Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Cardiovascular diseases (CVDs) are among the leading causes of morbidity and mortality worldwide. Unhealthy dietary habits have clearly been shown to contribute to the development of CVDs. Beyond the primary nutrients, a healthy diet is also rich in plant-derived compounds. Natural polyphenols, found in fruits, vegetables, and red wine, have a clear role in improving cardiovascular health.
- :polyphenols
- resveratrol
- oxidative stress
- inflammation
- cardiovascular diseases
1. Introduction
A variety of risk factors, including hypertension, dyslipidemia, diabetes mellitus, obesity, smoking, alcohol consumption, sedentary lifestyle, and unhealthy diet have been shown to contribute to the development and progression of CVDs [3]. These promote endothelial cell dysfunction, oxidative stress, smooth muscle cell, and fibroblast proliferation, and the transition of macrophages to foam cells. The resultant chronic inflammation ultimately leads to atherosclerotic plaque development across the vascular bed [4]. In addition, the complex interaction between chronic inflammation, oxidative stress, lipid metabolism, and endothelial dysfunction plays a crucial role in the development of HF [4,5,6].
Lifestyle modifications, such as improving dietary habits, are traditionally considered primary determinants of cardiovascular (CV) health. The potential benefits of dietary interventions and nutrient supplementation in improving CV outcomes have been investigated extensively over the past decades. The Mediterranean diet represents a special historical nutrition pattern consumed by populations living in the Mediterranean Basin. As demonstrated by several clinical trials in recent years, prolonged and strict adherence to this diet may reduce the incidence of CVDs by improving the CV risk profile, primarily due to its anti-inflammatory and antioxidant effects [7,8]. There is evidence that modern healthy dietary habits share many similarities with the Mediterranean diet, including the high intake of vegetables, fruits, legumes, seeds, nuts, whole grains, dairy foods, fish, seafood, and vegetable oils (e.g., olive oil) combined with the limited consumption of sweets, soft drinks, and red meat [9,10,11]. While heavy alcohol use can be detrimental, the regular but modest consumption of red wine, which is part of the Mediterranean diet, has well-documented health benefits [12]. As published by Renaud et al. in the early 1990s, the French population has a lower overall incidence and mortality from CVDs, despite a diet relatively rich in saturated fat compared to other developed countries. This observation, also known as the “French paradox”, may be attributable to the higher red wine consumption per capita in France [13].
In addition to the minerals and vitamins found in healthy diets, the role of plant-derived ingredients is also important to mention, such as natural polyphenols. These bioactive phytochemicals can be found in a wide variety of plants (e.g., fruits, vegetables, seeds, nuts), and are also present in wine, tea, spices, oils, and chocolate. They act as natural UV filters and protect from a variety of biotic (e.g., infection) and abiotic stresses (e.g., oxidative, or toxic damages, injury) owing to their antioxidant/anti-inflammatory activities [14,15,16,17,18,19]. Polyphenols also contribute to the flavor, taste, and color of plants. Their dietary intake has seen an increase in recent years with the concomitant decrease in the risk of chronic and age-related disorders, such as cancer, neurodegenerative conditions, inflammation, and CV diseases. Especially in recent years, most consumers prefer using natural ingredients as additives because of their broad availability and safety profile. Grape seed and olive oil extracts rich in polyphenols may be used as food additives [20,21].
2. Classification, Biosynthesis, and Metabolism of Polyphenols
2.1. Classification and Biosynthesis of Polyphenols
The classification of polyphenols is based on the number of phenolic rings and the structural elements attached to the rings. The majority are derived through the secondary metabolism of L-phenylalanine via the phenylpropanoid pathway. Alternatively, they can be synthesized through the shikimate and/or polyketide pathways, therefore approximately 10,000 structural variants may exist [14,29,30]. The bioactive polyphenols are divided into four major subclasses: (1) flavonoids; (2) non-flavonoid stilbenes; (3) phenolic acids; and (4) lignans. The main sources of relevant polyphenols are depicted in Figure 1 [31,32].
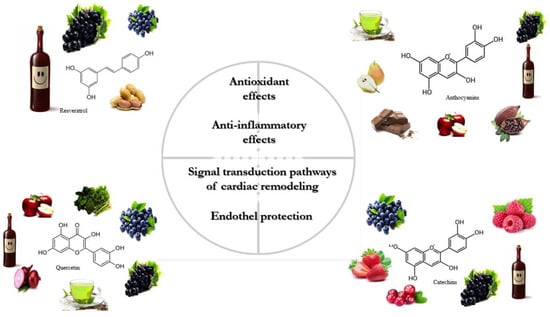
Figure 1. The primary sources and physiological effects of relevant polyphenols.
Stilbenes, including the most widely investigated resveratrol (RES), are produced via the general phenylpropanoid pathway. They contain two phenyl residues linked by a two-carbon methylene bridge (1,2-diphenylethylene nucleus). This basic structure can subsequently be glycosylated, methylated, or prenylated by specific enzymes. In addition to RES, other stilbenes include piceatannol, pinosylvin, pinostilbene, combretastatin, polydatin, mulberroside, and various oligostilbenes [33,34]. Overall, a relatively small fraction of habitual diets contain these compounds when compared to flavonoids and phenolic acids (peanuts, berries, grapes, and red wine) [35].
Flavonoids are composed of two aromatic rings and a heterocyclic ring with a typical C6-C3-C6 skeleton structure. They are generally classified into 7 subgroups based on the degree of oxidation, hydroxylation, glycosylation, and substitutions of the central heterocyclic ring: (1) flavonols; (2) flavones; (3) isoflavones; (4) anthocyanidins; (5) flavanones; (6) flavanols; (7) chalcones. Approximately two-third of the polyphenolic compounds are flavonoids and are ubiquitous to most plants [36,37,38].
Phenolic or phenolcarboxylic acids produced by the phenylpropanoid pathway contain aromatic phenolic rings and one carboxylic acid group in their structure. They are among the main groups of polyphenols and are present in one-third of plants. They are divided into two subclasses: (1) hydroxybenzoic acid (e.g., gallic acid); and (2) hydroxycinnamic acid (e.g., caffeic acid, ferulic acid). Their aromatic phenolic rings have the ability to lose an electron, which explains their high antioxidant activity [39,40,41].
Lignans are biphenolic compounds derived from the combination of two phenylpropanoid C6–C3 units synthetized through the shikimate pathway. The ether, lactone, or carbon bonds linked to the biphenolic ring are responsible for the biological activity of these moieties. The primary compounds that are also the most studied include secoisolariciresinol, matairesinol, and pinoresinol [42,43].
2.2. Metabolism of Polyphenols
The bioavailability of polyphenols is poor in general. They undergo rapid and extensive metabolism in the gastrointestinal tract and liver that alters their molecular structure. These changes are naturally responsible for the low plasma levels of intact (unmetabolized) forms [44,45] that, according to the literature, rarely exceed 1 µM after consuming 10 to 100 mg of a polyphenolic compound [46]. The main mechanisms of action of stilbenes are shown in detail in Figure 2.
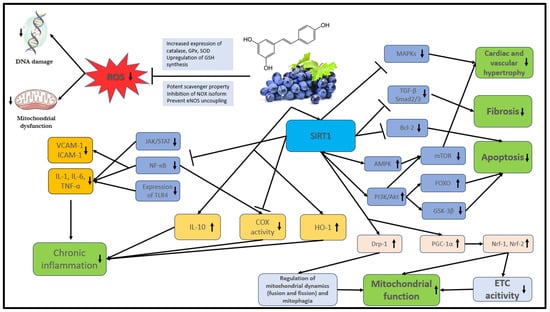
Figure 2. The main mechanisms of action of stilbenes. Akt: protein kinase B; AMPK: AMP-activated protein kinase; COX: cyclooxygenase; Bcl-2: B-cell lymphoma 2, DNA: deoxyribonucleic acid; Drp1: dynamin-related protein 1; ETC: electron transport chain; FOXO: Forkhead box O; GPx: glutathione peroxidase; GSH: glutathione; GSK-3β: glycogen synthase kinase-3 beta; eNOS: endothelial nitric oxide synthase; HO-1: heme oxygenase-1; ICAM: intracellular cell adhesion molecule; IL: interleukin; JAK: Janus kinase; MAPK: mitogen-activated protein kinase; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor kappa B; NOX: NADPH oxidases; Nrf: nuclear respiratory factor; PI3K: phosphatidylinositol 3-kinase; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha; ROS: reactive oxygen species; SIRT1: NAD-dependent deacetylase sirtuin-1; SOD: superoxide dismutase; STAT: signal transducer and activator of transcription proteins; TGF-β: transforming growth factor; TLR4: toll-like receptor 4; TNF: tumor necrosis factor; VCAM: vascular cell adhesion molecule.
Polyphenols are ingested primarily in a conjugated form and are absorbed from the stomach, intestine, and colon with the rest eliminated through feces. The metabolism of glycosylated polyphenols (O-glycosides) is initiated by oral glycosidase enzymes, which is followed by conversion into aglycone form (free form) by hydroxylation in the stomach and small intestine. This process is facilitated by intestinal microbiota (phase I metabolism) [47,48]. Aglycone polyphenols are absorbed from the lumen of the small intestine into the cytosol of enterocytes by passive diffusion or by utilizing protein carriers. However, selected polyphenols (such as hydroxycinnamic acids) are resistant to enzymatic digestion and transition unchanged into the colon, where microbiota metabolize them into aglycone form. Following absorption, aglycone polyphenols are transported to the liver via the portal vein, where they are conjugated to O-sulphate or O-glucuronide forms (phase II metabolism).
3. Stilbenes and CVDs
3.1. Mechanism of Action
Stilbenes, and particularly RES, have been extensively investigated over the past decades owing to their potent antioxidant, anti-inflammatory, anti-proliferative, anti-apoptotic and mitochondrial protective effects. Collectively, these are thought to be responsible for their derived CV benefits.
Reactive oxygen (ROS) and nitrogen species (RNS) play an important role in physiological processes (cell homeostasis, signaling pathways) when present at low concentrations. However, high ROS levels promote oxidative stress within the cells and may lead to permanent damage of critical biomolecules (lipids, proteins, DNA) [65]. Although the bioavailability and plasma concentration of stilbenes are low, several in vitro studies have demonstrated that they are important antioxidant agents. Their efficacy is explained by two separate mechanisms: (1) direct accumulation in cardiac and vascular tissues; and (2) active metabolite formation [66,67]. RES has been shown to directly scavenge excessive ROS and RNS, including superoxide (O2·−), hydroxyl radical (·OH), hydrogen-peroxide (H2O2), and peroxynitrite [68,69,70].
ES was proven to attenuate ROS formation by inhibiting a range of NADPH oxidases (NOX isoforms) and by modulating the activity of the mitochondrial respiratory chain enzymes [77,78,79]. It reduces NOX isoform activity through the upregulation of SIRT1 (NAD+-dependent histone/protein deacetylase sirtuin 1) that, in turn: (1) prompts deacetylation of transcription factor NF (nuclear factor)-κB [80]; (2) upregulates the Forkhead box O1 (FOXO1) transcription factor-mediated synthesis of superoxide dismutase (SOD), as well as the expression of catalase (CAT) and glutathione peroxidase 1 (GPx1) [81,82,83]. Dysfunction of the endothelial nitric oxide synthase (eNOS) due to tetrahydrobiopterin (BH4) deactivation (uncoupling of eNOS) can also contribute to increased ROS production and consequent oxidative damage. RES can reverse eNOS uncoupling by upregulating GTP cyclohydrolase 1 (GCH1) expression, therefore increasing BH4 biosynthesis [84,85].
The effective inhibition of inflammatory processes is a key function of stilbenes [88,89]. NF-κB and STAT (signal transducers and activators of transcription) transcription factors play an important role in the regulation of physiological and inflammatory processes as well as apoptosis in macrophages. The activation of these factors is mediated by TLRs (toll-like receptors—transmembrane proteins of macrophages) and by their downstream signaling pathways, which lead to increased expression of pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α [90,91]. As a potent anti-inflammatory agent, RES can inhibit the expression of pro-inflammatory cytokines [92,93] by suppressing NF-кB and JAK/STAT through the activation of SIRT1 [94,95].
The expression of adhesion molecules, such as VCAM-1, ICAM-1, and E-selectin, are important steps in leukocyte activation. According to Zhang et al., RES can successfully inhibit adhesion molecules by suppressing the TNF-α-induced NF-κB activation [101].
COX-1 and COX-2 isoforms of the cyclooxygenase enzyme are key catalysts of prostaglandin (PG) biosynthesis and they play an important role in inflammatory processes. Vascular endothelial and smooth muscle cells serve as the major source of PG production. Arachidonic acid is released by endothelial cell membrane phospholipids with the cleavage of phospholipase A2 (PLA2) and then metabolized by COX enzymes to generate PGs (such as PGD2, PGE2, PGI2) and thromboxane (TX) A2. Proinflammatory cytokines (IL-1, TNF-α) play a crucial role in the activation of PG biosynthesis in states of chronic inflammation by upregulation of the NF-κB and MAPK signaling pathways. The ability of RES to inhibit the COX-1 and COX-2 enzymes can contribute to its marked anti-inflammatory (COX-2) and antiplatelet effects (COX-1) [106,107]. RES can reduce COX-2 expression by the downregulation of p65, c-Jun, Fos, and NF-κB transcription factors via SIRT1 activation, thus decreasing eicosanoid production (PGE2, TXA2) and consequently reducing inflammation. Moreover, SIRT1 can also inhibit (acetylation) the AP-1 (activated by MAPKs) transcription factors that are essential for COX-2 gene expression and for PGE2 release in inflammation as well as for endothelial proliferation [108].
Stilbenes, especially RES, affect different signal transduction pathways that have the ability to modify a variety of cellular functions, including cardiac remodeling, cell survival, apoptosis, maladaptive hypertrophy, fibrosis, and mitochondrial function. It can also regulate the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pro-survival pathway by activating SIRT1. Enhanced Akt activation can inhibit ROS-mediated apoptosis and improve cardiomyocyte survival through upregulation of FOXO transcription factors, inhibition of the Bcl-2 transcription factor family as well as glycogen synthase kinase-3β (GSK-3β) [109,110]. In addition, RES suppresses the ROS-mediated activation of MAPKs, which are important factors in controlling several cellular processes such as proliferation and apoptosis under both normal and pathological conditions. Overexpression of MAPKs induced by ROS has been shown to be involved in pathological cardiac hypertrophy and remodeling [111]. RES can stimulate the production of MAPK phosphatase-1 (MKP-1), which is the major negative regulator of MAPK activity [112,113]. However, it can inhibit cardiac remodeling through other pathways as well. RES can reduce Ang II-induced cardiac hypertrophy by inhibiting adverse NF-κB signaling activation [114].
Endothelial cells regulate vascular tone, vascular resistance, blood pressure, and hemostasis through the release of vasoactive substances, such as PGs, NO, and vasoconstrictive endothelin-1 (ET-1). Therefore, they play an essential and dynamic role in regulating the CV system. [122,123]. Normal vascular homeostasis can be deranged by various noxious insults and under different pathologic conditions, such as chronic inflammation and oxidative stress. The abnormal upregulation of ET-1 and the reduction in NO levels were shown to be associated with atherosclerosis development and CVDs. Besides their anti-inflammatory and antioxidant properties, stilbenes can modulate the activity of several signaling pathways and improve endothelial function. The beneficial effects of stilbenes on endothelial function and vascular wall tension have been previously linked to increased NO production and decreased ET-1 synthesis. RES enhances NO synthesis in endothelial cells by upregulating eNOS expression and preventing eNOS uncoupling through multiple mechanisms, such as SIRT1 activation [124,125,126].
3.2. Pre-Clinical and Human Clinical Trials with RES Related to CVDs
3.2.1. Pre-Clinical Trials
Vascular Function, Hypertension
Based on several pre-clinical studies, RES has a potent vasorelaxant and thus antihypertensive effect. Multiple mechanisms contribute to this favorable property, including SIRT1 activation, AMPK phosphorylation, increased eNOS activity, and reduced ROS production [185,186,187,188]. Per Dolinsky et al., 320 mg/kg daily RES intake for 5 weeks significantly reduced the blood pressure in spontaneously hypertensive rats (SHRs) as well as mice receiving angiotensin II (AT-II) infusion. Improved vascular relaxation by enhanced eNOS activity and reduced oxidative stress contributed to this finding [189].
Cardiac Function, Remodeling
It is well established that oxidative damage and chronic inflammation stimulate specific intracellular signal transduction pathways resulting in adverse cardiac remodeling characterized by fibrosis, cardiomyocyte hypertrophy, apoptosis, and necrosis [199]. Given its potent antioxidant and anti-inflammatory properties, RES was extensively evaluated in different murine models of cardiac remodeling and HF. Gu et al. studied the efficacy of RES at a dose of 2.5 mg/kg/day administered for 16 weeks in a mouse model of HF caused by myocardial infarction (surgical left coronary artery ligation). The results showed improved cardiac function and survival in the group receiving RES, potentially due to SIRT1-mediated upregulation of the AMPK signaling pathway [200]. These results are in agreement with another post-infarction, pre-clinical trial performed by Ahmed et al. [201]. It this study, long-term (10-month) RES treatment at a daily dose of 5 mg/kg significantly improved left ventricular (LV) systolic function in rats [201]. Similar results were published by Kanamori and colleagues [202].
3.2.2. Human Clinical Trials
Hypertension
The success of RES in pre-clinical studies prompted a range of human clinical trials involving populations with various diseases. Theodotou et al. investigated the efficacy of RES supplementation in patients with essential hypertension, adding it on top of standard therapy. A total of 97 patients were enrolled, divided into two groups, and followed for 2 years: (1) RES (50 mg/day) + standard treatment; (2) standard of care alone. The long-term administration of RES led to a reduction in blood pressure when initiated on top of standard medical therapy [145]. Conversely, Walker et al. were unable to detect a significant biological effect of RES given at 1 g/day for 35 days on blood pressure in patients with metabolic syndrome [146].
Vascular Protection
Although the vascular protective effects of RES are well-established in pre-clinical experiments, strong human evidence is lacking. In a pilot trial by Wong et al., RES given at 270 mg/day for 4 weeks led to a significant improvement in endothelial function as measured by flow-mediated dilatation (FMD) of the brachial artery in overweight and hypertensive individuals [147]. Similarly, in a study published by Fujitaka et al., RES (100 mg/day for 3 months) improved endothelial function as measured by FMD in 34 patients with metabolic syndrome [228]. However, no significant change was noted in blood pressure [228].
Heart Failure
In contrast to the high number of pre-clinical studies with RES in various HF models, merely a few human clinical trials have been performed to date in populations with HF. In a double-blind, placebo-controlled, randomized clinical trial, Magyar et al. investigated the efficacy of RES intake in post-MI patients with Stage B HF and preserved ejection fraction (baseline EF: 54.77 ± 1.64% in the treated group) [27]. Not unexpectedly, 10 mg/day RES for 3 months did not significantly impact the ejection fraction. However, it significantly improved the diastolic function parameters compared to placebo [27].
4. Effect of Relevant Flavonoid Compounds on CVDs
4.1. Mechanism of Action
Flavonoids have several, extensively investigated favorable biochemical effects such as anti-inflammatory, anti-aging, and anti-cancer properties. However, the primary biological activity of these compounds is their profound antioxidant capacity exerted via multiple mechanisms: (1) elimination of ROS; (2) prevention of ROS and RNS production; (3) activation of the antioxidant systems [233,234]. Flavonoids exhibit a marked scavenger activity. The functional hydroxyl group attached to the central ring can donate an electron to peroxynitrite, hydroxyl, and peroxyl radicals through resonance, stabilize these, and create a relatively stable flavonoid radical [235,236,237]. Furthermore, certain flavonoids can directly inhibit the activity of ROS-generating enzymes such as xanthine oxidase, NOS, and NADPH oxidases (NOX isoforms) [238,239,240]. They can also increase the activity of the antioxidant defense system through functional upregulation of selected enzymes such as NADPH-quinone oxidoreductase, glutathione S-transferase, and UDP-glucuronyl transferase [241,242].
Several trials reported that flavonoids have potent anti-inflammatory properties. They inhibit prostaglandin synthesis, thereby decreasing leukocyte infiltration and edema development [246]. Some block arachidonic acid release through the inhibition of phospholipase A2 (PLA2) as well as phospholipase C1 (PLC1). Moreover, Qct can block the synthesis of prostaglandins, leukotrienes, and thromboxanes by inhibiting the cyclooxygenase (COX) and lipoxygenase enzymes [247,248,249]. Many flavonoids (e.g., catechin, Qct) can decrease pro-inflammatory cytokine expression, such as IL-6, IL-8, TNF-α, and IL-1β, in macrophages and T-cells while enhancing the production of anti-inflammatory cytokines (e.g., IL-10) [247,250,251].
Flavonoids have the ability to modulate numerous intracellular signaling factors and signal transduction pathways, such as NF-κB, AP-1, Nrf2, MAPKs, ERK, PI3K/Akt, to reduce oxidative stress and inflammation, thereby acting as cardioprotective agents [253,254,255,256]. NF-κB regulates the expression of several inflammation-associated genes. Inhibition of this central transcription factor can therefore be of significant benefit. Flavonoids inhibit the nuclear translocation of p50 and p65 subunits of NF-κB in macrophages thus decreasing the expression of pro-inflammatory cytokines, adhesion molecules, NOS, and COX-2 [257,258]. In addition, the flavonoid-induced blockade of IκB-α protein phosphorylation can lead to NF-κB pathway inactivation, thereby suppressing TNF-α-induced apoptosis and inflammation [259,260].
4.2. Pre-Clinical and Human Clinical Trials with Selected Flavonoids Related to CVDs
4.2.1. Quercetin
One of the most widely studied flavonoids is quercetin (Qct) and its derivatives (Qct-glycosides, Qct-ethers, Qct-prenyls) found in apple, blueberry, onion, pepper, broccoli, and tea. They have potent anti-inflammatory and antioxidant effects that are primarily responsible for their positive CV effect.
re-Clinical Trials
-
Cardiac function, remodeling
The cardioprotective ability of Qct has been broadly examined using in vivo experimental models. Arumugam et al. demonstrated that Qct dosed at 10 mg/kg can protect against the progression of experimental autoimmune myocarditis to dilated cardiomyopathy in rats by inhibiting oxidative stress through modulation of the endothelin 1/MAPK signaling pathways [276]. In another rat model, Qct (25 mg/kg/day) decreased myocardial remodeling and fibrosis by reducing mitochondrial ROS production and total Ca2+ content [277]. According to Kumar et al., pre-treatment with Qct (50 mg/kg/day) for 14 days in rats significantly attenuated the isoproterenol-induced acute myocardial injury and inflammation [278].
-
Vascular function
The vascular endothelial benefits of Qct are also well demonstrated in pre-clinical trials. Machha et al. found in diabetic rats that the daily intake of 10 mg/kg protected vascular function via antioxidant activity and increased endothelium-derived NO levels [285]. According to a pre-clinical trial by Sánchez et al., Qct administration (10 mg/kg for 13 weeks) improved blood pressure and endothelial function in spontaneously hypertensive rats via enhancing NO production and suppressing the NADPH oxidase-mediated ROS generation [286].
-
Hypertension
A limited number of human clinical trials evaluated the beneficial effects of Qct on hypertension. Edwards et al. were the first to investigate the potential benefits of this compound in humans. In a randomized, double-blind, placebo-controlled, crossover study, 19 subjects with pre-hypertension and 22 with stage 1 disease were enrolled and administered 730 mg Qct or placebo daily for 4 weeks. There was no significant blood pressure change in the pre-hypertension group, but a significant reduction was documented in those with stage 1 hypertension: systolic (−7 ± 2 mmHg) and diastolic (−5 ± 2 mmHg) [149].
-
Myocardial ischemia
The number of studies evaluating the effect of Qct in HF and CAD is limited. Chekalina et al. investigated the potential benefits of Qct on myocardial ischemia and hemodynamic parameters in a population with stable CAD receiving standard-of-care therapy in a randomized, placebo-controlled trial. A total of 85 subjects were randomized into two groups: (1) 120 mg daily Qct supplementation for 2 months; (2) continued standard treatment for CAD. LVEF increased by 4.5% and 3.2%, respectively on echocardiography which also revealed an improvement in diastolic function parameters in both groups except for mitral inflow deceleration time (DT), which only changed in the cohort receiving Qct.
4.2.2. Catechins
Pre-Clinical Trials
-
Vascular function, hypertension
The antihypertensive and vascular protective effects of catechins are well demonstrated in pre-clinical trials. Catechins were shown to decrease blood pressure, improve endothelial function, and induce vasorelaxation [273,274,294,295]. In a recent trial, Munoz et al. investigated the beneficial effects of green tea extract on the CV system in animal models. Supplementation for 20 weeks prevented the development of hypertension and improved endothelial function in obese mice by decreasing arterial inflammation and oxidative stress [296].
-
Cardiac function, remodeling
Ischemia-induced oxidative damage of the myocardium leads to cardiomyocyte hypertrophy, myocardial fibrosis, and apoptosis. The myocardial protective effects of catechins during I/R insults may be explained by their antioxidant and anti-inflammatory properties. Ferenczyová et al. provided a thorough summary of the most relevant results related to I/R injury [304]. It is important to emphasize that catechin doses used in pre-clinical studies varied broadly (1–200 mg/kg/day).
Human Clinical Trials
-
Hypertension
Several human clinical trials have investigated the vasoactive and anti-hypertensive activity of catechins [309,310,311]. Bogdanski et al. evaluated the effect of green tea extract on cardiovascular risk factors in obese, hypertensive individuals. In this double-blind, placebo-controlled study, patients (n = 56) received green tea extract (379 mg/day) or placebo for 3 months. Both systolic (−4.9 ± 5.7 mmHg) and diastolic (−4.7 ± 3.2 mmHg) blood pressure values were significantly lower in the treatment group compared to placebo at the end of the follow-up period [158].
-
Atherosclerosis and myocardial ischemia
Catechins can also favorably influence the progression of atherosclerosis through CV risk factor modification, such as diabetes and lipid metabolism [152,160,164,312]. It is well known that elevated oxLDL levels induced by oxidative stress are primarily responsible for the development and progression of atherosclerosis and in general, CVDs. In a randomized, placebo-controlled, double-blind trial, Suzuki-Sugihara examined the antioxidant capacity of catechins in healthy male volunteers with 19 subjects eating a total of 1 g of catechin each. Plasma total antioxidant capacity was elevated at one hour following ingestion and LDL oxidizability was significantly reduced [165].
-
Heart failure
Data on the effect of catechin supplementation in HF are limited. In a recent trial, Dural et al. examined the benefits of chocolate (containing high amounts of catechins) in patients with systolic HF. They found that NT-proBNP levels decreased significantly with the use of both milk chocolate (356 ± 54.2 vs. 310 ± 72.1 pg/mL; p = 0.007) and dark chocolate (341 ± 57 vs. 301 ± 60.1 pg/mL; p = 0.028) compared to placebo. However, six-minute walking distance was unaffected [169].
4.2.3. Anthocyanins
Anthocyanins or anthocyanidins (aglycon form) include cyanidin, delphinidin, pelargonidin, petunidin, and malvidin. They are naturally occurring, water-soluble pigments responsible for the color of fruits and vegetables, especially berries (predominantly blueberry, cranberry, and strawberry), grapes, and carrots. The habitual intake of anthocyanins is variable worldwide (12–44 mg/day), but wine consumption contributes to approximately 14.4–24.5% of the total intake in Europe.
Pre-Clinical Trials
-
Cardiac function, remodeling
Several pre-clinical studies have recently demonstrated the favorable effect of anthocyanins on myocardial hypertrophy, fibrosis, and remodeling by limiting oxidative stress, reducing inflammation, and modulating cellular energy metabolism. In an in vivo murine model by Chen et al., high-dose delphinidin (15 mg/day for 8 weeks) successfully reversed pressure-overload-induced cardiac hypertrophy, LV dysfunction, and dilation, as provoked by transverse aortic constriction (TAC) in mice. One of the key possible mechanisms for these findings is the attenuation of TAC-induced oxidative stress by delphinidin. Notably, no significant benefit could be detected when it was administered at a lower dose (5 mg/day) [314].
-
Vascular function, hypertension
The beneficial effects of anthocyanins on vascular function have also been described. Petersen et al. demonstrated that the use of strawberry extract can limit vascular dysfunction by increasing endothelium-dependent vasorelaxation. In addition, it prompts a reduction in blood pressure by inhibiting chronic endothelial inflammation as well as ROS production [323]. Several other authors have published on the anti-inflammatory and vascular protective effects of anthocyanins [324].
-
Atherosclerosis and risk factors
Several pre-clinical trials have reported on the potent anti-atherogenic properties of anthocyanin-rich fruit extracts, likely related to their lipid-lowering, anti-inflammatory, and antioxidant actions [331,332,333,334,335]. When considering acute CV events, such as MI or stroke, platelet activation (aggregation and adhesion) represents a pivotal event. Therefore, effective inhibition of platelet hyperactivity may be considered a favorable benefit in improving CV outcomes and reducing the risk of adverse events [239].
Human Clinical Trials
-
Myocardial ischemia
The relationship between anthocyanin supplementation and CVD prevention is relatively well-studied. Cassidy et al. followed 93,600 women (25 to 42 years of age) who were healthy at baseline from the Nurses’ Health Study and examined the association between anthocyanin intake and the risk of MI. During 18 years of follow-up, 405 MI cases were reported and higher anthocyanin intake was inversely associated with the risk of MI after multivariate adjustment (HR: 0.68; 95% CI: 0.49, 0.96; p = 0.03 highest vs. lowest quintiles). These data suggest that high habitual anthocyanin intake may effectively reduce the risk of MI in a population of predominantly young women [170].
-
Hypertension
As potent vasorelaxant and antioxidant agents, anthocyanins can improve vascular function and reduce blood pressure. In a human clinical trial (n = 1898), Jennings et al. found that anthocyanin-rich food intake led to a significant reduction in systolic blood pressure and improvement in central hemodynamic parameters, such as augmentation index (AI) and pulse wave velocity (PWV) as measured by non-invasive methods [176]. McKay et al. reported that anthocyanin-rich hibiscus tea consumption for 6 weeks by 65 adults with grade I hypertension reduced systolic blood pressure significantly when compared to placebo (−7.2 ± 11.4 vs. −1.3 ± 10.0 mmHg; p = 0.030). However, diastolic blood pressure did not change significantly [177].
Evaluating the acute effects of high anthocyanin intake (480 mL cranberry juice with 94 mg anthocyanin content), Dohadwala et al. found a beneficial effect on vascular function as measured by FMD in subjects with CAD [181]. Several other studies also reported a similar improvement in FMD parameters after short- and long-term consumption of anthocyanin-rich extracts from different berries [340,341,342,343].
5. The Association between CV Mortality and Polyphenol Intake
The number of trials investigating the association between clinical cardiovascular outcomes and polyphenol use is limited. Sesso et al. performed a large, randomized, double-blind, placebo-controlled clinical trial (COcoa Supplement and Multivitamin Outcomes Study; COSMOS) aiming to evaluate the effect of cocoa intake on CVD prevention [345]. Researchers randomized 21,442 adults aged 60 years or older from the US with no prior history of MI or stroke. Participants received daily cocoa extract supplements (500 mg cocoa flavanols, including 80 mg epicatechin) and/or multivitamin supplements or matching placebo. The primary composite outcome was the incidence of stroke, MI, unstable angina requiring hospitalization, need for coronary revascularization, CV mortality, carotid artery surgery, and peripheral artery surgery. After a median follow-up of 3.6 years, 866 participants had a confirmed CV event. Although there was a favorable trend, the study failed its primary composite endpoint (HR: 0.90; 95% CI: 0.78, p = 1.02). Analyzing the secondary outcomes, there was a significant reduction in CVD deaths in the group treated with cocoa extract compared to placebo [345].
6. Red Wine Consumption and Its Association with CVDs
Wine is a complex hydro-alcoholic solution typically derived from fermented grapes. Since ancient times, wine, particularly red wine, has been part of various diets with known health benefits when consumed in moderate amounts [12]. Red wine contains a large variety of bioactive ingredients including polyphenols such as flavonoids (catechin, quercetin, and anthocyanins) as well as stilbenes (RES). These are thought to be responsible for its beneficial effects on the CV system [349]. The polyphenol content of wines depends strongly on the species and the interaction between environmental factors.
Renaud et al. were the first to report in 1992 that moderate red wine intake is inversely associated with the prevalence of ischemic heart disease and CV mortality despite the high saturated fat intake in southern France. This phenomenon is known as the “French paradox” [13]. Since then, several human clinical and epidemiological trials have confirmed that regular, long-term red wine consumption in moderate amounts exerts cardioprotective effects and can positively influence CV risk factors [351], lipid profile, and type-2 diabetes [352,353,354,355,356,357]. A group has shown that moderate red wine consumption at dinner (200 mL for 3 weeks) has a beneficial effect on hemorheological parameters, including red blood cell aggregability and deformability, in healthy volunteers. This may have a positive effect on the microcirculation thereby reducing the risk of CVDs [358].
Gronbaek et al. reported in 2000 that those consuming low (1–7 glasses per week) and moderate (8–21 glasses per week) amounts of red wine have 20% and 24% lower all-cause mortality compared to those not consuming wine, respectively [360]. However, a J-shaped relationship was observed between total alcohol use and mortality.
7. Adverse Effects of the Most Relevant Polyphenols
While polyphenols are well-tolerated compounds overall, it is important to mention a few relevant adverse effects that have been reported in clinical trials.
The side effect profile of stilbenes is the most thoroughly investigated when compared to other polyphenols. The development of adverse effects depends primarily on the duration of administration, achieved plasma concentration, as well as the primary cell/tissue type affected [368,369,370,371,372,373]. RES has been administered over a wide dose range in pre-clinical and human studies, however, the cut-off dose for toxicity remains unknown. The most commonly reported adverse effects were gastrointestinal. Brown et al. found in humans that emesis, diarrhea, and mild hepatic dysfunction may occur at a daily dose of 2.5 g and above [374].
Only a few dose-dependent side effects were reported for flavonoids. These generally include gastrointestinal (emesis, diarrhea, abdominal pain, and bloating), in addition to insomnia, headache, and palpitations [377]. Younes et al. published that catechin taken as a dietary supplement in doses exceeding 800 mg/day induces a significant increase in serum transaminases [378].
8. Conclusions
Unhealthy dietary habits are known to contribute to the development and progression of CVDs and mortality. Numerous in vitro and in vivo pre-clinical studies as well as human clinical trials have shown that the consumption of polyphenol-rich diet, including moderate red wine consumption, may be beneficial in the prevention and treatment of CVDs. Their antioxidant and anti-inflammatory properties, and ability to influence various intracellular processes are relatively well described. In pre-clinical trials, RES and flavonoids could effectively reduce blood pressure, enhance vascular function, and attenuate LV hypertrophy and remodeling while improving LV function. In addition, these polyphenols have potent anti-atherogenic effects and can positively influence several CV risk factors such as lipid profile, diabetes, and vascular inflammation. A large number of clinical trials have also been performed confirming the potent cardioprotective effects of these polyphenols in humans.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11112888
This entry is offline, you can click here to edit this entry!
