The COVID-19 pandemic has affected the world unprecedentedly, with both positive and negative impacts. COVID-19 significantly impacted the immune system, and understanding the immunological consequences of COVID-19 is essential for developing effective treatment strategies. The immunological aspects of long COVID-19 is a phenomenon where individuals continue to experience a range of symptoms and complications, even after the acute phase of COVID-19 infection has subsided. The immune system responds to the initial infection by producing various immune cells and molecules, including antibodies, T cells, and cytokines. However, in some patients, this immune response becomes dysregulated, leading to chronic inflammation and persistent symptoms.
- COVID-19
- long COVID-19
- immune responses
- autoimmunity
1. Introduction
2. Long COVID-19 and Immune Pathology
2.1. Definition and Prevalence of Long COVID-19
2.2. Overview of the Immune Response to COVID-19 and How It Relates to Long COVID-19
2.3. Mechanism behind Post-COVID-19 Immune Pathology
2.4. Impact of Post-COVID-19 Immune Pathology on Patient Outcomes
3. Autoimmunity in COVID-19 Patients
4. Autoimmunity in Post-COVID-19 Patients
Mechanisms behind the Development of Autoimmunity in Post-COVID-19 Patients
5. Treatments for Long COVID-19
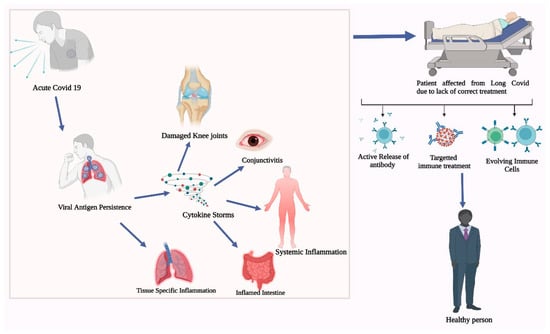
6. Promising New Treatments, Immune Therapies, and Their Limitations
7. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/life13112121
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current state of the science. Immunity 2020, 52, 910–941.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of Coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel Coronavirus from patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733.
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; Tumani, H.; Otto, M. Guillain-Barré syndrome spectrum associated with COVID-19: An up-to-date systematic review of 73 cases. J. Neurol. 2021, 268, 1133–1170.
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020, 383, 334–346.
- Gnanaraj, J.; Parnes, A.; Francis, C.W.; Go, R.S.; Takemoto, C.M.; Hashmi, S.K. Approach to pancytopenia: Diagnostic algorithm for clinical hematologists. Blood Rev. 2018, 32, 361–367.
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144.
- Lopez-Leon, S.; Wegman-Ostrosky, O.B.; Nissen, J.; Cantwell, L.; Schwinn, M.; Tulstrup, M.; Westergaard, D.; Ullum, H.; Brunak, S.; Tommerup, N.; et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 2021, 11, 13153.
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758.
- Paces, J.; Strizova, Z.; Smrz, D.; Cerny, J. COVID-19 and the immune system. Physiol. Res. 2020, 69, 379–388.
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2021, 268, 113426.
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631.
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211.
- The Lancet. Facing up to long COVID. Lancet 2020, 396, 1861.
- Zhang, D.; Guo, R.; Lei, L.; Liu, H.; Wang, Y.; Wang, Y.; Qian, H.; Dai, T.; Zhang, T.; Lai, Y.; et al. Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2021, 109, 13–22.
- Alwan, N.A. The road to addressing Long Covid. Science 2021, 373, 491–493.
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 Coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e15.
- Elgellaie, A.; Thomas, S.J.; Kaelle, J.; Bartschi, J.; Larkin, T. Pro-inflammatory cytokines IL-1α, IL-6 and TNF-α in major depressive disorder: Sex-specific associations with psychological symptoms. Eur. J. Neurosci. 2023, 57, 1913–1928.
- Gallais, F.; Velay, A.; Nazon, C.; Wendling, M.J.; Partisani, M.; Sibilia, J.; Candon, S.; Fafi-Kremer, S. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg. Infect Dis. 2021, 27, 113–121.
- Xie, J.; Ding, C.; Li, J.; Wang, Y.; Guo, H.; Lu, Z.; Wang, J.; Zheng, C.; Jin, T.; Gao, Y.; et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J. Med. Virol. 2020, 92, 2004–2010.
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435.
- Wiech, M.; Chroscicki, P.; Swatler, J.; Stepnik, D.; De Biasi, S.; Hampel, M.; Brewinska-Olchowik, M.; Maliszewska, A.; Sklinda, K.; Durlik, M.; et al. Remodeling of T cell dynamics during Long COVID is dependent on severity of SARS-CoV-2 infection. Front. Immunol. 2022, 13, 886431.
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288.
- Zimmermann, P.; Pittet, L.F.; Curtis, N. How common is Long COVID in children and adolescents? Pediatr. Infect Dis. J. 2021, 40, e482–e487.
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2 specific immune memory persists after mild COVID-19. Cell 2021, 184, 169–183.e17.
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950.
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427.
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect Dis. 2021, 53, 737–754.
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94.
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N. Engl. J. Med. 2021, 385, 320–329.
- Asadi-Pooya, A.A.; Nemati, H.; Shahisavandi, M.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; et al. Long COVID in children and adolescents. World J. Pediatr. 2021, 17, 495–499.
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062.
- Rahmati, M.; Yon, D.K.; Lee, S.W.; Udeh, R.; McEVoy, M.; Kim, M.S.; Gyasi, R.M.; Oh, H.; López Sánchez, G.F.; Jacob, L.; et al. New-onset type 1 diabetes in children and adolescents as postacute sequelae of SARS-CoV-2 infection: A systematic review and meta-analysis of cohort studies. J. Med. Virol. 2023, 95, e28833.
- Fernández-de-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining post-COVID symptoms (Post-acute COVID, long COVID, persistent post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621.
- Rathore, S.; Dahiya, P. Drug repurposing using computational tools and preventive strategies for COVID-19 and future pandemics. J. Health Manag. 2023, 24, 43–52.
- Wu, L.P.; Wang, N.C.; Chang, Y.H.; Tian, X.Y.; Na, D.Y.; Zhang, L.Y.; Zheng, L.; Lan, T.; Wang, L.F.; Liang, G.D. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect Dis. 2007, 13, 1562–1564.
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M.; et al. COVID-19: A multidisciplinary review. Front. Public Health 2020, 8, 383.
- Chow, K.W.; Pham, N.V.; Ibrahim, B.M.; Hong, K.; Saab, S. Autoimmune Hepatitis-like syndrome following COVID-19 vaccination: A systematic review of the literature. Dig. Dis. Sci. 2022, 67, 4574–4580.
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999.
- Vuille-Lessard, É.; Montani, M.; Bosch, J.; Semmo, N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J. Autoimmun. 2021, 123, 102710.
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; Hanauer, D.A.; James, J.A.; Kanthi, Y.; Ladd, V.; et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021, 131, e154886.
- Fagyas, M.; Nagy, B., Jr.; Ráduly, A.P.; Mányiné, I.S.; Mártha, L.; Erdősi, G.; Sipka, S., Jr.; Enyedi, E.; Szabó, A.Á.; Pólik, Z.; et al. The majority of severe COVID-19 patients develop anti-cardiac autoantibodies. Geroscience 2022, 44, 2347–2360.
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643.
- Mobasheri, L.; Nasirpour, M.H.; Masoumi, E.; Azarnaminy, A.F.; Jafari, M.; Esmaeili, S.A. SARS-CoV-2 triggering autoimmune diseases. Cytokine 2022, 154, 155873.
- Vahabi, M.; Ghazanfari, T.; Sepehrnia, S. Molecular mimicry, hyperactive immune system, and SARS-CoV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. Int. Immunopharmacol. 2022, 112, 109183.
- Peng, M.Y.; Liu, W.C.; Zheng, J.Q.; Lu, C.L.; Hou, Y.C.; Zheng, C.M.; Song, J.Y.; Lu, K.C.; Chao, Y.C. Immunological aspects of SARS-CoV-2 infection and the putative beneficial role of Vitamin-D. Int. J. Mol. Sci. 2021, 22, 5251.
- Zhou, Y.; Han, T.; Chen, J.; Hou, C.; Hua, L.; He, S.; Guo, Y.; Zhang, S.; Wang, Y.; Yuan, J.; et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin. Transl. Sci. 2020, 13, 1077–1086.
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection. JAMA Netw. Open. 2021, 4, e2128568.
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64.
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627.
- Hampshire, A.; Trender, W.; Chamberlain, S.R. Cognitive deficits in people who have recovered from COVID-19 relative to controls: An N=84,285 online study. medRxiv 2021.
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959.
- Negrini, F.; Ferrario, I.; Mazziotti, D. Neuromuscular and balance symptoms as late effects after COVID-19: A case series. Eur. J. Phys. Rehabil. Med. 2021, 57, 306–309.
- Lee, M.J.; Sayers, A.E.; Drake, T.M.; Singh, P.; Bradburn, M.; Wilson, T.R.; Murugananthan, A.; Walsh, C.J.; Fearnhead, N.S.; NASBO Steering Group; et al. Malnutrition, nutritional interventions and clinical outcomes of patients with acute small bowel obstruction: Results from a national, multicentre, prospective audit. BMJ Open 2019, 9, 029235.
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning. immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181.
- World Health Organization. Water, Sanitation, Hygiene, and Waste Management for SARS-CoV-2, the Virus that Causes COVID-19. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-WASH-2020.4 (accessed on 29 July 2020).
- Shah, K.; Saxena, D.; Mavalankar, D. Secondary impact of COVID-19 on nutrition transition and food security in India. Front. Public Health 2020, 8, 89699.
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135.
- Chauvet-Gélinier, J.C.; Novella, J.L.; Jolly, D. Impact of major depression on chronic medical illness. Encephale 2010, 36, D59–D70.
- Alsubaie, M.M.; Abbott, R.; Dunn, B.; Dickens, C.; Keil, T.F.; Henley, W.; Kuyken, W. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: A systematic review. Clin. Psychol. Rev. 2017, 55, 74–91.
- Dennis, C.L. Peer support within a health care context: A concept analysis. Int. J. Nurs. Stud. 2003, 40, 321–332.
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605.
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179.
- Gentry, C.A.; Nguyen, P.; Thind, S.K.; Kurdgelashvili, G.; Williams, R.J. Characteristics and outcomes of US Veterans at least 65 years of age at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents. J. Infect. 2023, 86, 248–255.
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 229–237.
- Gottlieb, A.B.; Armstrong, A.W.; FitzGerald, O.; Gladman, D.D. Prologue: Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) 2022 Annual Meeting. J. Rheumatol. 2023, jrheum.2023-0496.
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 2021, 325, 632–644.
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect Dis. 2021, 102, 501–508.
- Wada, H.; Shiraki, K.; Shimpo, H.; Shimaoka, M.; Iba, T.; Suzuki-Inoue, K. Thrombotic mechanism involving platelet activation, hypercoagulability and hypofibrinolysis in coronavirus disease 2019. Int. J. Mol. Sci. 2023, 24, 7975.
- Bhargava, A.; Dahiya, P. COVID-19 pandemic: Assessment of current strategies and socio-economic impact. J. Health Manag. 2020, 24, 466–477.
- Piechotta, V.; Chai, K.L.; Valk, S.J.; Kimber, C.; Monsef, I.; Doree, C.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; McQuilten, Z.; et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database Syst Rev. 2020, 7, CD013600.
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228.
- Shang, L.; Zhao, J.; Hu, Y.; Du, R.; Cao, B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020, 395, 683–684.
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944, Erratum in Lancet 2023, 401, 644.
- Zhou, Q.; Chen, V.; Shannon, C.P.; Wei, X.S.; Xiang, X.; Wang, X.; Wang, Z.H.; Tebbutt, S.J.; Kollmann, T.R.; Fish, E.N. Interferon-α2b Treatment for COVID-19. Front. Immunol. 2020, 11, 1061.
- Tripathi, D.; Sodani, M.; Gupta, P.K.; Kulkarni, S. Host directed therapies: COVID-19 and beyond. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100058.
