Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
Polyhedral oligomeric silsesquioxanes (POSS) and hybrid organo-halide perovskites are two important types of hybrid nanoscale frameworks with great potential in materials chemistry. Both are currently under intensive investigation for a wide range of possible applications.
- perovskite
- silsesquioxane
- POSS
- hybrid materials
1. Introduction
Polyhedral oligomeric silsesquioxanes (POSS) of general the chemical composition (RSiO3/2)n are an important and extensively studied class of hybrid nanoscale materials. While the inorganic cage of the best-known POSS is usually octahedral, the chemical structure of organic functional groups R on the silicon atoms may vary (Scheme 1) [1]. The unique structure of POSS makes them a versatile component of various organic-inorganic hybrid materials. Functionalized POSS were thus applied for the synthesis of inorganic complexes and catalysts [2,3,4,5,6], including extended MOF architectures [7,8] as well as photoactive [9], self-healing [10] and self-assembling systems [11], hybrid polymer composites [12,13,14,15,16,17], and materials for biomedical applications [18,19,20,21].
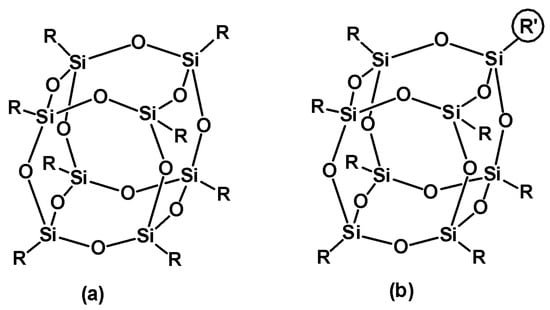
Scheme 1. Chemical structure of (a) monofunctional (RSiO3/2)8 with functional the group R and (b) bifunctional POSS (RSiO3/2)7(R′SiO3/2)1 where R can be iBu or Ph and R′ is a specific organic substituent.
Interesting results obtained in the field of materials engineering have inspired the use of polyhedral silsesquioxanes to improve the structure and properties of hybrid organo-halide perovskites. Materials based on this type of perovskite material are of great interest in the field of organic electronics, both in basic research and in a wide range of applications [22,23,24,25]. Techniques for their preparation are facile and relatively inexpensive, and thus they offer significant potential for thin-film solar cell technologies. Their characteristic structural features are sheets of organic and inorganic components alternately stacked on the molecular scale [26]. Alkylammonium cations are often used as layer separators in metal halide perovskites, acting as a barrier against magnetic and electronic coupling. Three-dimensional perovskites with the formula ABX3 (A: amine, B: metal, and X: halide) are typically obtained when small organic amine molecules are used as the precursors of cationic species, whereas A2BX4 two-dimensional perovskite structures are produced when larger amine molecules are employed.
Inorganic cesium lead halide CsPbX3 (X = Cl, Br, and I or mixed halide) perovskite nanocrystals (NCs) are dominant in this field, owing to their high photoluminescence quantum yield (PLQY, up to 90% in solution) and narrow emission peaks, as well as their easy solution processing [27]. The exceptional optoelectronic characteristics of CsPbX3 NCs, and their photovoltaic performance, make them valuable emissive materials in electroluminescent (EL) devices, including light emitting diodes (LED), solar cells, lasers, and photodetectors [28]. Small intrinsic defects do not act as electronic trap states [29], and their characteristic emission colors can be adjusted across the visible range by tuning the size of NCs and their chemical features [30,31,32,33].
2. Structure and Properties
2.1. Layered POSS-Metal Halide Hybrid Perovskites
The interfacial layers play an important role in determining the efficiency and stability of perovskite structures, and various strategies have been developed to cope with the problem of surface engineering [35]. The successful application of functionalized trialkoxysilanes for the formation of siloxane networks between perovskite layers and interface passivation was reported [36,37,38,39,40]. Cage-like POSS molecules, that exhibit a characteristic tendency to self-organize, can also be a suitable interlayer component of perovskite structures. The advantage of POSS over the random silsesquioxane networks obtained in the sol-gel process with trialkoxysilanes is the uniform morphology of the inorganic layer due to the defined size of silsesquioxane nanocages.
A new class of low-dimensional layered POSS-metal halide hybrid perovskites was prepared under ambient conditions using octa(ammoniumpropyl)octasilsesquioxane chloride (A-POSS) and several metal halide salts (CuCl2, PdCl2, PbCl2, and MnCl2) [41]. The components were first dissolved in aqueous hydrochloric acid or water, and then the complexes were isolated as microcrystalline precipitates. The structural features of the hybrid complexes indicate the dependence of the stability of octahedral POSS on the synthesis conditions. The cage silsesquioxane structure mostly remained intact in the complexes, with metal halides formed in the process. Solid-state 29Si MAS NMR recorded for the hybrid species showed a slight upfield shift of the peak attributed to the T3-type silicon atoms in the cubic POSS structure. The extent of the shift depended on the type of perovskite cation (δSi = −63.9 ppm; −67.1 ppm; −65.1 ppm; −64.4 ppm; and −64.1 ppm for, respectively, A-POSS, Cu-A-POSS; Pd-A-POSS; Pb-A-POSS; and Mn-A-POSS). A slight peak broadening was attributed to the magnetic effect of the metal halide layers, especially in the case of the Cu and Mn complexes. For Pb-A-POSS, the broadening of the main peak was accompanied by the appearance of a weak resonance peak around −55 ppm. The chemical shift range indicates that the band may have arisen from the partial degradation of the silsesquioxane cage. Pd-A-POSS was an exception, as their 29Si NMR spectra featured a sharp T3 peak.
Interestingly, the use of octa(propylammonium)octasilsesquioxane bromide (Br-POSS) or iodide (I-POSS) for the co-crystallization with PbCl2 did not involve the degradation of the silsesquioxane cube [43]. The plate-like products precipitated quite rapidly, and their PXRD diffractograms indicated layered structures with interlayer spacings (1.61 nm and 1.62 nm, respectively) similar to those calculated for Pb-A-POSS. The ionic silsesquioxane interlayers stabilized the excited electron-hole pairs in the semiconducting Pb-X-POSS lead halide perovskites (X = Cl−NH3+; Br−H+; I−H+) with well isolated layers, even if they lacked porosity. This effect is important for potential applications of hybrid POSS-perovskite materials as light-emitting diodes and light absorbers in solar cells. The absorption and the exciton emission peak positions shifted towards longer wavelengths by changing the ionic group from Cl−NH3+ to Br−H+ to I−H+. For Pb-Br-POSS and Pb-I-POSS, strong absorption bands were observed at ~390 and 510 nm, while sharp PL peaks appeared at 424 and 526 nm, respectively, when excited with λex = 355 nm. These results showed that by selecting the composition of hybrid complexes, it is possible to adjust both the structure and the properties of NCs. This may have a significant impact on the design process of, e.g., solar cell technology.
The observed phenomena were explained by detailed structural analysis. The PXRD patterns showed differences between the interlayer thicknesses of the perovskites obtained from EA (1.06 nm) or PEA (1.86 nm) alone and those containing a mixture of one of the amines and A-POSS (1.71 nm regardless of the type of organic amine). Increasing the amount of EA resulted in a product of bimodal structure with the layer distances of 1.06 nm (EA) and 1.71 nm (A-POSS or a mixture of A-POSS and EA), as indicated by the characteristic diffraction patterns. The results of the N2 sorption studies corroborated the PXRD data. All the hybrid materials containing A-POSS (also those admixed with EA) contained micropores smaller than 2 nm and sorption/desorption isotherms of type I were obtained. The amount of adsorbed N2 was greater for Cu-A-POSS/EA than Cu-A-POSS, and the micropore volume and BET surface increased linearly with the replacement ratio (x) of POSS with EA(44% increment if x = 0.202). When x was >20%, the micropore volume and BET surface area decreased and an increase in the fraction of nonporous layered perovskites was observed with PXRD (EA-derived layered perovskites are nonporous). On the contrary, the shrinkage of the interlayer pores was observed in the case of the layered perovskites separated by mixtures of the cubic silsesquioxanes and PEA (comparing to Cu-PEA). It implies the influence of π–π stacking of the aromatic amine molecules on the porosity of the layered perovskites. The reduction in the interlayer distance suggests that the ordering of PEA was altered in the presence of A-POSS. The effect allows for the precise structural engineering of the interlayer distance and, correspondingly, the properties of such complex systems.
2.2. Encapsulation with POSS to Increase Brightness and Stability of Perovskites
Perovskite nanocrystals of the CsPbX3 structure are promising and valuable emissive materials in electroluminescent devices. However, the quality of an as-prepared film of NCs may be relatively poor due to the presence of the long-chain surface ligands used during the coating process, which hampers the efficiency of the charge injection. The processing problems may lead to uneven, patchy film coverage over the device sublayers. Perovskite nanocrystals can be protected from moisture by encapsulation in a hydrophobic polymer matrix, which provides a physical barrier [45,46,47,48]. However, the formation of stable perovskite-polymer composites is not trivial due to the phenomena of phase separation and nanocrystal agglomeration.
Functionalized POSS can be a solution in the engineering of perovskite materials to enhance the low stability of the ionic nanocrystal lattice. Hydrophobic polyhedral silsesquioxanes of the (RSiO3/2)7(R′SiO3/2)1 (R = i-Bu and R′ a functional group) structure can play a beneficial role as materials that can encapsulate the perovskite NCs or form an intermediate hydrophobic passivation layer for their thin films. Monofunctional POSS of this type can improve the surface coverage and the morphological features of the perovskite films and possibly improve their miscibility with polymer matrices. For example, molecules of (iBuSiO3/2)7[HS(CH2)3SiO3/2] were used as a surface protecting additive to the CsPbX3 (X = Br or I) nanocrystals [49,50]. However, low amounts of POSS were required since the silsesquioxane cages may act as insulators.
The treatment provided the moisture resistant hybrid perovskite nanopowders-quantum dots (PQD) that can be used as solid state luminophores in all-perovskite white light-emitting devices. The hybrid silsesquioxane molecules acted as a hole-blocking layer between the thin coating made of the perovskite NCs and the 1,3,5-tris(N-phenylbenzimidazol-2-yl) benzene (TPBi) film that operated as the electron-transporting layer. The POSS-PQD exhibited (HRTEM and PXRD) the lattice plane distance of 0.58 nm, characteristic for cubic phase CsPbBr3 perovskite, and high output performance. Moreover, the silsesquioxane coating prevented anion exchange between the perovskite nanocrystals in the solid state, thereby increasing the stability of the mixtures of the perovskite NC powders with the different halide compositions. The distinct emission spectra of the different POSS-passivated CsPbX3 were preserved, while the uncoated NCs underwent ion exchange, resulting in a broadening of their characteristic PL signals in the solid state. It should be noted that their PLQY in toluene solutions slightly decreased to 62% (X = Br) and 45% (X = Br/I) upon passivation with POSS, but the absolute PLQY in the solid state did not change and remained very high (respectively, 61% and 45%).
This strategy yielded single layer, all-perovskite devices that emitted white light by mixing the nanopowders of the green-emitting POSS-CsPbBr3 and the red-emitting POSS-CsPb(Br/I)3. The POSS-passivated perovskites were thus used as solid state luminophores for the fabrication of all-perovskite (a single down-conversion layer) white LEDs with a CIE chromaticity coordinate of (0.349, 0.383), CRI = 81, and luminous efficiency of 14.1 lm W−1. The characteristic electroluminescence spectrum is a combination of the three emission peaks (the blue one originated from the blue-emitting InGaN LED chip).
The improvement in the morphology and coverage of the CsPbBr3 NCs in the presence of POSS did not always lead to luminance enhancement. If the POSS molecules were present in the active layer, they acted as insulators. As a result, the external quantum efficiency (EQE) value was not high and the average PL lifetimes were reduced (from 434 to 134 ns, and from 115 to 63 ns for the suspension and supernatant solutions, respectively). Nevertheless, with the optimized POSS concentrations, higher loading of the separated NCs resulted in an increase in the overall LED brightness.
It was more beneficial to use POSS as a separate layer on top of the active perovskite NC layer. In this case, the average PL decay time only decreased from 434 to 342 ns, and the average recombination rate increased by 21%. The effect of the upper POSS layer was attributed to the more efficient electron and hole recombination in the NCs zone and the blocking of hole transport between the perovskite NCs and the TPBi layers. In this case, the peak LED luminance was almost eight times higher (2983 cd/m2 at 11.5 V; LE = 1.20 cd/A; EQE = 0.35%) than that obtained without the hole-blocking layer. In addition, POSS enhanced the stability of the LED devices and their operation lifetime was five times longer.
MA-NCs can further participate in radical polymerization reactions. The copolymerization with methyl methacrylate and/or (iBuSiO3/2)7[(H3C)H2C=CC(O)O(CH2)3SiO3/2] yielded composite materials (PMPNC and PMPOPNC, respectively). Their diffraction patterns corresponded to that of the orthorhombic perovskite NCs crystals. The thin films cast from the dispersions of PMPOPNC in the organic solvents contained well-dispersed crystalline nanoparticles with a size of ∼12 nm, embedded within the amorphous PMMA-co-P(ME-POSS) (PMPO) matrix. No phase separation was observed. The nanoparticles of PMPOPNC were larger (average size 68 nm) than the free ME-NCs and their size distribution was wider. The PL spectra of PMPNC and PMPOPNC corroborated those of the free ME-NCs and the ME-NC/PMMA composites (λe at 516 nm). Their PLQY (respectively, 68% and 72%) was similar to that of the free ME-NCs (PLQY above 80%) and larger than the PLQY of the MENCs/PMMA blend (ca. 54%). The emission wavelength and PLQY of the PMPOPNC can be tuned by adjusting the ratio of halides in the perovskite structure CsPbX3 by using different lead halide precursors.
The PMPOPNC film was hydrophobic (static water contact angle of 120°, comparing to 105° of PMPNC and 104° of ME-NCs/PMMA blend). The effect was assigned to the micro-nanorough features revealed in the SEM micrographs, caused by the migration of the POSS-containing fraction to the air-solid interface (confirmed by XPS gradient concentration data).
2.3. POSS As a Moisture Barrier in Perovskite Films
As was mentioned in the previous section, apart from the employment of the POSS coated perovskite NCs as solid state luminophores, the large size of the POSS macromolecules and the presence of hydrophobic organic groups grafted to Si atoms can be an advantage with regard to their barrier action against moisture. The significant potential in this area is offered by monofunctional polyhedral silsesquioxanes of type (RSiO3/2)7(R′SiO3/2), where R = iBu and R′ is a reactive organic residue that enables the adsorption of POSS on the surface of perovskite crystals or thin films. The passivated perovskites can be applied as water resistant light-emitting materials.
It was also shown that [3-(2-aminoethyl)amino]propyl-heptaisobutyl substituted POSS (iBuSiO3/2)7[H2N(CH2)3SiO3/2] (POSS-NH2) can be applied as a capping ligand for (CH3NH3)PbBr3 (MAPbBr3) (MA—methylammonium) [52]. POSS-NH2 passivated the surface of the MAPbBr3 NCs, controlling the crystal size and increasing the perovskite material stability in the LED devices. The importance of the presence of sterically demanding POSS ligands was demonstrated by the comparison of the structure and properties of hybrid NCs with their analogs modified with (3-aminopropyl)triethoxysilane (APTES) as the caping ligands.
POSS-NH2 made the surface of a thin layer of the perovskite NCs waterproof, influenced the structure of the film, and helped to tune its optoelectronic behaviour [53]. The photovoltaic performance with a power conversion efficiency (PCE) over 20% was observed. It was also shown that POSS-NH2 can effectively passivate the surface of thin coatings of NCs composed of the mixed halide perovskites, MAxFA1−xPbI3−yBry (FA—formamidinium), as well as influence the crystal grain boundary and the number of ionic defects [54]. The temperature-dependent admittance measurements proved that the presence of POSS-NH2 reduced the charge trap density and, as a result, the trap-state energy level of 0.045 eV was achieved. Those features were reflected in an enhancement of the open-circuit voltage (VOC) and power conversion efficiency from 18.1% to 20.5%.
As was already mentioned, the presence of POSS is beneficial, but its quantity must be optimized because the inorganic cube acts also as an insulator. It was shown that the surface composition of the FA0.85MA0.15Pb(I0.85Br0.15)3 films and their morphology changed in a regular manner in the presence of POSS-NH2 as a result of its interaction with the perovskite film [55]. When the amount of silsesquioxane exceeded the optimum concentration (10 mg/mL), the quality of the perovskite film surface morphology was compromised by the formation of cracks. The AFM height profiles displayed by the variations in the root mean square (RMS) values show that the size of NCs changed on modification with POSS-NH2 to a degree that depended on the amount of POSS used for the modification.
2.4. Application of Water-Resistant Hybrid POSS-Perovskite Systems
The stability of the MAPbI3-based solar cells, based on the passivated films under ambient environment, was shown by the stable VOC and JSC values during 90 days. After that time, the solar cells made of the perovskites passivated by POSS-NH2 and POSS-SH retained, respectively, 100% and 94% of their initial JSC (fill factors around 89% and 88%, respectively, of their original performance and 72% of the initial FF for the non-passivated devices). The better protection offered by POSS-NH2 corroborated the stronger interactions of this compound with the perovskite film. The protective effect provided by POSS was less pronounced in the case of the solar cells based on (FA)0.85(MA)0.15Pb(I3)0.85(Br3)0.15 with formamidinium ions embedded in the perovskite structure. Passivation with POSS played a minor role in such stable systems, which already had good moisture resistance.
[3-(2-Aminoethyl)amino]propyl-heptaisobutyl-POSS was used for the synthesis of an amphiphilic copolymer (ap-POSS-PMMA-b-PDMAEMA, methylmethacrylate, MMA, and 2-(dimethylamino)ethylmethacrylate, DMAEMA) further applied to prepare the stable core-shell colloidal perovskite nanocrystal-polymer micelle composites (ap-POSS-PMMA-b-PDMAEMA@CsPbBr3) [58]. The presence of the hydrophobic POSS-PMMA segment of ap-POSS-PMMA-b-PDMAEMA was crucial in the process of self-assembling into the “reverse” micelles in DMF/toluene. The reverse micelles acted as confined nanoreactor templates during the perovskite crystallization, passivating the perovskite surface with a multidentate capping shell.
3. Conclusions
Polyhedral oligomeric silsesquioxanes can be effectively used for the modification of inorganic cesium-halide perovskites CsPbX3 (X = Cl, Br, I), both nanocrystals and thin films. Depending on the chemical structure of POSS, they can act as an interlayer component of perovskite structures (ionic octafunctional POSS) or as a passivating and structure-controlling agent of perovskite nanocrystals (bifunctional polyhedral silsesquioxanes of (iBuSiO3/2)7(R′SiO3/2)1 type). The literature reports show that the crystallization kinetics of perovskites can be modified by the presence of structure-directing POSS. This approach results in a reduction in the number of crystallographic defects in the perovskite-based materials and, consequently, an improvement/modification in their optoelectronic properties. In addition, POSS can enhance the hydrolytic and thermal stability of perovskite structures.
This entry is adapted from the peer-reviewed paper 10.3390/ma16196531
This entry is offline, you can click here to edit this entry!
