Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Neuropsychiatric disorders (NDs) are a diverse group of pathologies, including schizophrenia or bipolar disorders, that directly affect the mental and physical health of those who suffer from them, with an incidence that is increasing worldwide. Most NDs result from a complex interaction of multiple genes and environmental factors such as stress or traumatic events, including the recent Coronavirus Disease (COVID-19) pandemic.
- bipolar disorders
- lipocalins
- mayor depression disorders
1. Introduction
In the past decades, there has been growing interest in the study of neuropsychiatric disorders (NDs) since they have become the first cause of disability and the second cause of death worldwide [1]. A European meta-analysis published in 2011 estimated that 38.2% of the population suffers from at least one brain disorder each year, i.e., mood or developmental, which means around 164 million people [2][3][4]. The most frequent disorder is anxiety (14%), followed by insomnia (7%), major depression (6.9%), somatoform disorders (6.3%), and alcohol and drug dependence (4%); however, attention deficit hyperactivity disorder (ADHD) stands out in the youngsters (5%) [2]. Most recent studies, based on data from the World Health Organization (WHO), place the incidence of this group of pathologies at almost 50%, an increase that would be related to increasingly frequent natural disasters or conflict-induced humanitarian crises in numerous countries, as well as the recent Coronavirus Disease (COVID-19) pandemic, among many other factors [5][6][7].
From a clinical point of view, there are several circumstances that could be behind the neuropsychiatric symptoms, including metabolic alterations, the consumption of poisons/toxins, and general medical conditions such as neoplasm, trauma or infections [8]. This complexity constitutes a handicap for the diagnosis and treatment of NDs. Primary information provided from gene expression or brain activity imaging techniques is useful but with limitations. Thus, there is a current effort focusing on the discovery of potential biomarkers for improving prevention, diagnosis, drug response, and drug development for NDs. In this sense, finding biomarkers for NDs would help to predict outcomes, stratify groups of patients, and make decisions regarding treatments and therapies. Undoubtedly, an ideal biomarker is an intracellular or extracellular molecule that shows measurable differences between physiological and pathological states. Protein profiling in serum, plasma, urine, saliva, or cerebrospinal fluid (CSF) in NDs is a promising field of research [4]. In addition, not only proteomics but all the “omics”, including genomics, transcriptomics, metabolomics, and epigenetic techniques, are powerful tools in the search of biomarkers [9]. The big question is whether there is any molecule that meets all these requirements and can be used in clinical practice.
The apolipoprotein (Apo) family is a specialized group of proteins that associates with lipids and mediates several steps in lipid metabolism. So far, there are 22 known members of the family but only Apo E [10], Apo J [11], and Apo D [12] are expressed at high levels in the nervous system [13]. Several studies indicate that of candidate psychiatric biomarkers detected using proteomic techniques, cholesterol and associated proteins, specifically apolipoproteins, may be of interest. Cholesterol is necessary for brain development and its synthesis continues at a lower rate in the adult brain. Specifically, Apo D is the component of lipoproteins responsible for lipid transport [14][15], whose implication in NDs has gained interest in recent years [16][17][18][19][20][21].
2. Role of Apo D as a Biomarker in Neuropsychiatric Disorders
Current evidence strongly suggests the increase of Apo D expression in NDs such as Schizophrenia (SZ), Bipolar Disorders (BPD), Major Depressive Disorders (MDD), or Autism Spectrum Disorder (ASD), a group of diseases that shares some cardinal pathological features including oxidative stress, excitotoxicity, myelin dysfunction, cholesterol imbalance, and apoptosis, and where it seems to play a fundamental role as a neuroprotective protein. The existing lack of biomarkers for the effective and early detection of NDs makes it necessary to search for new predictive molecules, and, in this sense, Apo D could be a good candidate. An updated view of the role of Apo D as a potential biomarker for different NDs, summarized in Figure 1.
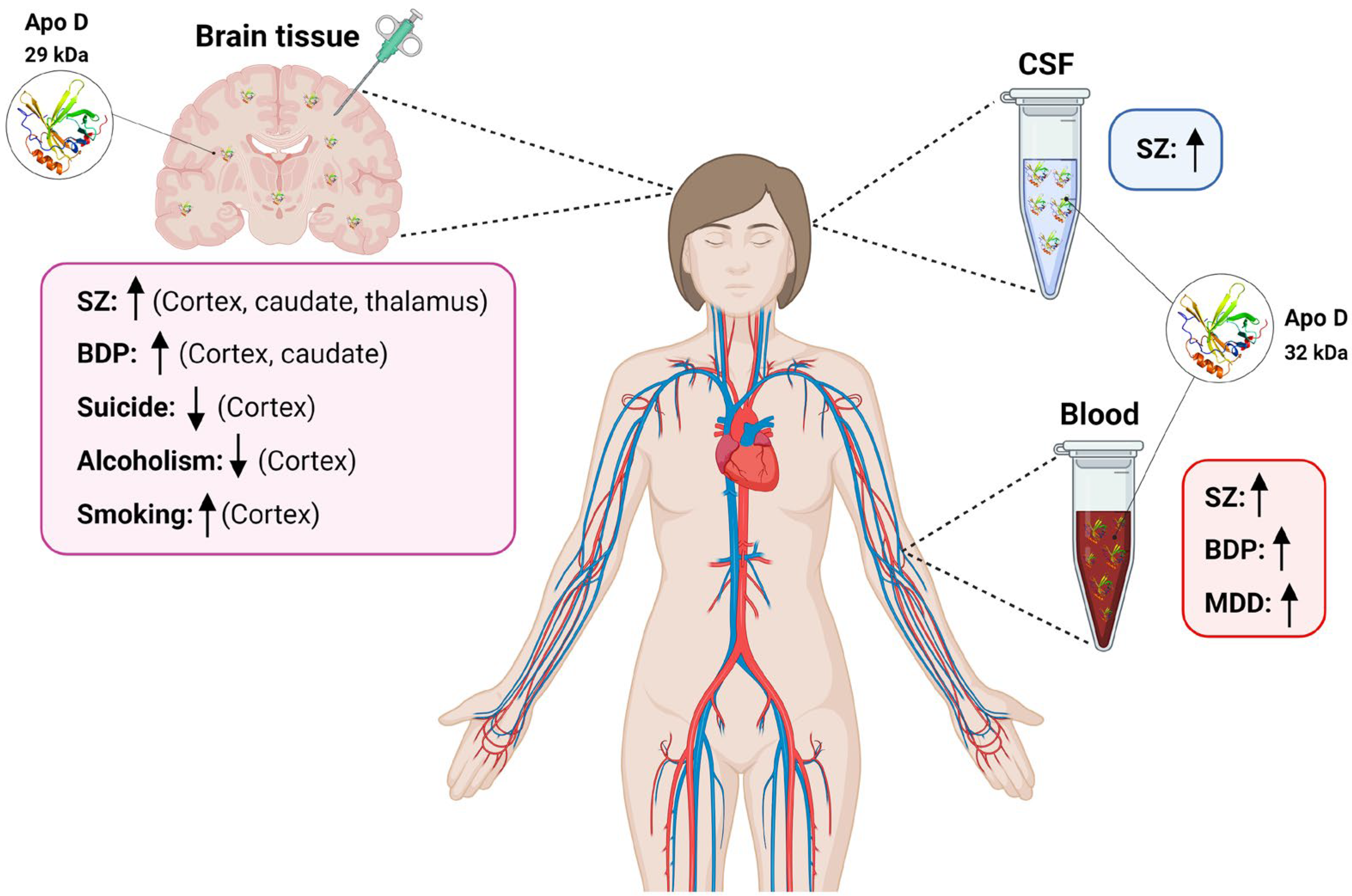
Figure 1. Scheme summarizing variations in Apo D levels in blood, CSF, and human brain tissues found in different neuropsychiatric diseases. SZ: schizophrenia; BPD: Bipolar disorders; CSF: cerebrospinal fluid; MDD: Major depressive disorder. ↑: increased Apo D levels; ↓: decreased Apo D levels.
2.1. Apo D in Schizophrenia
SZ is a severe and long-term brain condition characterized by changes in how a person thinks, perceives, and interprets reality that affects approximately 20 million individuals globally [22]. Major symptoms that may appear continuously or in relapsing episodes include psychosis, hallucinations, delusions, disorganized speech, lack of motivation, or cognitive deficits [23]. Researchers speculate that a variety of genetic and environmental factors contribute to the development of SZ [24]. While there is no cure for SZ, ongoing research is paving the way for innovative and more secure treatment options. However, the success of these treatments involves, without a doubt, adequate diagnostic methods. Nowadays, clinical diagnosis predominantly relies on subjective evidence, encompassing self-reported experiences and observed behavioral abnormalities, followed by psychiatric evaluations [25]. Consequently, there is a pressing need for objective and specific diagnostic tests based on biomarkers to enhance the accuracy of SZ diagnosis in clinical practice [22]. Although the pathological mechanisms of the disease are not fully understood, multiple lines of evidence indicate that disruptions in membrane lipids and their metabolism caused by excessive oxidative stress likely play a role in the development of SZ, affecting neurotransmission and complex brain function [23][26].
Taking this into consideration, Thomas et al., in 2001, described, for the first time, a selective increase of Apo D quantity in some brain areas of patients with SZ [27]. They took samples from the left hemisphere of 20 subjects diagnosed with SZ and analyzed Apo D levels using Western blot (WB) and ELISA techniques. They found significant increases in Apo D amount in the prefrontal cortex (BA9 and BA46; 46% increase), orbitofrontal cortex (BA11; 23.9%), amygdala (42.8%), thalamus (31.7%), and caudate, precisely the brain areas most affected by the pathophysiological changes related with SZ [28]. Remarkably, Apo D serum levels were lower in these patients than in control ones, suggesting that the local Apo D increment may act as a kind of compensatory response against systemic insufficiencies in lipid metabolism. However, it is important to keep in mind that most of the patients in these studies were medicated with neuroleptic drugs, and a gradual accumulation in both Apo D mRNA and protein was reported in response to chronic clozapine (CLZ) administration in the brains of rodents [29]. In humans, a significant increment of Apo D serum levels was found in never-medicated patients when compared with controls and even higher in those chronic patients treated with the atypical antipsychotic drug CLZ versus drug-free ones [30]. These findings seem to support the view that Apo D could be acting as an antioxidant neuroprotective molecule to prevent AA peroxidation and, at the same time, as a biological marker in an indirect way [31].
Furthermore, Apo D high levels have also been found in a study that looked for biomarkers of psychosis risk in blood. In fact, Apo D has been identified, together with matrix metalloproteinase 7, as the only two molecules of 117 (related to hormonal responses, inflammation, growth, oxidative stress, and metabolism) that showed a significant difference between people with clinical high-risk symptoms who developed psychosis and those who, despite presenting symptoms, did not do so [32]. A meta-analysis for exploring potential biomarkers of SZ in human peripheral fluids confirmed Apo D as one of the 20 proteins that were altered in the serum and plasma of SZ patients compared with controls. Despite these results, authors proposed several proteins as possible biomarkers for SZ, but Apo D was not among them [33]. Relatedly, Raiszadeh et al., in 2012, performed a proteomic analysis of sweat in SZ subjects [34]; Apo D was one of the five proteins that sweat and serum had in common. However, expression levels of Apo D in sweat samples were not high enough to take into consideration the analysis of this lipocalin as an SZ biomarker. These results are quite surprising as Apo D has been reported by other authors, after dermicidin and Apo J, as the third most abundant protein in human sweat, representing 15% of the total of secreted proteins [35].
The differences in the results of the mentioned studies could be explained by different factors such as the analysis methodologies, the number of subjects, the phase of the disease, or the medication. Once again, data about pharmacological treatments of patients is interesting since it has been shown that some drugs, like haloperidol (HAL), reduce Apo D expression in the hippocampus, piriform cortex, and caudate-putamen, while others, such as risperidone (RISP) or olanzapine (OLZ), produce the opposite effects in rat brain [36]. In fact, the negative effects of HAL over cell morphology, as well as Apo D quantities, could be restored with RISP or OLZ post-treatment. Even more, pre-treatments with these atypical antipsychotic drugs also prevent Apo D reduction caused by HAL but not in such an effective way as post-treatments. It is known that CLZ, RISP, and OLZ are better choices than HAL for SZ treatment due to the negative effects on cognition and extra-pyramidal symptoms caused by the last one. Therefore, Apo D increase could be involved in the molecular mechanisms that underlie the positive effects of CLZ, RISP, and OLZ in SZ treatment, e.g., to sequester free AA in the cell, preventing its release and its entry into inflammatory pathways such as the cyclooxygenase [18][36][37][38]. Anyway, it would be interesting to know the effect of more drugs on Apo D expression to determine if this lipocalin could be a good biomarker for SZ.
In the CSF, Apo D has been identified as a potential biomarker for cerebral amyloid angiopathy [39], chronic pain [40], MS [41], and idiopathic normal pressure hydrocephalus [42], among other brain-related pathologies. When CSF was analyzed in the search for SZ markers, using iTRAQ-based proteomic and mass spectrometry techniques, Apo D was identified as one of the ten proteins that showed differential expression across the clinical dopaminergic spectrum but did not show linearity or statistical significance to be considered as a biomarker probably due to the low number of patients included in these experiments [43].
While it is true that due to the invasiveness of the techniques, using Apo D as a biomarker for diagnosing SZ in brain tissue or CSF does not provide an advantage over other biomarkers, the ability to detect changes in Apo D levels with just a blood sample and a protein profiling assay places it in an advantageous position compared to other molecular markers whose detection involves methodologically complex and expensive assays, such as RNA sequencing.
2.2. Apo D in Bipolar Disorders
BPDs are a group of chronic mental health conditions that cause abnormal shifts in mood, ranging from extreme highs (mania) to extreme lows (depression) episodes [24]. BPDs affect the quality of life of millions of adults worldwide, negatively impacting day-to-day social interactions and potentially leading to premature mortality from cardiovascular disease or suicide [44]. The exact cause of these pathologies is unknown, but genetics play a key role, accounting for 70% of the risk for BPD as heritable [45]. Regarding BPD physiopathology, recent studies suggest that immune-inflammatory changes induce structural brain alterations at the limbic network that compromise dopamine and serotonin neurotransmitter signaling, which would explain the symptoms and the cognitive deterioration of patients [45][46].
The study of the Apo D brain levels in post-mortem samples of BPD subjects, in the same areas as the authors did previously for SZ, demonstrated differences between the two NDs [28]. In the case of BPD, an increase of Apo D expression was observed in the dorsolateral prefrontal (BA9; 273% increment), lateral prefrontal (BA46; 111% increment), and in the parietal cortices (BA40; 123% increment) of BPD patients vs. healthy controls. In contrast, no significant increase was observed in the orbitofrontal (BA11; 37.9% increment) and cingulate cortices (BA24; 57.7% increment), and no changes were appreciated in the amygdala or the thalamus [27][28].
In regard to the potential of Apo D as a serum biomarker for BPD, results found in the literature are quite contradictory. Dean and co-workers, in 2008, using ELISA immunological assays, did not observe differences in Apo D levels between BPD vs. SZ patients or their controls [47]. Interestingly, medications did not seem to have an effect on the amount of Apo D in the plasma of BPD subjects. Contrarily, Knöchel et al., in 2017, performed a nanoliquid chromatography–multiple reactions monitoring mass spectrometry (nano-LC–MRM-MS) and observed that BPD patients exhibited higher Apo D levels in serum than those diagnosed with SZ (p < 0.050) [48]. Similar results have been reported in a recent study; Apo D is found elevated in the serum of BPD patients compared to controls [49]. In this case, the proteins were separated by gel electrophoresis and then analyzed by mass spectrometry. At this point, it would be interesting to know the type of BPD of the patients and the number of subjects included in these studies, as well as to explore a little further the real influence of methodologies on the results obtained.
Notwithstanding, it is important to remember that Apo D conformation is not the same in the post-mortem brains and in plasma, and, consequently, their behavior may be completely different. Thus, a systematic review and a meta-analysis on MS-based proteomics applied to human peripheral fluids to assess potential biomarkers of BPD showed that Apo D is present in plasma and serum, and it was among the 258 proteins differentially expressed in blood-related samples (plasma, serum, and PBMCs) when studying BPD patients vs. controls [33]. Finally, Apo D has not been studied in other human fluids from BPD subjects.
2.3. Apo D in Major Depressive Disorder
MDD or clinical depression is a ND characterized by a persistent low mood, feelings of sadness, and loss of interest that condition the daily functioning and quality of life of patients [50]. It is considered the most common mental illness worldwide; the number of cases has increased by almost 50% over the past 30 years [51]. Since MDD is very variable in the lifetime course and recurrences are frequent, the early diagnosis of the initial depressive episode is instrumental in prescribing individualized treatments and preventing multiple episodes [50]. Until today, all attempts carried out to identify an applicable biomarker panel to the disease have failed [52].
The MDD pathophysiology includes dysfunction of the hypothalamic–pituitary–adrenal (HPA) axis, neurotransmitter metabolism disorder, oxidative stress, and neuroinflammation [53], processes in which Apo D could participate thanks to its function as a neuroprotective protein. However, there is no data about Apo D levels in brain tissue from patients with MDD since all studies have been done in serum samples. In this sense, Apo D was first reported in relation to MDD by Xu et al. in 2012, when these authors observed a 1.69-fold change increase in Apo D levels in MDD first-episode never-medicated patients compared with healthy controls using iTRAQ 2D LC-MS/MS. Nonetheless, when the proteins were studied by WB, the differences were not significant [54]. In a similar study (first-onset drug-naïve MDD patients) using multiplexed immunoassay profiling and LC-MS(E), despite Apo D being present above the limits of detection in all samples studied, no significant changes in Apo D levels were found in MDD patients vs. controls [55]. Surprisingly, in 2016, Apo D was identified as one of the six proteins that could be used as serum biomarkers in MDD, with a 68% accuracy, according to the authors [56]. In this study, blood samples of 50 non-medicated MDD patients and their corresponding controls were analyzed by LC–MS/MS to obtain a serum proteome profiling. With this method, a group of differentially expressed proteins was found, most of them implicated in inflammatory response and lipid transport, Apo D among them [33][54]. Moreover, the authors tested, with a logistic regression model, the robustness of these proteins (Apo B, Apo D, CP, GC, HRNR, and PFN1) in terms of accuracy, sensitivity, and specificity, obtaining values of 68, 67, and 69%, respectively [33]. Overall, the study not only detects a group of proteins that could be used as biomarkers of MDD but also implies that the modulation of inflammatory and immune systems, as well as lipids metabolism, are implicated in the pathophysiology of MDD. In this way, high levels of Apo D in the serum of non-medicated depressed patients may reflect a neuroprotective response to oxidative stress, systemic inflammation, neurovascular dysfunction, and BBB permeability [57]. It is important to bear in mind that certain treatments for depressive disorders have effects on lipid metabolism, so it would be interesting to know what happens with Apo D in MDD-medicated patients [58][59].
The study of Lee et al., performed by liquid chromatography–tandem mass spectrometry and label-free quantification in plasma from BPD and MDD subjects, took into account the effects of medication over protein expression (antipsychotics, mood stabilizers, antidepressants, or benzodiazepines) and demonstrated that Apo D expression was not affected for any of the antipsychotic drugs. However, it was negatively associated with the total scores of the Hamilton depression rating scale (HAM-D) and with the anhedonia/retardation and guilt/agitation scores of this scale [60]. In fact, Apo D expression seems to decrease with the worsening of symptoms, which would make it impossible to exert its neuroprotective role.
Clinical evidence suggests that some people suffering from MDD attempt suicide. It has been reported that alterations in cholesterol levels could play a crucial role in suicidal behavior, as shown by the significant association between (i) low levels of peripheral circulating cholesterol in patients with suicidal and violent conduct [61][62], and (ii) low levels of central cortical cholesterol in violent suicide completers [63]. There is little data about Apo D and suicidal behavior. Genome-wide expression profiling using DNA from samples of the prefrontal cortex (BA47) of ten suicidal subjects and eight matching controls showed that six genes were downregulated in the suicides, including Apo D (−1.57-fold change). Unfortunately, Apo D data were not validated by real-time PCR, as happened with Apo E (−1.63-fold change) or sortilin 1 (1.86-fold change). Anyway, Apo D and Apo E low levels could be an indicator of failure in cholesterol transport, as expected in this type of disorder [64].
This entry is adapted from the peer-reviewed paper 10.3390/ijms242115631
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480.
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679.
- Susser, E.; Ritsner, M.S. Brain protection in neuropsychiatric disorders: Past, present and future challenges. In Brain Protection in Schizophrenia, Mood and Cognitive Disorders; Springer: Dordrecht, The Netherlands, 2010; pp. 3–25. ISBN 9789048185535.
- Woods, A.G.; Sokolowska, I.; Taurines, R.; Gerlach, M.; Dudley, E.; Thome, J.; Darie, C.C. Potential biomarkers in psychiatry: Focus on the cholesterol system. J. Cell. Mol. Med. 2012, 16, 1184–1195.
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet 2019, 394, 240–248.
- Harrison, P.J.; Taquet, M. Neuropsychiatric disorders following SARS-CoV-2 infection. Brain 2023, 146, 2241–2247.
- Global Burden of Disease Collaborative Network. VizHub—GBD Results. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 26 September 2023).
- Taber, K.H.; Hurley, R.A.; Yudofsky, S.C. Diagnosis and treatment of neuropsychiatric disorders. Annu. Rev. Med. 2010, 61, 121–133.
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432.
- Elshourbagy, N.A.; Liao, W.S.; Mahley, R.W.; Taylor, J.M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc. Natl. Acad. Sci. USA 1985, 82, 203–207.
- De Silva Harshini, V.; Harmony, J.A.K.; Stuart, W.D.; Gil, C.M.; Robbins, J. Apolipoprotein J: Structure and Tissue Distribution. Biochemistry 1990, 29, 5380–5389.
- Drayna, D.; Fielding, C.; McLean, J.; Baer, B.; Castro, G.; Chen, E.; Comstock, L.; Henzel, W.; Kohr, W.; Rhee, L. Cloning and expression of human apolipoprotein D cDNA. J. Biol. Chem. 1986, 261, 16535–16539.
- Elliott, D.A.; Weickert, C.S.; Garner, B. Apolipoproteins in the brain: Implications for neurological and psychiatric disorders. Clin. Lipidol. 2010, 5, 555–573.
- Forero, D.A.; López-León, S.; González-Giraldo, Y.; Dries, D.R.; Pereira-Morales, A.J.; Jiménez, K.M.; Franco-Restrepo, J.E. APOE gene and neuropsychiatric disorders and endophenotypes: A comprehensive review. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018, 177, 126–142.
- Charnay, Y.; Imhof, A.; Vallet, P.G.; Kovari, E.; Bouras, C.; Giannakopoulos, P. Clusterin in neurological disorders: Molecular perspectives and clinical relevance. Brain Res. Bull. 2012, 88, 434–443.
- Björkhem, I.; Meaney, S.; Fogelman, A.M. Brain Cholesterol: Long Secret Life behind a Barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815.
- Pfrieger, F.W. Outsourcing in the brain: Do neurons depend on cholesterol delivery by astrocytes? BioEssays 2003, 25, 72–78.
- Davison, J.; O’Gorman, A.; Brennan, L.; Cotter, D.R. A systematic review of metabolite biomarkers of schizophrenia. Schizophr. Res. 2018, 195, 32–50.
- Kim, H.W.; Rapoport, S.I.; Rao, J.S. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol. Psychiatry 2011, 16, 419–428.
- Parekh, A.; Smeeth, D.; Milner, Y.; Thuret, S. The role of lipid biomarkers in major depression. Healthcare 2017, 5, 5.
- Rassart, E.; Desmarais, F.; Najyb, O.; Bergeron, K.F.; Mounier, C. Apolipoprotein D. Gene 2020, 756, 144874.
- Lin, P.; Sun, J.; Lou, X.; Li, D.; Shi, Y.; Li, Z.; Ma, P.; Li, P.; Chen, S.; Jin, W.; et al. Consensus on potential biomarkers developed for use in clinical tests for schizophrenia. Gen. Psychiatry 2022, 35, e100685.
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486.
- Mandal, P.K.; Gaur, S.; Roy, R.G.; Samkaria, A.; Ingole, R.; Goel, A. Schizophrenia, Bipolar and Major Depressive Disorders: Overview of Clinical Features, Neurotransmitter Alterations, Pharmacological Interventions, and Impact of Oxidative Stress in the Disease Process. ACS Chem. Neurosci. 2022, 13, 2784–2802.
- Pagsberg, A.K. Schizophrenia spectrum and other psychotic disorders. Eur. Child Adolesc. Psychiatry 2013, 22, 3–9.
- Li, M.; Gao, Y.; Wang, D.; Hu, X.; Jiang, J.; Qing, Y.; Yang, X.; Cui, G.; Wang, P.; Zhang, J.; et al. Impaired Membrane Lipid Homeostasis in Schizophrenia. Schizophr. Bull. 2022, 48, 1125–1135.
- Thomas, E.A.; Dean, B.; Pavey, G.; Sutcliffe, J.G. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: Implications for the pathophysiology of psychiatric disorders. Proc. Natl. Acad. Sci. USA 2001, 98, 4066–4071.
- Thomas, E.A.; Dean, B.; Scarr, E.; Copolov, D.; Sutcliffe, J.G. Differences in neuroanatomical sites of apoD elevation discriminate between schizophrenia and bipolar disorder. Mol. Psychiatry 2003, 8, 167–175.
- Thomas, E.A.; Danielson, P.E.; Austin Nelson, P.; Pribyl, T.M.; Hilbush, B.S.; Hasel, K.W.; Gregor Sutcliffe, J. Clozapine increases apolipoprotein D expression in rodent brain: Towards a mechanism for neuroleptic pharmacotherapy. J. Neurochem. 2001, 76, 789–796.
- Mahadik, S.P.; Khan, M.M.; Evans, D.R.; Parikh, V.V. Elevated plasma level of apolipoprotein D in schizophrenia and its treatment and outcome. Schizophr. Res. 2002, 58, 55–62.
- Fyfe-Desmarais, G.; Desmarais, F.; Rassart, É.; Mounier, C. Apolipoprotein D in Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1027.
- Perkins, D.O.; Jeffries, C.D.; Addington, J.; Bearden, C.E.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; Mathalon, D.H.; McGlashan, T.H.; Seidman, L.J.; et al. Towards a Psychosis Risk Blood Diagnostic for Persons Experiencing High-Risk Symptoms: Preliminary Results from the NAPLS Project. Schizophr. Bull. 2015, 41, 419–428.
- Rodrigues, J.E.; Martinho, A.; Santa, C.; Madeira, N.; Coroa, M.; Santos, V.; Martins, M.J.; Pato, C.N.; Macedo, A.; Manadas, B. Systematic Review and Meta-Analysis of Mass Spectrometry Proteomics Applied to Human Peripheral Fluids to Assess Potential Biomarkers of Schizophrenia. Int. J. Mol. Sci. 2022, 23, 4917.
- Raiszadeh, M.M.; Ross, M.M.; Russo, P.S.; Schaepper, M.A.; Zhou, W.; Deng, J.; Ng, D.; Dickson, A.; Dickson, C.; Strom, M.; et al. Proteomic analysis of eccrine sweat: Implications for the discovery of schizophrenia biomarker proteins. J. Proteome Res. 2012, 11, 2127–2139.
- Csosz; Emri, G.; Kallõ, G.; Tsaprailis, G.; Tozsér, J. Highly abundant defense proteins in human sweat as revealed by targeted proteomics and label-free quantification mass spectrometry. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2024–2031.
- Khan, M.M.; Parikh, V.V.; Mahadik, S.P. Antipsychotic drugs differentially modulate apolipoprotein D in rat brain. J. Neurochem. 2003, 86, 1089–1100.
- Morais Cabral, J.H.; Atkins, G.L.; Sánchez, L.M.; López-Boado, Y.S.; López-Otin, C.; Sawyer, L. Arachidonic acid binds to apolipoprotein D: Implications for the protein’s function. FEBS Lett. 1995, 366, 53–56.
- Thomas, E.A.; George, R.C.; Gregor Sutcliffe, J. Apolipoprotein D modulates arachidonic acid signaling in cultured cells: Implications for psychiatric disorders. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 421–427.
- Kuiperij, H.B.; Hondius, D.C.; Kersten, I.; Versleijen, A.A.M.; Rozemuller, A.J.M.; Greenberg, S.M.; Schreuder, F.H.B.M.; Klijn, C.J.M.; Verbeek, M.M. Apolipoprotein D: A potential biomarker for cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2020, 46, 431–440.
- Khoonsari, P.E.; Ossipova, E.; Lengqvist, J.; Svensson, C.I.; Kosek, E.; Kadetoff, D.; Jakobsson, P.J.; Kultima, K.; Lampa, J. The human CSF pain proteome. J. Proteom. 2019, 190, 67–76.
- Kroksveen, A.C.; Guldbrandsen, A.; Vedeler, C.; Myhr, K.M.; Opsahl, J.A.; Berven, F.S. Cerebrospinal fluid proteome comparison between multiple sclerosis patients and controls. Acta Neurol. Scand. 2012, 126, 90–96.
- Li, X.; Miyajima, M.; Mineki, R.; Taka, H.; Murayama, K.; Arai, H. Analysis of potential diagnostic biomarkers in cerebrospinal fluid of idiopathic normal pressure hydrocephalus by proteomics. Acta Neurochir. 2006, 148, 859–864.
- Gupta, A.K.; Pokhriyal, R.; Khan, M.I.; Kumar, D.R.; Gupta, R.; Chadda, R.K.; Ramachandran, R.; Goyal, V.; Tripathi, M.; Hariprasad, G. Cerebrospinal fluid proteomics for identification of α2-macroglobulin as a potential biomarker to monitor pharmacological therapeutic efficacy in dopamine dictated disease states of Parkinson’s disease and schizophrenia. Neuropsychiatr. Dis. Treat. 2019, 15, 2853–2867.
- Dong, M.; Lu, L.; Zhang, L.; Zhang, Q.; Ungvari, G.S.; Ng, C.H.; Yuan, Z.; Xiang, Y.; Wang, G.; Xiang, Y.T. Prevalence of suicide attempts in bipolar disorder: A systematic review and meta-analysis of observational studies. Epidemiol. Psychiatr. Sci. 2019, 29, e63.
- McIntyre, R.S.; Alda, M.; Baldessarini, R.J.; Bauer, M.; Berk, M.; Correll, C.U.; Fagiolini, A.; Fountoulakis, K.; Frye, M.A.; Grunze, H.; et al. The clinical characterization of the adult patient with bipolar disorder aimed at personalization of management. World Psychiatry 2022, 21, 364–387.
- Magioncalda, P.; Martino, M. A unified model of the pathophysiology of bipolar disorder. Mol. Psychiatry 2022, 27, 202–211.
- Dean, B.; Digney, A.; Sundram, S.; Thomas, E.; Scarr, E. Plasma apolipoprotein E is decreased in schizophrenia spectrum and bipolar disorder. Psychiatry Res. 2008, 158, 75–78.
- Knöchel, C.; Kniep, J.; Cooper, J.D.; Stäblein, M.; Wenzler, S.; Sarlon, J.; Prvulovic, D.; Linden, D.E.J.; Bahn, S.; Stocki, P.; et al. Altered apolipoprotein C expression in association with cognition impairments and hippocampus volume in schizophrenia and bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 199–212.
- Smirnova, L.; Seregin, A.; Boksha, I.; Dmitrieva, E.; Simutkin, G.; Kornetova, E.; Savushkina, O.; Letova, A.; Bokhan, N.; Ivanova, S.; et al. The difference in serum proteomes in schizophrenia and bipolar disorder. BMC Genom. 2019, 20, 535.
- Monroe, S.M.; Harkness, K.L. Major Depression and Its Recurrences: Life Course Matters. Annu. Rev. Clin. Psychol. 2022, 18, 329–357.
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140.
- Dadkhah, M.; Jafarzadehgharehziaaddin, M.; Molaei, S.; Akbari, M.; Gholizadeh, N.; Fathi, F. Major depressive disorder: Biomarkers and biosensors. Clin. Chim. Acta 2023, 547, 117437.
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065.
- Xu, H.B.; Zhang, R.F.; Luo, D.; Zhou, Y.; Wang, Y.; Fang, L.; Li, W.J.; Mu, J.; Zhang, L.; Zhang, Y.; et al. Comparative proteomic analysis of plasma from major depressive patients: Identification of proteins associated with lipid metabolism and immunoregulation. Int. J. Neuropsychopharmacol. 2012, 15, 1413–1425.
- Stelzhammer, V.; Haenisch, F.; Chan, M.K.; Cooper, J.D.; Steiner, J.; Steeb, H.; Martins-de-Souza, D.; Rahmoune, H.; Guest, P.C.; Bahn, S. Proteomic changes in serum of first onset, antidepressant drug-naïve major depression patients. Int. J. Neuropsychopharmacol. 2014, 17, 1599–1608.
- Lee, M.Y.; Kim, E.Y.; Kim, S.H.; Cho, K.C.; Ha, K.; Kim, K.P.; Ahn, Y.M. Discovery of serum protein biomarkers in drug-free patients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 69, 60–68.
- Najjar, S.; Pearlman, D.M.; Devinsky, O.; Najjar, A.; Zagzag, D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: A review of clinical and experimental evidence. J. Neuroinflammation 2013, 10, 142.
- Pan, S.J.; Tan, Y.L.; Yao, S.W.; Xin, Y.; Yang, X.; Liu, J.; Xiong, J. Fluoxetine induces lipid metabolism abnormalities by acting on the liver in patients and mice with depression. Acta Pharmacol. Sin. 2018, 39, 1463–1472.
- Benton, C.S.; Miller, B.H.; Skwerer, S.; Suzuki, O.; Schultz, L.E.; Cameron, M.D.; Marron, J.S.; Pletcher, M.T.; Wiltshire, T. Evaluating genetic markers and neurobiochemical analytes for fluoxetine response using a panel of mouse inbred strains. Psychopharmacology 2012, 221, 297–315.
- Lee, H.; Rhee, S.J.; Kim, J.; Lee, Y.; Kim, H.; Lee, J.; Lee, K.; Shin, H.; Kim, H.; Lee, T.Y.; et al. Predictive protein markers for depression severity in mood disorders: A preliminary trans-diagnostic approach study. J. Psychiatr. Res. 2021, 142, 63–72.
- Atmaca, M.; Kuloglu, M.; Tezcan, E.; Ustundag, B. Serum leptin and cholesterol values in violent and non-violent suicide attempters. Psychiatry Res. 2008, 158, 87–91.
- Olié, E.; Picot, M.C.; Guillaume, S.; Abbar, M.; Courtet, P. Measurement of total serum cholesterol in the evaluation of suicidal risk. J. Affect. Disord. 2011, 133, 234–238.
- Lalovic, A.; Levy, E.; Luheshi, G.; Canetti, L.; Grenier, E.; Sequeira, A.; Turecki, G. Cholesterol content in brains of suicide completers. Int. J. Neuropsychopharmacol. 2007, 10, 159–166.
- Freemantle, E.; Mechawar, N.; Turecki, G. Cholesterol and phospholipids in frontal cortex and synaptosomes of suicide completers: Relationship with endosomal lipid trafficking genes. J. Psychiatr. Res. 2013, 47, 272–279.
This entry is offline, you can click here to edit this entry!
