The World Health Organization identifies tuberculosis (TB), caused by Mycobacterium tuberculosis, as a leading infectious killer. Although conventional treatments for TB exist, they come with challenges such as a heavy pill regimen, prolonged treatment duration, and a strict schedule, leading to multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains. Nanocarriers, such as lipid nanoparticles, nanosuspensions, liposomes, and polymeric micelles, facilitate targeted delivery of anti-TB drugs. The benefits of nanocarriers include reduced drug doses, fewer side effects, improved drug solubility, better bioavailability, and improved patient compliance, speeding up recovery.
1. Introduction
Among the most important global health challenges are infectious diseases such as tuberculosis (TB), acquired immunodeficiency syndrome, and human immunodeficiency virus infection [
1,
2].
Mycobacterium tuberculosis, an aerobic, Gram-positive, non-motile, acid-fast tubercular, rod-shaped bacillus, causes airborne TB, which mostly affects the lungs but may also impact extra-pulmonary regions [
3]. Due to their lipid-rich cell walls, mycobacteria may live within alveolar macrophages [
4]. The tubercle bacillus,
M. tuberculosis, which is spread via airborne droplets and can remain, live, and divide every 16–20 h inside alveolar macrophages, is the principal method of transmission for this dangerous illness [
5]. Per the latest report by World Health Organization (WHO), about 10.6 million cases of TB were reported in 2021, comprising 6 million men, 1.2 million children, and 3.4 million women [
6]. It was estimated that about 1.6 million people died from TB in 2021 throughout the world. About 80% people infected by TB reside in low- and middle-income countries. The main causes for TB include weakened immune system, chewing of tobacco, undernourishment, and other complications such as diabetes and HIV infection [
6]. In 2021, 2.2 million new TB cases were attributed to undernourishment, 740,000 to alcohol use disorders, and 690,000 to smoking throughout the globe. To reach the global goal set at a high-level UN meeting on TB in 2018, USD 13 billion is required annually for TB prevention, diagnosis, treatment, and care. It is expected that TB detection and treatment saved 74 million lives between 2020 and 2021 [
7]. TB is the second highest infectious cause of death after COVID-19 and the thirteenth major cause of mortality across the world [
8]. TB exists in all nations and among all age groups, but it can be treated and avoided. One of the Sustainable Development Goals (SDGs) of the United Nations is to end the TB epidemic by 2030 [
9].
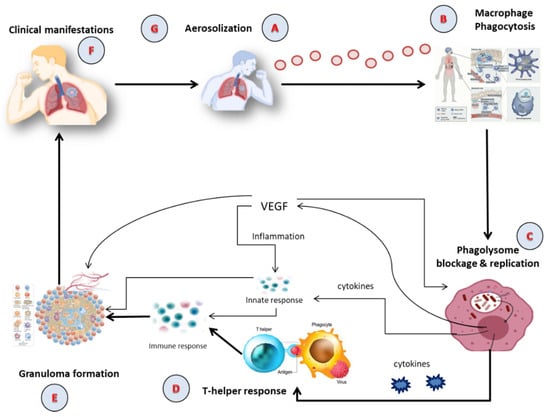
Tubercle bacilli nuclei in droplets that reach the lungs’ alveoli during breathing cause infection, in a step called aerosolization (
Figure 1A) [
22,
23]. These tubercle bacilli are ingested by alveolar macrophages, the majority of which are killed or inhibited (
Figure 1B) [
24]. After preventing the acquisition of the phagosome and lysosome,
M. tuberculosis reproduces intracellularly inside the macrophages (
Figure 1C). Asymmetric cell division is a special kind of cell division seen in
M. tuberculosis [
25,
26]. Those bacteria may spread, if they are alive, through the lymphatic system or the circulation to the regional lymph nodes, the apex of the lung, kidneys, brain, and bony parts of the body, where TB sickness is most likely to develop. This process of dissemination sets the immune system for an expanded response (
Figure 1D) [
27]. To use an analogy, a bacterial jail called a granuloma aims to isolate a bacterium beneath an enclosure of immune cells. Both macrophages and lymphocytes that surround and enclose
M. tuberculosis constitute the granuloma itself. TH1, natural killer (NK) cells, dendritic cells, macrophage, regulatory T cells (Treg), foam cells, giant cells, epithelioid macrophage, neutrophils, and B cells are some of the cells implicated in the granuloma (
Figure 1E). In clinical significance, primary and secondary TB are the two forms of TB (
Figure 1F). In immunocompromised individuals, primary infection is the one that develops when the immune system cannot handle it. At this point (
Figure 1G), the infected person releases infectious aerosols of
M. tuberculosis and infects the next susceptible person. Suppose
M. tuberculosis is present but not eradicated by the immune system or granuloma. In such instances, the illness is believed to be latent and could turn into secondary TB.
Figure 1. The Pathophysiology of active TB; (A) Aerosolization; (B) Macrophage phagocytosis; (C) Blockage and replication of phagolysosome; (D) T-helper response; (E) Granuloma formation; (F) Clinical manifestation; (G) Infection of susceptible person via aerosolization. VEGF: Vascular Endothelial Growth Factor.
2. Nano-Based Drug Delivery in TB
Due to various benefits such as lower doses, improved dosage regimens, reduced adverse effects, decreased drug degradation, improved solubility and bioavailability, and improved patient compliance over conventional therapy, nanomedicines have been shown to be effective therapies and result in encouraging outcomes for the treatment of TB [
43,
44,
45,
46]. A wide variety of drug delivery systems using different types of nanocarriers have proven to be successful [
45]. Controlled and sustained drug release is one advantage of nanocarrier-based anti-TB medications over free medicines [
47]. They also decrease dosing frequency and address the issue of poor compliance [
16]. Physical encapsulation, adsorption, or chemical conjugation are all ways that therapeutic drugs can be introduced into nanocarriers. Significantly, it is possible to target host cells utilizing nanocarriers via either passive accumulation or active targeting [
48].
Nanosized drug delivery systems advantages can be summarized as follows:
-
Targeted Drug Delivery: Nanocarriers can deliver drugs directly to the infected site, ensuring that the therapeutic agents reach the desired location in the body, thus increasing the efficacy and reducing potential side effects [
48].
-
Enhanced Bioavailability: Nanoformulations can increase the solubility of poorly water-soluble anti-TB drugs, leading to better absorption and higher bioavailability [
16,
43].
-
Reduced Drug Dosage: Due to their efficient delivery and release mechanisms, nanosized drug delivery systems can often achieve therapeutic effects with reduced drug doses, minimizing potential side effects and toxicity [
16].
-
Overcoming Drug Resistance: Nanocarriers can be engineered to counter multidrug-resistant TB strains by co-delivering multiple drugs or by protecting the drug from degradation [
43,
44].
-
Controlled Release: The drugs encapsulated in nanocarriers can be released in a sustained manner over time, ensuring consistent drug levels and potentially reducing the frequency of dosing [
47].
-
Reduced Side Effects: Targeted delivery and reduced dosage mean that healthy tissues are less exposed to the drug, which can minimize side effects [
43,
44].
-
Improved Patient Compliance: Nanosized drug delivery systems can offer simplified dosing regimens, leading to better patient adherence to the treatment, especially considering the long treatment courses required for TB [
43,
44].
-
Penetration of Biological Barriers: Nanocarriers can be designed to penetrate tough biological barriers, such as the blood–brain barrier, enabling treatment of TB manifestations in difficult-to-reach sites [
43,
44].
-
Protection from Degradation: Some nanocarriers can protect the encapsulated drugs from enzymatic or pH-mediated degradation in the body, ensuring the drug remains active longer [
43,
44].
-
Versatility: Nanosized delivery systems can be adapted or modified to carry various types of drugs, including small molecules, proteins, or even nucleic acids, offering flexibility in treatment strategies [
43,
44].
-
Co-delivery of Multiple Drugs: Certain nanocarriers can encapsulate multiple anti-TB agents, allowing for combination therapy, which is often more effective and can reduce the likelihood of developing drug resistance [
43,
44].
By harnessing these advantages, nanosized drug delivery systems hold potential to revolutionize TB treatment strategies, addressing many of the challenges posed by conventional therapy.
2.1. Polymeric Nanoparticles (PNPs)
PNPs are appealing nanocarriers to increase the effectiveness of chemotherapeutics and reduce the toxic effects of anti-TB drugs by encapsulating and conjugating the therapeutic drugs [
49]. The delivery of anti-TB drugs via encapsulation in PNPs improves the efficacy and proficiency of TB treatment due to their possession of various characteristics of improved bioavailability, reduced dose frequency, smaller size, greater drug loading capacity, improved stability, higher surface volume ratio, biocompatibility, biodegradability, and ease of modification [
50,
51]. PNPs provide reduced systemic toxicity owing to direct delivery of a drug to a specific site, which allows for minimal exposure of other organs to the drug. PNPs enable the reduction of drug resistance by enhancing intracellular uptake and concentration of the drug at the infected site [
51].
PNPs are composed of natural and synthetic polymers [
53]. Albumin, collagen, chitosan, hemoglobin, and alginate are examples of natural polymers; however, they are not frequently used due to their high cost or low purity [
54]. Synthetic polymers which are biodegradable, biocompatible, and stable include poly(amides), poly(amino acids), poly(alk-l-cyanoacrylates), poly(esters), and poly(orthoesters) [
55]. PLGA (poly(lactide-co-glycolide)) copolymers are among those mentioned above that are frequently employed in anti-TB drug delivery. The primary benefit of these polymers is that they may break down inside the body via metabolic pathways and eliminated. PLGA has also been authorized for use in a number of medical applications.
2.2. Solid Lipid Nanoparticles (SLNs)
Lipid nanoparticles have received a great deal of attention from researchers because they are at the cutting edge of the rapidly developing field of nanotechnology and show tremendous promise for reaching the goal of regulated and targeted medication delivery in the treatment of various kinds of infectious diseases [
58,
59,
60]. SLNs offer a number of noteworthy advantages, including increased solubility, reduced side effects, increased bioavailability of pharmaceuticals, adaptation of encapsulation of both hydrophilic and hydrophobic medications, better stability, higher specificity, and increased likelihood of large-scale manufacture [
16]. SLNs have similar properties to PNPs, but the main point is that they possess a better safety profile [
61].
The more beneficial properties of SLNs are their size (less than 400 nm), simplicity of functionalization, chemical and mechanical stability, and improved transport of lipophilic therapeutic drugs [
62]. Additionally, SLNs are able to penetrate many physiological barriers that prevent drugs from reaching infected sites and can bypass the multidrug resistance mechanisms that are common to TB treatment [
63]. Due to their increased permeability and long retention times, SLNs have the unique inherent ability to concentrate the drug with precision at the infected site.
These are made up of either solid lipids or a combination of lipids and surfactants [
64]. They may also contain an aqueous phase, surface modifiers, co-surfactants, stealthing agents, and cryoprotective chemicals [
65]. A hydrophobic medication, or combination of hydrophobic drugs, is confined within the solid lipid matrix of SLNs, providing physical stability by shielding the molecule from chemical deterioration. These enhance the half-life of medications in blood circulation and alter their release pattern, increasing the therapeutic potency of the drugs [
66].
2.3. Nanostructured Lipid Carriers (NLCs)
NLCs have demonstrated their efficacy as advanced drug carriers in the treatment of infectious diseases to address a number of the aforementioned shortcomings of SLNs [
70,
71]. Due to their unique qualities, which include improved drug encapsulation, long-term chemical and physical stability of the encapsulated drug, surface changes, and site-specific targeting, they have a wide range of applications as drug carriers [
72,
73]. Since they combine liquid and solid lipids, their crystallinity is decreased, and their matrix system is loosely packed. Due to the overall improvement in drug entrapment capability and higher stability, further research is required [
73].
2.4. Liposomes
Liposomes have become a potential drug delivery vehicle because they have a variety of properties, including the ability to encapsulate high doses, to deliver hydrophilic and hydrophobic drugs, to extend the circulation time of the drug, to generate low adverse effects, to control drug delivery, to increase rate of dissolution, and to target drugs to specific cells; biocompatibility; biodegradability; ease of manufacturing; and versatility [
56]. Phospholipids and sterols are common components of liposomal substances, which provide the vesicles with stability and unique properties [
11]. These qualities make liposomes an appealing choice for delivering a range of antimycobacterial medicines. Liposomes are frequently used to treat bacterial infections because they stop drug breakdown, provide a controlled drug release, and, in some circumstances, allow medication transport through the bacterial membrane to the intracellular milieu [
76]. Additionally, liposomes can provide a promising delivery method for negatively charged oligonucleotides since these molecules can electrostatically interact with cationic liposomes to form complexes [
77].
In addition to conventional drug delivery, new potential for direct nasal administration of anti-TB drugs to the lungs has been made possible by nanodrug delivery systems based on liposomes. This technique has the advantage of delivering drug concentrations that are pharmacologically effective in alveolar macrophages, producing better therapeutic outcomes [
78].
Despite various benefits of liposomes, such as their safety and biocompatibility, their primary disadvantage as nanocarriers is their instability in plasma. Selective serum proteins (opsonins) bind to the surface of liposomes when they enter the bloodstream, indicating their presence. The mononuclear phagocyte system (MPS), which seizes liposomes and expels them from the bloodstream, recognizes this signal. Although the opposite has also been recorded, in general, larger liposomes are cleared from blood circulation more quickly than smaller ones, and negatively charged liposomes have a shorter half-life in the bloodstream than neutral liposomes [
11]. However, it can be combated using functionalization with polyethylene glycol (PEG) and various kinds of ligands such as antibodies and other molecules which increase specificity to the infected site.
2.5. Nanoemulsions (NEs)
Nano-emulsions are thought to be one of the most promising options for increasing the oral bioavailability of anti-TB medicines to increase their therapeutic efficacy. A nano-emulsion loaded with anti-TB medicines can quickly overcome biological barriers to enter systemic circulation and, as a result, achieve the target for lowering the load of
M. tuberculosis. Additionally, the lipidic composition of such systems makes it easier to target the medications to the lymph nodes, improving drug absorption and reducing the frequency of administration [
12,
81]. In addition, based on the possession of special characteristics such as physical stability, increased surface area, prolonged circulation time, amphiphilicity, specific drug targeting, tumor imaging properties, optical clarity, biodegradability, improved aqueous solubility, and bioavailability, researchers’ focus has shifted to nano-emulsions [
82,
83].
2.6. Polymeric Micelles (PMs)
Due to their promising outcomes, polymeric micelles are frequently used to deliver anti-TB medicines. These formulations are known to make poorly soluble drugs more soluble and increase bioavailability, stability, extended circulation, and controlled release while simultaneously reducing toxicity, antigenicity, and immunogenicity with improved tractability [
85]. This versatile nanocarrier enables the delivery of anti-TB drugs via the oral, ophthalmic, parenteral, and intranasal routes to accomplish the site-specific delivery of the drugs. The ability to maintain a steady concentration for an extended period of time is made possible by the sustained release of the drug from the miceller structure. Their nanometric size range of 10–200 nm allows them to pass through blood capillaries without being detected by the reticuloendothelial system (RES) while also preventing premature excretion via glomerular filtration [
86].
2.7. Dendrimers
Dendrimers are polybranched, three-dimensional, nanometric, monodispersed, star-shaped vesicles comprising numerous branches on interior surface, a central core, and various functional groups on exterior surface [
91]. They have certain structural and chemical characteristics such size (less than 100 nm), shape, and molecular weight. Dendrimers have the potential to prolong drug release, increase the solubility of hydrophobic compounds, and improve the permeability of nanoconjugates across a variety of biological barriers [
65]. Although several dendrimers have been applied for drug delivery in various diseases, polyamidoamine (PAMAM) and polypropylene imine (PPI) are extensively used owing to their hydrophilic nature, biocompatibility, and non-immunogenicity [
92]. A combination of drugs can be included in dendrimers thanks to the functional groups found on their external surface. It is possible to modify these functional groups to offer drug targeting at the particular site [
93].
Dendrimers display several advantages as drug carriers, but they also exhibit hemolytic and cytotoxic capabilities, raising serious concerns regarding their safety. Surface functionalization of functional groups found on the external surface of dendrimers can lessen these harmful effects. Polyethene glycols can be used to functionalize the surface of dendrimers, which enhances drug circulation time due to EPR and reduces toxic effects [
65].
2.8. Carbon Nanotubes (CNTs)
Researchers have given CNTs a great deal of attention as a potential drug carrier to deliver anti-TB medicines because of a variety of properties including reduced size, increased surface area, high drug loading capability, regulated and sustained release of the pharmaceuticals, and drug targeting [
95]. Nanodrug delivery via functionalized CNTs can be used to avoid bacterial multidrug resistance and lower drug dosage. Both single-walled CNTs (SWCNT) and multiwalled CNTs (MWCNT) have been found in studies to be able to thwart the development of drug resistance to several drugs by destroying bacterial cell walls, inducing oxidative stress, and shattering bacterial DNA or macromolecules. Because nanofluids and nanoparticle suspensions have great dispersion stability and bioavailability, they can also be used to construct this CNT system and are hence a feasible drug delivery option [
96].
Due to their improved hydrophilicity and lower cytotoxicity, CNTs should be functionalized using various polymers, chemical groups, or biomolecules to assure their targeting ability and safety in the treatment of cancer. By covalent and non-covalently connecting different kinds of polymers and chemical groups to the surface of CNTs, functionalization of the CNTs’ surfaces can be accomplished [
65].
2.9. Metallic Nanoparticles (MNPs)
MNPs are one of the most effective methods of drug delivery against the contagious
M. tuberculosis. Due to its bacterial selectivity, reducible size, and extra antibacterial capabilities, this carrier represents a highly promising new carrier for the treatment of TB. When functionalized with targeting ligands that provide controlled deposition into infected cells, MNPs have also been reported to offer improved targeting, gene silencing, and drug delivery. Several MNPs, such as iron oxide nanoparticles (IONPs), zinc oxide nanoparticles (Zn ONPs), copper nanoparticles (Cu NPs), gold nanoparticles (Au NPs), and silver nanoparticles (Ag NPs), are used to treat TB.
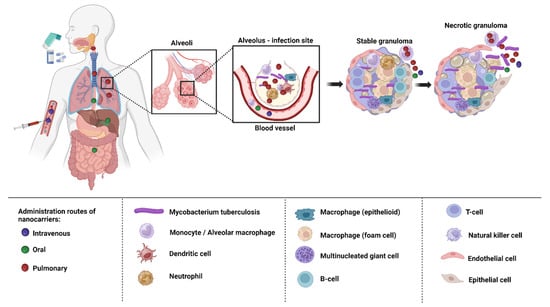
3. Delivery of Nanoformulations for TB Treatment
Depending on the biological milieu and obstacles that nanocarriers must overcome, the various ways of administering nanocarriers have varying restrictions. Delivery of nanocarriers via the pulmonary, oral, intravenous, and topical routes may be effective in treating TB. In order to attain the best efficacy, nanocarrier properties can be customized to the intended delivery method [
135]. The targeting of infected alveolar macrophages and granulomas employing nanocarriers via various routes of administration has been depicted in the
Figure 4.
Figure 4. Drug targeting macrophages and granulomas via different routes of administration.
The oral route is the preferred method of administering nanocarriers because it is noninvasive and more practical for patients to finish their therapy. Low pH and highly proteolytic conditions in the stomach medium, as well as hepatic first-pass metabolism, restrict the range of formulations that are achievable and lower bioavailability [
136,
137]. Intravenous administration enables the swift absorption of the drugs into systemic circulation and straight into the bloodstream without having to go through first-pass metabolism, which also offers a more accurate control of the dose that is provided [
137]. Nanocarriers injected intravenously are either cleared by the MPS or are associated with proteins (protein corona) [
138]. Both the oral and intravenous route of administration are associated with adverse effects [
139]. The non-invasive topical route enables prolonged release and local activity, resulting in fewer adverse systemic effects, and bypasses hepatic first-pass metabolism. Given that cutaneous TB is a rare illness, it might be advantageous, although little research has been carried out on this approach [
140].
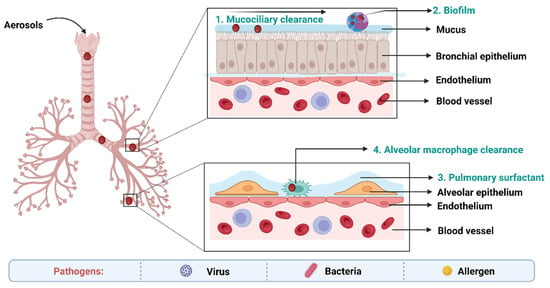
4. Biological Barriers to Delivery of Anti-TB Drugs
The main obstacles to drug absorption after pulmonary administration for medications administered via inhalation include numerous clearance systems which are exposed to pharmaceuticals. The barriers to delivery of anti-TB drugs to the lungs has been depicted in the Figure 5.
Figure 5. Biological barriers in targeted drug delivery to lungs in TB.
On the epithelium of the trachea and bronchial tree, the mucus layer is present. Its main components are water (90–95%), mucins (primarily MUC5AC and MUC5B), and other substances such as DNA, lipids, electrolytes, proteins, cells, and cell debris (3–8%) [
144]. High-viscoelasticity mucus, which is mostly attributed to mucins (high molecular weight (MW) glycoproteins), prevents xenobiotics such inhaled nanoformulations from invading [
145]. As a result, nanoformulations and mucus interact in a variety of ways when the former are inhaled into the respiratory system. With a pore size of around 340 nm, the physical barrier will prevent big nanoformulations from reaching the epithelium. Mucus’s negative charge makes it easier for it to interact electrostatically with positively charged nanoformulations, which has an impact on how those nanoformulations move around [
146].
Pulmonary surfactant (PS), an amphiphilic lipoprotein complex, is secreted by alveolar type II (AT-II) cells in the pulmonary epithelium. PS is made up of surfactant proteins and phospholipids [
148]. Surfactant proteins can promote the adhesion and agglutination of certain drugs by mucosal cilia, macrophages, and monocytes. Lung surfactants may also be used to remove therapeutic medications or drug carriers from the body [
149].
Inhaled nanoformulations initially come into contact with MPs and DCs in the lower respiratory tract, resulting in nanoformulations with smaller size, and are best taken up by MPs, whilst larger one are phagocytosed by DCs [
152]. The phagocytosis mechanisms of MPs and DCs are size-dependent and opsonin-dependent. The opsonized nanoformulations are more likely to be recognized by the MPs’ membrane receptors because the opsonin proteins (antibody, complement, and fibrinogen) are quickly adsorbed on the surface of nanoformulations [
153]. The identification and phagocytosis of inhaled nanomedicines by MPs play a more significant function than DCs in the lung clearance process because the optimal size of particle deposition in the deeper respiratory tract is larger than 500 nm.
5. Ligand Conjugated Nanoformulations for Circumvention of Pulmonary Barriers
Drug targeting is crucial because it ensures that the therapeutic agent is delivered directly to the site of disease or infection, maximizing its efficacy while minimizing potential side effects elsewhere in the body. Targeted delivery means that a smaller amount of drug can have a more potent effect, reducing the overall dosage and thereby lessening the chances of adverse reactions or drug toxicity.
The target organ or tissue is typically chosen based on the location of the disease or infection. For diseases such as TB, the primary target would be the lungs, where the TB bacteria predominantly reside. However, TB can also affect other organs, and thus, the choice of target would depend on the disease’s manifestation. Scientific research, patient diagnostics, and understanding of disease progression play significant roles in deciding the target.
The mechanism for targeting often involves the use of ligands that can bind specifically to receptors found on the desired target cells. In the context mentioned, nanocarriers are employed as the delivery vehicles. As they move through the body, processes such as opsonization and phagocytosis work to remove them. However, when these nanocarriers are coupled with specific ligands, they can bind to receptors on target cells, such as macrophages, enhancing their ability to deliver drugs directly to the desired location (Figure 6).
Figure 6. Drug targeting employing conjugation of ligands with drug-loaded nanocarriers in TB.
5.1. Mannose Targeting
A C-type lectin receptor called the mannose receptor (CD206) may detect ligands that include a terminal mannose,
N-acetylglucosamine, or fucose moiety. Both dendritic cells and the majority of tissue macrophages express this receptor in high amounts [
155]. It controls inflammatory signaling pathways as well as endocytosis and phagocytosis. Additionally, it is crucial for the phagocytosis of
M. tuberculosis, for preventing phagosome-lysosome fusion, and for the intracellular survival of the bacteria [
156]. Additionally, the mannose receptor contributes to the development of granulomas. Based on these results, targeting the mannose receptor for TB treatment appears promising, and intracellular co-localization of the bacteria and the nanocarrier is more likely given that they may use the same macrophage entry channel [
135].
5.2. Folic Acid Targeting
Folic acid is necessary vitamin required for cell growth [
161]. This essential molecule is required for DNA replication and repair and RNA synthesis and contributes to the metabolism of amino acids, phospholipids, and nucleotides [
162]. Via endocytosis, folic acid derivatives are taken up by cells via the action of foliate receptors. One of the several isoforms of folate receptors, folate receptor α, is overexpressed on the surface of activated macrophages associated with autoimmune and inflammatory illnesses as well as on tumor-associated macrophages (TAMs) [
163].
5.3. Hyaluronic Acid (HA) Targeting
Hyaluronic acid (also known as hyaluronan, or HA) is a glycosaminoglycan made up of repeating
N-acetyl-D-glucosamine and d-glucuronic acid disaccharide units [
165]. A significant part of the extracellular and pericellular matrix (ECM), HA is found in tissues throughout the body and, when in contact with immune cells, can signal whether an area is healthy or inflamed. In healthy tissues, high molecular weight HA (>1000 kDa) predominates and exhibits immunosuppressive and anti-inflammatory properties, whereas low molecular weight HA (500 kDa) forms in response to tissue damage or infection as the components of the ECM break down and exhibits immunostimulatory and pro-inflammatory properties [
166].
Activated immune cells increase the expression of CD44, the HA binding receptor, during an inflammatory response. The macrophage protein CD44 is abundantly expressed, and AMs can bind HA even under homeostatic (non-inflammatory) circumstances. HA is absorbed by macrophages in a CD44-dependent manner, after which it is moved to the lysosomes [
167]. Additionally,
M. tuberculosis can use HA as a carbon source for multiplication, and CD44 serves as a location for the macrophage to bind to
M. tuberculosis [
168].
5.4. Tuftsin Receptor Targeting
Tuftsin is a naturally occurring tetrapeptide that is created via the enzymatic cleavage of the CH2 domain of IgG. Tuftsin is harmless to humans and animals and has anticancer, chemotaxis, and phagocytosis-stimulating activities. By means of receptor-mediated endocytosis, tuftsin is taken up by macrophages and polymorphonuclear phagocytes. Tuftsin and its derivatives have been the subject of countless studies over the past few decades because of the broad range of biological actions they have [
172]. It was mentioned that neuropilin-1, which is present in most tissues and has, among others, angiogenesis and axonal guidance functions, binds to tuftsin as well [
173].
5.5. Mycolic Acid Targeting
Numerous substances found in the cell walls of mycobacteria could be used as targeted ligands. Due to their dominance as lipids in the outer cell wall envelope of mycobacterium species, mycolic acids (MAs) are among them and are hence attractive possibilities [
174]. It has been demonstrated that the different MA subtypes significantly influence the pathogen’s pathogenicity. Interesting biological processes carried out by MA from
M. tuberculsis include foam cell production and immunological steering towards Th1 cellular responses, as well as cholestroid-such as characteristics. MA was found to have immunogenic properties. Human CD4 and CD8 (double negative) T-lymphocyte proliferation takes place when CD1b molecules on dendritic cells are exposed [
175].
6. Conclusions
TB is a leading cause of death from infectious diseases worldwide. While various drug treatments exist to combat this disease, significant challenges remain, including poor patient compliance, a heavy pill burden, and notably, the development of MDR and XDR in TB patients. Additionally, physiological and pathophysiological barriers in the pulmonary region can hinder the efficient delivery of anti-TB drugs to the target site. The advent of drug nanocarriers offers hope for a more effective TB treatment, potentially reducing MDR and XDR. Nanotechnology-based drug formulations, including PNPs, SLNs, NLCs, NEs, PMs, CNTs, MNPs, liposomes, and dendrimers, offer several advantages over traditional treatments. These benefits include a reduced drug dose, fewer adverse effects, enhanced drug solubility and bioavailability, better patient compliance, and decreased drug resistance, all contributing to faster patient recovery from TB. While these nanoformulations can be administered through various methods, such as orally, intravenously, topically, or via the pulmonary route—each with its pros and cons—the most effective treatment for TB involves the direct delivery of anti-TB drugs to the lungs, the primary organ affected by TB. This direct approach offers several advantages over other methods of administration, including reduced doses, decreased frequency of dosing, and fewer side effects. However, barriers such as mucociliary clearance, biofilm formation, pulmonary surfactants, and alveolar macrophage clearance in the respiratory system challenge direct drug delivery to the lungs. Overcoming these obstacles is essential for efficient drug delivery. Conjugating drug-loaded nanocarriers with specific ligands, such as mannose, mycolic acid, folic acid, HA, and aptamers, allows these emerging therapeutic options to navigate these biological and structural barriers in the respiratory system. This targeted approach improves drug delivery, enhancing TB treatment using nanotechnology. In conclusion, TB treatment can benefit significantly from ligand-functionalized nanoformulations of anti-TB drugs. Nevertheless, despite advancements in TB treatment through nanotechnology-based methods, there’s a pressing need for novel drug therapies for more effective TB treatments, aiming to reduce TB cases worldwide.
This entry is adapted from the peer-reviewed paper 10.3390/ph16101360