; Chae, K.R.; Hwang, D.Y.; Cho, J.Y. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int. J. Mol. Med. 2008, 22, 529–539.
- Adlard, P.A.; Perreau, V.M.; Pop, V.; Cotman, C.W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 4217–4221.
- Vasconcelos-Filho, F.S.L.; da Rocha Oliveira, L.C.; de Freitas, T.B.C.; de Pontes, P.; Rocha, E.S.R.C.D.; Godinho, W.D.N.; Chaves, E.M.C.; da Silva, C.G.L.; Soares, P.M.; Ceccatto, V.M. Effect of involuntary chronic physical exercise on beta-amyloid protein in experimental models of Alzheimer’s disease: Systematic review and meta-analysis. Exp. Gerontol. 2021, 153, 111502.
- Belarbi, K.; Burnouf, S.; Fernandez-Gomez, F.J.; Laurent, C.; Lestavel, S.; Figeac, M.; Sultan, A.; Troquier, L.; Leboucher, A.; Caillierez, R.; et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol. Dis. 2011, 43, 486–494.
- Jullienne, A.; Trinh, M.V.; Obenaus, A. Neuroimaging of Mouse Models of Alzheimer’s Disease. Biomedicines 2022, 10, 305.
- Ferrari, C.; Sorbi, S. The complexity of Alzheimer’s disease: An evolving puzzle. Physiol. Rev. 2021, 101, 1047–1081.
- Piersson, A.D.; Mohamad, M.; Rajab, F.; Suppiah, S. Cerebrospinal Fluid Amyloid Beta, Tau Levels, Apolipoprotein, and (1)H-MRS Brain Metabolites in Alzheimer’s Disease: A Systematic Review. Acad. Radiol. 2021, 28, 1447–1463.
- Liang, K.Y.; Mintun, M.A.; Fagan, A.M.; Goate, A.M.; Bugg, J.M.; Holtzman, D.M.; Morris, J.C.; Head, D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann. Neurol. 2010, 68, 311–318.
- Stojanovic, M.; Jin, Y.; Fagan, A.M.; Benzinger, T.L.; Hassenstab, J.; Cruchaga, C.; Morris, J.C.; Head, D. Physical Exercise and Longitudinal Trajectories in Alzheimer Disease Biomarkers and Cognitive Functioning. Alzheimer Dis. Assoc. Disord. 2020, 34, 212–219.
- Zhang, X.L.; Zhao, N.; Xu, B.; Chen, X.H.; Li, T.J. Treadmill exercise inhibits amyloid-beta generation in the hippocampus of APP/PS1 transgenic mice by reducing cholesterol-mediated lipid raft formation. Neuroreport 2019, 30, 498–503.
- Elahi, M.; Motoi, Y.; Matsumoto, S.E.; Hasan, Z.; Ishiguro, K.; Hattori, N. Short-term treadmill exercise increased tau insolubility and neuroinflammation in tauopathy model mice. Neurosci. Lett. 2016, 610, 207–212.
- El Ouaamari, Y.; Van den Bos, J.; Willekens, B.; Cools, N.; Wens, I. Neurotrophic Factors as Regenerative Therapy for Neurodegenerative Diseases: Current Status, Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 3866.
- Blesch, A. Neurotrophic factors in neurodegeneration. Brain Pathol. 2006, 16, 295–303.
- Campos, C.; Rocha, N.B.; Lattari, E.; Paes, F.; Nardi, A.E.; Machado, S. Exercise-induced neuroprotective effects on neurodegenerative diseases: The key role of trophic factors. Expert. Rev. Neurother. 2016, 16, 723–734.
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4.
- Ruiz-Gonzalez, D.; Hernandez-Martinez, A.; Valenzuela, P.L.; Morales, J.S.; Soriano-Maldonado, A. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 2021, 128, 394–405.
- Cho, J.; Shin, M.K.; Kim, D.; Lee, I.; Kim, S.; Kang, H. Treadmill Running Reverses Cognitive Declines due to Alzheimer Disease. Med. Sci. Sports Exerc. 2015, 47, 1814–1824.
- Stein, A.M.; da Silva, T.M.V.; Coelho, F.G.M.; Rueda, A.V.; Camarini, R.; Galduroz, R.F.S. Acute exercise increases circulating IGF-1 in Alzheimer’s disease patients, but not in older adults without dementia. Behav. Brain Res. 2021, 396, 112903.
- Ozbeyli, D.; Sari, G.; Ozkan, N.; Karademir, B.; Yuksel, M.; Cilingir Kaya, O.T.; Kasimay Cakir, O. Protective effects of different exercise modalities in an Alzheimer’s disease-like model. Behav. Brain Res. 2017, 328, 159–177.
- Chuang, K.A.; Li, M.H.; Lin, N.H.; Chang, C.H.; Lu, I.H.; Pan, I.H.; Takahashi, T.; Perng, M.D.; Wen, S.F. Rhinacanthin C Alleviates Amyloid-beta Fibrils’ Toxicity on Neurons and Attenuates Neuroinflammation Triggered by LPS, Amyloid-beta, and Interferon-gamma in Glial Cells. Oxidative Med. Cell. Longev. 2017, 2017, 5414297.
- Walters, A.; Phillips, E.; Zheng, R.; Biju, M.; Kuruvilla, T. Evidence for neuroinflammation in Alzheimer’s disease. Prog. Neurol. Psychiatry 2016, 20, 25–31.
- Wang, M.; Zhang, H.; Liang, J.; Huang, J.; Chen, N. Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease. J. Neuroinflamm. 2023, 20, 76.
- Henrique, J.S.; Franca, E.F.; Cardoso, F.D.S.; Serra, F.T.; de Almeida, A.A.; Fernandes, J.; Arida, R.M.; Gomes da Silva, S. Cortical and hippocampal expression of inflammatory and intracellular signaling proteins in aged rats submitted to aerobic and resistance physical training. Exp. Gerontol. 2018, 110, 284–290.
- Tapia-Rojas, C.; Aranguiz, F.; Varela-Nallar, L.; Inestrosa, N.C. Voluntary Running Attenuates Memory Loss, Decreases Neuropathological Changes and Induces Neurogenesis in a Mouse Model of Alzheimer’s Disease. Brain Pathol. 2016, 26, 62–74.
- Aczel, D.; Gyorgy, B.; Bakonyi, P.; BukhAri, R.; Pinho, R.; Boldogh, I.; Yaodong, G.; Radak, Z. The Systemic Effects of Exercise on the Systemic Effects of Alzheimer’s Disease. Antioxidants 2022, 11, 1028.
- Kelly, A.M. Exercise-Induced Modulation of Neuroinflammation in Models of Alzheimer’s Disease. Brain Plast. 2018, 4, 81–94.
- Jensen, C.S.; Bahl, J.M.; Ostergaard, L.B.; Hogh, P.; Wermuth, L.; Heslegrave, A.; Zetterberg, H.; Heegaard, N.H.H.; Hasselbalch, S.G.; Simonsen, A.H. Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp. Gerontol. 2019, 121, 91–98.
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552.
- Maugeri, G.; D’Agata, V.; Magri, B.; Roggio, F.; Castorina, A.; Ravalli, S.; Di Rosa, M.; Musumeci, G. Neuroprotective Effects of Physical Activity via the Adaptation of Astrocytes. Cells 2021, 10, 1542.
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167.
- Nagele, R.G.; D’Andrea, M.R.; Lee, H.; Venkataraman, V.; Wang, H.Y. Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003, 971, 197–209.
- Gonzalez-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sanchez, K.; Ariza-Salamanca, D.; Mora-Munoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427.
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer’s disease. Afr. Health Sci. 2016, 16, 1045–1055.
- Kohut, M.L.; McCann, D.A.; Russell, D.W.; Konopka, D.N.; Cunnick, J.E.; Franke, W.D.; Castillo, M.C.; Reighard, A.E.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006, 20, 201–209.
- Herring, A.; Blome, M.; Ambree, O.; Sachser, N.; Paulus, W.; Keyvani, K. Reduction of cerebral oxidative stress following environmental enrichment in mice with Alzheimer-like pathology. Brain Pathol. 2010, 20, 166–175.
- Bernardo, T.C.; Marques-Aleixo, I.; Beleza, J.; Oliveira, P.J.; Ascensao, A.; Magalhaes, J. Physical Exercise and Brain Mitochondrial Fitness: The Possible Role Against Alzheimer’s Disease. Brain Pathol. 2016, 26, 648–663.
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661.
- Garcia-Mesa, Y.; Colie, S.; Corpas, R.; Cristofol, R.; Comellas, F.; Nebreda, A.R.; Gimenez-Llort, L.; Sanfeliu, C. Oxidative Stress Is a Central Target for Physical Exercise Neuroprotection Against Pathological Brain Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 40–49.
- de Farias, J.M.; Dos Santos Tramontin, N.; Pereira, E.V.; de Moraes, G.L.; Furtado, B.G.; Tietbohl, L.T.W.; Da Costa Pereira, B.; Simon, K.U.; Muller, A.P. Physical Exercise Training Improves Judgment and Problem-Solving and Modulates Serum Biomarkers in Patients with Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 4217–4225.
- Veronese, N.; Solmi, M.; Basso, C.; Smith, L.; Soysal, P. Role of physical activity in ameliorating neuropsychiatric symptoms in Alzheimer disease: A narrative review. Int. J. Geriatr. Psychiatry 2019, 34, 1316–1325.
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimer’s Dis. 2019, 6, 2–15.
- Manzano-Palomo, S.; De la Morena-Vicente, M.A.; Barquero, M.S. Neurotransmitters in Alzheimer’s disease. Rev. Neurol. 2006, 42, 350–353.
- Zong, B.; Yu, F.; Zhang, X.; Zhao, W.; Sun, P.; Li, S.; Li, L. Understanding How Physical Exercise Improves Alzheimer’s Disease: Cholinergic and Monoaminergic Systems. Front. Aging Neurosci. 2022, 14, 869507.
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895.
- Valenzuela, P.L.; Castillo-Garcia, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108.
- van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270.
- Leal-Galicia, P.; Chavez-Hernandez, M.E.; Mata, F.; Mata-Luevanos, J.; Rodriguez-Serrano, L.M.; Tapia-de-Jesus, A.; Buenrostro-Jauregui, M.H. Adult Neurogenesis: A Story Ranging from Controversial New Neurogenic Areas and Human Adult Neurogenesis to Molecular Regulation. Int. J. Mol. Sci. 2021, 22, 11489.
- Nicolis di Robilant, V.; Scardigli, R.; Strimpakos, G.; Tirone, F.; Middei, S.; Scopa, C.; De Bardi, M.; Battistini, L.; Saraulli, D.; Farioli Vecchioli, S. Running-Activated Neural Stem Cells Enhance Subventricular Neurogenesis and Improve Olfactory Behavior in p21 Knockout Mice. Mol. Neurobiol. 2019, 56, 7534–7556.
- Lee, W.D.; Wang, K.C.; Tsai, Y.F.; Chou, P.C.; Tsai, L.K.; Chien, C.L. Subarachnoid Hemorrhage Promotes Proliferation, Differentiation, and Migration of Neural Stem Cells via BDNF Upregulation. PLoS ONE 2016, 11, e0165460.
- Saraulli, D.; Costanzi, M.; Mastrorilli, V.; Farioli-Vecchioli, S. The Long Run: Neuroprotective Effects of Physical Exercise on Adult Neurogenesis from Youth to Old Age. Curr. Neuropharmacol. 2017, 15, 519–533.
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544.
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821.
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 2014, 35 (Suppl. 2), S20–S28.
- Thomas, A.G.; Dennis, A.; Bandettini, P.A.; Johansen-Berg, H. The effects of aerobic activity on brain structure. Front. Psychol. 2012, 3, 86.
- Wang, R.; Holsinger, R.M.D. Exercise-induced brain-derived neurotrophic factor expression: Therapeutic implications for Alzheimer’s dementia. Ageing Res. Rev. 2018, 48, 109–121.
- Frederiksen, K.S.; Gjerum, L.; Waldemar, G.; Hasselbalch, S.G. Effects of Physical Exercise on Alzheimer’s Disease Biomarkers: A Systematic Review of Intervention Studies. J. Alzheimer’s Dis. 2018, 61, 359–372.
- Moreno-Jimenez, E.P.; Terreros-Roncal, J.; Flor-Garcia, M.; Rabano, A.; Llorens-Martin, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553.
- Babcock, K.R.; Page, J.S.; Fallon, J.R.; Webb, A.E. Adult Hippocampal Neurogenesis in Aging and Alzheimer’s Disease. Stem Cell Rep. 2021, 16, 681–693.
- Suwabe, K.; Byun, K.; Hyodo, K.; Reagh, Z.M.; Roberts, J.M.; Matsushita, A.; Saotome, K.; Ochi, G.; Fukuie, T.; Suzuki, K.; et al. Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc. Natl. Acad. Sci. USA 2018, 115, 10487–10492.
- Shepherd, A.; Zhang, T.D.; Zeleznikow-Johnston, A.M.; Hannan, A.J.; Burrows, E.L. Transgenic Mouse Models as Tools for Understanding How Increased Cognitive and Physical Stimulation Can Improve Cognition in Alzheimer’s Disease. Brain Plast. 2018, 4, 127–150.
- Ryan, S.M.; Kelly, A.M. Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Ageing Res. Rev. 2016, 27, 77–92.
- Voss, M.W.; Soto, C.; Yoo, S.; Sodoma, M.; Vivar, C.; van Praag, H. Exercise and Hippocampal Memory Systems. Trends Cogn. Sci. 2019, 23, 318–333.
- Duzel, E.; van Praag, H.; Sendtner, M. Can physical exercise in old age improve memory and hippocampal function? Brain 2016, 139, 662–673.
- Du, Z.; Li, Y.; Li, J.; Zhou, C.; Li, F.; Yang, X. Physical activity can improve cognition in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Interv. Aging 2018, 13, 1593–1603.
- Panza, G.A.; Taylor, B.A.; MacDonald, H.V.; Johnson, B.T.; Zaleski, A.L.; Livingston, J.; Thompson, P.D.; Pescatello, L.S. Can Exercise Improve Cognitive Symptoms of Alzheimer’s Disease? J. Am. Geriatr. Soc. 2018, 66, 487–495.
- Demurtas, J.; Schoene, D.; Torbahn, G.; Marengoni, A.; Grande, G.; Zou, L.; Petrovic, M.; Maggi, S.; Cesari, M.; Lamb, S.; et al. Physical Activity and Exercise in Mild Cognitive Impairment and Dementia: An Umbrella Review of Intervention and Observational Studies. J. Am. Med. Dir. Assoc. 2020, 21, 1415–1422.e1416.
- Apatiga-Perez, R.; Soto-Rojas, L.O.; Campa-Cordoba, B.B.; Luna-Viramontes, N.I.; Cuevas, E.; Villanueva-Fierro, I.; Ontiveros-Torres, M.A.; Bravo-Munoz, M.; Flores-Rodriguez, P.; Garces-Ramirez, L.; et al. Neurovascular dysfunction and vascular amyloid accumulation as early events in Alzheimer’s disease. Metab. Brain Dis. 2022, 37, 39–50.
- Soto-Rojas, L.O.; Pacheco-Herrero, M.; Martinez-Gomez, P.A.; Campa-Cordoba, B.B.; Apatiga-Perez, R.; Villegas-Rojas, M.M.; Harrington, C.R.; de la Cruz, F.; Garces-Ramirez, L.; Luna-Munoz, J. The Neurovascular Unit Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2022.
- Maliszewska-Cyna, E.; Oore, J.; Xhima, K.; Thomason, L.A.M.; Steinman, J.; McLaurin, J.; Sled, J.G.; Stefanovic, B.; Aubert, I. P1-015: Evaluation of Effects of Physical Exercise on Vascular and Cerebral Pathology, Plasticity and Function in a Mouse Model of Alzheimer’s Disease. Alzheimer’s Dement. 2016, 12, P404–P405.
- Andrade-Guerrero, J.; Orta-Salazar, E.; Salinas-Lara, C.; Sánchez-Garibay, C.; Rodríguez-Hernández, L.D.; Vargas-Rodríguez, I.; Barron-Leon, N.; Ledesma-Alonso, C.; Diaz-Cintra, S.; Soto-Rojas, L.O. Effects of Voluntary Physical Exercise on the Neurovascular Unit in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 11134.
- Kook, S.Y.; Seok Hong, H.; Moon, M.; Mook-Jung, I. Disruption of blood-brain barrier in Alzheimer disease pathogenesis. Tissue Barriers 2013, 1, e23993.
- Pedrinolla, A.; Venturelli, M.; Fonte, C.; Tamburin, S.; Di Baldassarre, A.; Naro, F.; Varalta, V.; Giuriato, G.; Ghinassi, B.; Muti, E.; et al. Exercise training improves vascular function in patients with Alzheimer’s disease. Eur. J. Appl. Physiol. 2020, 120, 2233–2245.
- Thomas, B.P.; Tarumi, T.; Sheng, M.; Tseng, B.; Womack, K.B.; Cullum, C.M.; Rypma, B.; Zhang, R.; Lu, H. Brain Perfusion Change in Patients with Mild Cognitive Impairment After 12 Months of Aerobic Exercise Training. J. Alzheimer’s Dis. 2020, 75, 617–631.
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743.
- Yan, X.; Hu, Y.; Wang, B.; Wang, S.; Zhang, X. Metabolic Dysregulation Contributes to the Progression of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 530219.
- Kang, S.; Lee, Y.H.; Lee, J.E. Metabolism-Centric Overview of the Pathogenesis of Alzheimer’s Disease. Yonsei Med. J. 2017, 58, 479–488.
- Park, H.J.; Rhie, S.J.; Shim, I. The effects of physical exercise therapy on weight control: Its regulation of adipocyte physiology and metabolic capacity. J. Exerc. Rehabil. 2023, 19, 141–148.
- Martinez-Montoro, J.I.; Benitez-Porres, J.; Tinahones, F.J.; Ortega-Gomez, A.; Murri, M. Effects of exercise timing on metabolic health. Obes. Rev. 2023, 24, e13599.
- Richter, E.A.; Derave, W.; Wojtaszewski, J.F. Glucose, exercise and insulin: Emerging concepts. J. Physiol. 2001, 535, 313–322.
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148.
This entry is offline, you can click here to edit this entry!
