Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Congenital heart diseases (CHDs) are structural or functional defects present at birth due to improper heart development. Current therapeutic approaches to treating severe CHDs are primarily palliative surgical interventions during the peri- or prenatal stages, when the heart has fully developed from faulty embryogenesis.
- stem cells
- placenta and heart development
- congenital heart diseases
1. Introduction
The development of the human heart is a complex process that is initiated early during embryogenesis, orchestrated by intricate molecular and cellular events. Congenital heart diseases (CHDs), which result from improper embryonic development, are a group of structural and functional cardiac defects that affect approximately 1% of live births [1]. CHDs may present variable degrees of severity, ranging from minor defects with minimal or no clinical impact to severe malformations requiring immediate medical intervention at birth. Some cardiac conditions may necessitate lifelong treatment and care, frequently resulting in long-term morbidity and increased mortality rates [2]. At present, therapeutic approaches to the treatment of CHDs primarily focus on palliative interventions that improve cardiac structure and function, alleviate symptoms, and reduce the risk of long-term complications. Even surgical interventions, such as cardiac repair or transplantation, which significantly improve survival rates for patients, do not address the underlying genetic abnormalities associated with CHDs. As a result, the risk of CHDs in the children of patients who survived critical CHDs increases to 2–5% [1]. Consequently, there has been a shift from high infant mortality rates to increased survival of adults with complex CHDs, who are at risk for subsequent cardiovascular complications potentially leading to heart failure and the need for lifelong medical care [3]. In the United States, although pediatric admissions for patients with CHDs only account for 3.7% of total admissions, their annual cost is approximately $5.6 billion, which is about 15% of overall costs for pediatric patients [4]. Thus, CHDs are major and increasing health and economic burdens worldwide, and novel strategies to reliably diagnose, refine treatments for, and, if possible, prevent CHDs are urgently needed.
Current prenatal screening programs, genetic counselling, and optimization of maternal health and care, both before and during pregnancy, play a pivotal role in preventing CHDs [5]. The accuracy of prenatal tests to identify cardiac fetal anomalies and the development of innovative medical procedures have opened possibilities for fetal cardiac interventions (FCIs) in utero, at a critical developmental stage when the heart is still growing and developing. FCIs aim to correct fetal cardiac malformations that are either prone to progress with severe complications during mid- or late gestation, or to carry a high risk of fetal demise or life-threatening conditions at birth. FCIs also intend to improve neonatal and prenatal survival of the offspring, and limit lifelong morbidity and mortality [6][7][8][9].
Thus, the limitations of surgical and medicinal treatments of cardiovascular diseases have stimulated the scientific and medical field to search for novel strategies. Consequently, human stem cells have emerged as a promising source of cells for cardiac regeneration in patients to complement current medical and surgical interventions, in addition to serving as cellular models to uncover the underlying mechanisms of CHDs [10][11]. To date, numerous preclinical and clinical studies have been performed to treat CHDs using embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells, such as cardiac progenitor cells and mesenchymal stem cells [12][13][14][15][16]. Although the outcomes of these trials remain largely unsatisfactory, the usage of autologous and allogenic stem cells to complement surgical interventions in infants with CHDs have recently shown positive results, particularly in patients with hypoplastic left heart syndrome (HLHS) [3][12][17][18][19]. To further reduce surgical interventions in patients, the tissue engineering research field is actively developing cell-seeded clinical patches that are able to either grow in synchronization with the cardiovascular structures or to be gradually replaced by the newly formed tissues of treated infants [3]. In addition to their differentiation abilities, stem cells secrete a wide range of bioactive molecules (secretomes) and release extracellular vesicles, including exosomes, which have promising potential in facilitating the repair and regeneration of damaged adult cardiac tissues and improving heart function [20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35]. The secretomes and exosomes released by various cells have significant roles in (cardiac) development and tissue repair, and hold promising potential toward reversing CHDs, or at least mitigating their severity [18][36][37][38][39][40].
The causes of CHDs are often multifactorial and may involve inherited genetic mutations, sporadic developmental errors, and/or environmental factors [41][42][43][44][45]. Mutations or other genomic abnormalities have been shown to occur in genes that play crucial roles in cardiac development, including genes encoding for transcriptional and epigenetic/chromatin remodeling factors (such as NKX2.5, TBX5, GATA4, CITED2, TBX20, p300, and CBP), cell signaling and adhesion proteins (such as ACVR1, NOTCH1, and PDGFRA), and structural sarcomere proteins (such as MYH6, MYH7, and ACTC1) [46][47]. Chromosomal anomalies resulting in syndromic complications, such as Down syndrome (trisomy 21), Turner syndrome (monosomy X), and DiGeorge syndrome (22q11.2 deletion), are often associated with increased risk of CHDs [46][47].
Maternal/embryonic–fetal environmental factors, including maternal diabetes, medications taken during pregnancy, infections, alcohol or drug abuse, exposure to tobacco smoke, chemicals or toxins, may also increase the risk for CHDs [5][47]. Of interest, embryonic heart and placental development are interrelated and concomitant, sharing regulatory molecules and signaling pathways. Placental defects are also more common in pregnancies with CHDs, and the inadequate placental function can contribute to persistent cardiac defects postnatally [48][49][50].
Despite the complexity of embryonic heart formation and the high incidence of CHDs, cardiogenesis is a remarkably robust developmental process. Indeed, dysfunctions of more than one gene and/or environmental insults are often necessary to drastically impair heart development and lead to severe CHDs or death during gestation [51][52][53]. The resilience of this process certainly relies on the complex and evolutionary conserved gene regulatory network, which is orchestrated by key signaling pathways and transcription factors, that belong to families of proteins sometimes displaying overlapping or redundant functions [54]. Their robustness also depends on the existence of multiple sources of cardiac progenitors with functional redundancy, ensuring the production of heart cells even in the event of loss or defects in formation, expansion, and differentiation of certain progenitors [55][56][57]. For instance, despite ablation of over 50% of emerging First Heart Field (FHF) and Secondary Heart Field (SHF) cardiac progenitors at early stages of cardiogenesis, mouse embryos survived and developed into adult animals without any apparent cardiac abnormalities [56][58]. Therefore, the dysfunction or loss of cardiac progenitors and cardiomyocytes in mouse embryonic hearts can truly be compensated by alternative embryonic mechanisms, which include the expansion and migration of other unaffected cardiac cells [56][58][59][60][61].
Recent studies, using animal models and in vitro differentiation of human pluripotent stem cells, have also suggested that cardiac cell lineages are pre-determined during the early stages of development. Both positioning and functions of cardiac cells in the heart may be defined even before gastrulation, and orchestrated by multiple signaling pathways and environmental cues [55][62][63][64][65][66][67]. This idea is also supported by the ability of pluripotent stem cells to generate self-organizing cardiac organoids when provided with external cues from the extracellular matrix (ECM) and signaling molecules [68]. Moreover, in the absence of maternal tissues in vitro, human blastocysts attached to instructive supports and/or, stimulated by signaling molecules (such as WNT and NODAL/ACTIVIN), autonomously self-organize and originate structures resembling the proper embryo, with key landmarks of normal development, including bilaminar disc formation, primitive streak formation, lineage commitment, and extraembryonic annexes [69][70][71][72]. Therefore, future clinical strategies that would consistently provide correct developmental signals at early embryonic stages have the potential to compensate for defective mechanisms, thereby significantly reducing the incidence of CHDs.
2. Navigating Early Mammalian Embryonic Heart and Placental Development
2.1. Placental Development
Placental and embryonic heart developments occur simultaneously, forming a placenta–heart axis, and sharing developmental pathways and common susceptibility to genetic defects [49]. Thus, the developing heart is highly vulnerable to early placental insufficiency. During early human embryogenesis, at embryonic days 4 to 5 (E4-5), the blastocyst attaches to the uterine wall. Trophoblast cells, encasing the inner cell mass that will originate the embryo proper, differentiate into inner cytotrophoblast cells, which are key for blastocyst implantation in the uterus, and stem cells that will also originate the outer syncytiotrophoblast [73][74]. The syncytiotrophoblast is responsible for the exchange of nutrients, gases, and waste products between the maternal and embryonic circulations, and also acts as a barrier against pathogens and harmful substances [75]. Interactions between the cytotrophoblast and syncytiotrophoblast are essential for proper placental development and function. Aberrant vascular placental development can lead to placental insufficiency and compromise fetal growth due to inefficient blood supply, and may result in preeclampsia, gestational diabetes, intrauterine growth restriction (IUGR), and placental abruption [76][77][78][79][80]. These complications are associated with an increased risk of adverse fetal outcomes, including CHDs [81][82][83][84]. Vascular endothelial growth factor (VEGF) and its receptors, hypoxia-inducible factors (HIFs), which regulate the cellular response to hypoxia, play a crucial role in placental and heart development, as well as in vascular remodeling, and their dysfunction increases the risk for CHDs [73][85][86][87]. Immune modulation is also essential for placental development and for fetal tolerance by the maternal immune system [88][89].
2.2. Heart Development
During embryogenesis, the heart is progressively built with multiple mature and functional cells, originating from many molecularly distinct progenitor cells that arise at different times and structures from the developing embryo [55][63][64][65][90][91]. The heart primarily develops from three main pools of embryonic progenitors, which are the FHF, SHF, and the proepicardium cells (Figure 1). The FHF and SHF derive from the earliest MESP1-marked cardiac mesodermal progenitors, which emerge shortly after gastrulation [55][62][63][64][65]. However, precardiac progenitor cells in the nascent mesoderm are, in fact, a heterogeneous population, composed of molecularly distinct progenitors that leave the primitive streak in a sequential manner, with contributions to specific and overlapping parts of the heart [91][92][93].
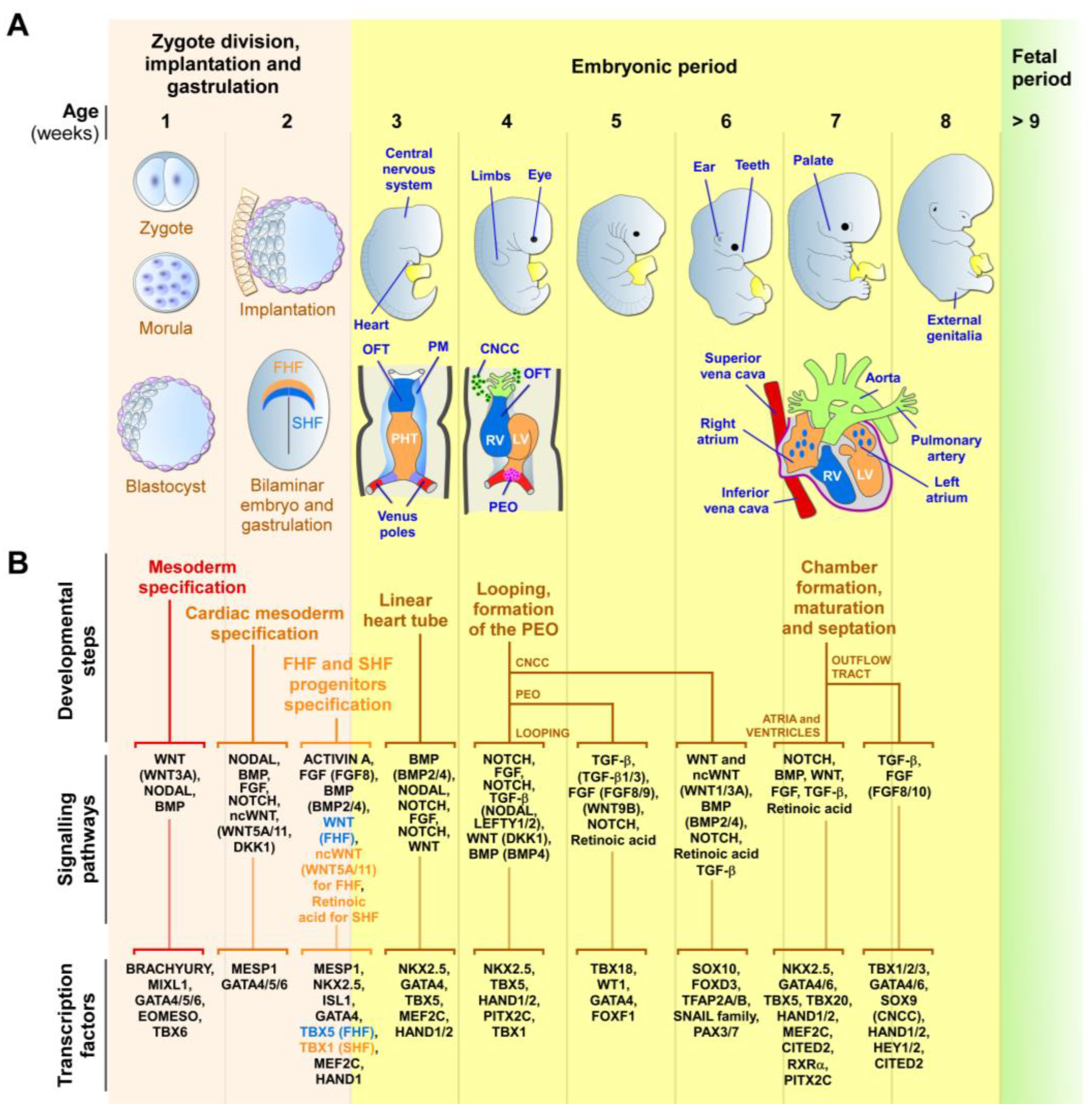
Figure 1. Early human cardiac development. (A) Schematic representation of the zygotic and embryonic stages of human development, highlighting key developmental programs and milestones. Gestational times are indicated in weeks. The initial steps of heart, central nervous system, limb, eye, ear, tooth, and genital development are shown. The primary (FHF—blue) and secondary (SHF—orange) heart field cells, the cardiac neural crest cells (CNCCs—green), and the proepicardial organ (PEO—purple), and their respective derivatives, are illustrated. During week 2, the cardiac mesoderm gives rise to progenitors of the FHF and SHF, which form the cardiac crescent. The FHF originates the primary heart tube (PHT), which subsequently contributes to the left ventricle (LV) and parts of the right and left atria. SHF cells migrate through the pharyngeal mesoderm (PM) and contribute to the elongation of the PHT by ingression at both atrial and venous poles. SHF cells contribute to the development of the right ventricle (RV), outflow tract (OFT), atria, and inflow myocardium. Cells originating from the venous poles (red) give rise to the superior and inferior vena cava. The PEO cells contribute to the epicardium and coronary vessels. CNCCs migrate from the dorsal neural tube into the cardiac OFT, where they contribute to the formation of the septum, separating the truncus arteriosus into the aorta and pulmonary artery, as well as contributing to heart valve formation and parasympathetic innervation. (B) Overview of the secretomes, main signaling pathways, and transcription factors that regulate each listed stage of heart development. The noncanonical WNT pathway is indicated as ncWNT.
The first cells to leave the primitive streak, emerging at approximately E15-16 in humans, are FHF cells that give rise to the cardiac crescent structure, which migrates and fuses at the midline to form the primitive linear heart tube (at around E19 in humans) [41][55][62][63][64][91][94][95]. The linear heart tube, destined to generate the bulk of the atrial chambers and left ventricle, contains an outer myocardium and an inner endocardium, separated by a specific ECM known as the cardiac jelly. Rhythmic contractions are initiated by cardiomyocytes of the heart tube at around E21 in humans. At approximately E19-20, human SHF cells migrate into the heart tube and differentiate into cardiomyocytes, smooth muscle, and endothelial cells. The SHF contributes to the outflow tract (OFT), the right ventricular region, the atrioventricular canal structures, and the atria [41][55][62][63][64][91][94][95].
Around E28, the human heart tube undergoes a rightward looping to initiate the formation of the four-chambered heart. At this stage, the conductive system also begins to develop from cardiac progenitors, which differentiate into specialized myocytes with high conductance to form the sinoatrial node, atrioventricular node, bundle of His, and Purkinje fibers, rather than working cardiomyocytes [96][97]. As the heart develops, conduction cells establish connections to form a functional electrical system that coordinates the contraction and relaxation of the cardiac muscle. Further maturation and refinements of the conductive system occur throughout fetal development, with further structural and functional changes happening after birth and during postnatal growth [96][97]. In humans, ventricular septation starts around E50 and originates from the myocardium, which generates the ventricular septum and establishes the right and left atrio-ventricular canals. Atrial septation starts at E60 and derives from the septum primum and septum secundum, concomitantly with OFT septation [41][65][98][99].
OFT septation, which results in the formation of the aorta and the pulmonary artery, and their respective connections to the left and right ventricles, is accomplished by SHF cells and cardiac neural crest cells (CNCCs), a subpopulation of neural crest cells that delaminate from the neural tube [99][100]. CNCCs also give rise to the tip of the interventricular septum and OFT cushions, which will differentiate into the aortic and pulmonary valves and the parasympathetic coronary innervation [100]. The proepicardial organ is an extracardiac structure, which develops distinctly from the heart tube (at E9.5 in mouse) and contributes to the epicardial cells located around all heart chambers [93][101][102]. Other early multipotent progenitor cells, marked by KDR/FLK1/VEGFR2 expression, which are a source for endocardium, myocardium, and hematopoietic progenitors, were also detected in the primitive streak [55][103]. The major functional structures of the human heart are completed by E60. After this stage, the heart undergoes progressive growth, as well as structural, metabolic, and functional maturation processes, which are vital for its function during a lifetime [68].
3. Unveiling the Potential of Secretomes and Exosomes for CHD Prevention
3.1. Exogenous Instructive Molecules to Mitigate CHDs
Multiple signaling pathways, which may display overlapping or redundant functions, are involved in generating cardiac progenitors and other cell lineages [55][62][63][64][65][66][67]. All issues considered, it is tempting to propose that the supplementation of exogenous cardiogenic instructive molecules at key moments during early embryonic stages may compensate for defective pathways and/or lineage decisions of cardiovascular progenitors to mitigate CHDs. To date, only a few experimental reports on animal models and stem cells support this idea. For instance, prolonged hypoxia causes DNA damage, premature senescence, impaired angiogenesis, and fibrosis associated with the upregulation of TGF-β1 expression in human fetuses with HLHS [104]. In vitro, hypoxia exposure of a human HLHS-derived iPSC disease cell model promotes the differentiation into cardiac fibroblasts instead of cardiac progenitors, cardiomyocytes, and endothelial cells [104]. Inhibition of TGF-β1 activity by its antagonist compound SB431542, in HLHS-derived iPSC exposed to hypoxia, prevented senescence and promoted genomic stability [104], suggesting that early interventions to inhibit TGF-β1 could improve ventricular growth and overcome pathways dysregulated in HLHS. IUGR is another gestational defect that hinders fetal growth in the uterus. IUGR is associated with increased placental vascular resistance, which forces the workload onto the fetal heart and increases the risk of cardiovascular disease [105]. Maternal administration of IGF-1 and IGF-2, which are known to stimulate placental and fetal growth in animal models, showed promising potential to treat IUGR in cases where downregulation of IGF-1 receptors in the placenta is not observed [105][106].
3.2. Exosomes, a Non-Cellular Approach for Correcting Embryonic Cardiac Defects
Emerging evidence suggests that the beneficial effects of stem cells in medical applications may primarily result from paracrine signaling molecules and the release of extracellular vesicles, rather than from the direct integration of stem cells or their derived cells into the target tissue [107]. Exosomes, which can be isolated and characterized by well-established approaches [108][109], present advantages such as scalable production, easy storage, consistent morphology and function, compliance with regulatory standards, and possible reduction in the variability of outcomes associated with cell therapies [110]. The therapeutic potential of exosomes and secretomes derived from hormonally primed human endometrial epithelial cells was tested in mouse models, and proven to enhance embryonic growth, development, and implantation [111]. This study also suggested that dysfunctions of the endometrial secretomes, which hinder implantation in cases of infertility, can be amended through the supply of exogenous exosomes. Also, maternal diabetic pregnancies in mouse models showed a higher occurrence of neural tube defects associated with exosomes produced from vascular progenitor cells expressing FLK1 derived from mesoderm cells lacking Survivin [112]. Interestingly, delivery into the amniotic cavity of Survivin-enriched vascular progenitor exosomes prevented neural tube defects in diabetic pregnancies [112], demonstrating the capacity of modified exosomes delivered in utero to limit neuronal pathology. Thus, exosomes are a promising non-cellular alternative to cells for clinical applications to correct embryonic defects. Exosomes can also be engineered to carry specific cargo, such as miRNAs or even gene-editing tools more suitable for the correction of genetic abnormalities associated with CHDs [113][114]. Notably, exosomes derived from the cardiac progenitors of children undergoing reconstructive heart surgeries showed promising effects in promoting angiogenesis, reducing fibrosis, resolving hypertrophy, and improving cardiac function in a rat model of heart arrhythmias caused by ischemia-reperfusion injury [38], showing the cardioprotective effect of exosomes from infant cardiac cells. Interestingly, exosomes from neonatal progenitors improved cardiac function regardless of oxygen levels, while exosomes from older children were only reparative in hypoxic conditions. Therefore, further research is needed to determine the most suitable cell sources and culture conditions for the production and modification of exosomes that would yield the best personalized clinical outcomes in the treatment of CHDs [39][112].
4. Harnessing Secreted Factors in Embryogenesis for Protection against CHDs
Like in many diseases, the early correction of a CHD is likely to result in a better clinical outcome, through early normalization of cardiogenesis. Reports on stem cell differentiation and heart development have suggested that cell positioning and functions within the heart may be determined prior to gastrulation, through precise signaling pathways and environmental cues [55][62][63][64][65][66][67]. Therefore, early interventions aiming to target the blastocyst at the pre-gastrulation/pre-implantation stage, very early embryonic states, may reduce the occurrence and severity of CHDs. Interestingly, pioneering studies on Inhibitors of Differentiation (ID) genes have revealed that double knockout of any pair of these genes in mouse models results in prominent cardiac defects and midgestational lethality of the embryos [115][116]. However, the injection of wild-type ESCs, either in ID-null blastocysts to form a chimera, or intraperitoneally in females prior to conception, rescued cardiac malformations and viability of ID-null embryos through upregulation of IGF-1 and WNT5A, without incorporation of wild-type ESCs into ID-null embryos [115][116]. Similarly, a chimeric embryo formation, using wild-type ESCs, improved the morphogenetic defects of RA signaling-deficient embryos by increasing RA expression [117]. Interestingly, transient exogenous WNT5A and WNT11, supplied at the 1-cell stage, efficiently rescued the viability and cardiac defects of CITED2-depleted zebrafish embryos and cardiac differentiation of mouse ESCs [118]. Dysfunctions of CITED2, a transcriptional modulator, have been widely associated with zebrafish, mouse, and human CHDs, as well as with embryonic lethality [53][118][119][120][121][122][123][124][125][126]. At the cellular level, CITED2 regulates the expressions of numerous genes involved in early cardiogenesis, such as BRACHYURY, MESP1, ISL1, GATA4, TBX5, MEF2C, NODAL, LEFTY1/2, PITX2C, VEGFA, WNT5A, and WNT11, among others [118][119][120]. Thus, the supplementation of WNT5A and WNT11 at the blastocyst stage in utero holds potential for rescuing CHDs associated with CITED2 dysfunction in mammals, since the function of CITED2 is conserved across vertebrates [118][120][122][127]. This strategy may also prevent CHDs triggered by the dysfunction of many genes other than CITED2, such as CITED2-target cardiogenic genes, including WNT5A and WNT11.
Moreover, WNT5A and WNT11 trigger many congenital cardiac anomalies, and contribute to DiGeorge syndrome, when defective [128][129][130][131][132][133][134]. Indeed, the WNT5A and WNT11 proteins are central for proper gestation and successful pregnancy, and their expressions are naturally highly increased and localized in the uterine luminal epithelium prior to and during blastocyst attachment to the uterus [135][136]. These proteins also promote embryonic uterine implantation and survival [135], as well as placental growth [136]. WNT5A and WNT11 are also important in late gastrulation, for the regulation of the anterior–posterior axis elongation, notochord extension, and proper patterning of the neural tube and somites [137]. In early mouse gastrulation, WNT11 is initially expressed in endoderm progenitors, and later, during mid-gastrulation, it plays a role in the formation of the embryonic and extraembryonic endothelia, as well as the formation of the endocardium in all chambers of the developing heart [138]. During late gastrulation, WNT11 showed successive waves of expression in different regions of the myocardium, important to originate left ventricle precursors (FHF progenitors) from E7.0–8.0, right ventricle progenitors (SHF progenitors) from E8.0–9.0, and the superior wall of the OFT from E8.5–10.5 (also SHF progenitors) [138]. Other studies have implicated WNT5A and WNT11 in the development of cardiac progenitors in vitro and in vivo [139][140][141][142], proper fetal hematopoiesis [143][144], kidney development [145], and guidance of sympathetic neurons to their innervation targets in vivo [146], among other processes. Altogether, the broad range of effects exhibited by WNT5A and WNT11 during early development emphasizes their high potential in limiting CHDs and other birth abnormalities when supplied exogenously at early embryonic stages. Another recent study highlighted the role of a subset of human amniotic epithelial cells (AECs) in mesoderm formation at E8.0–9.0 during early post-implantation stages [147]. Interestingly, impaired mesoderm formation and lethality due to loss of ISL1 expression in non-human primate embryos, or in human AEC differentiation in vitro due to the decrease in BMP4 expression, was partially restored through supplementation of BMP4 [147]. Overall, these findings demonstrate the potential for biomolecules, such as WNT5A/WNT11, BMP4, and inhibitors of the TGF-β pathway (SB431542, for instance), to reduce CHDs when administered at very early stages of development.
This entry is adapted from the peer-reviewed paper 10.3390/jpm13081263
References
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900.
- Gilboa, S.M.; Devine, O.J.; Kucik, J.E.; Oster, M.E.; Riehle-Colarusso, T.; Nembhard, W.N.; Xu, P.; Correa, A.; Jenkins, K.; Marelli, A.J. Congenital Heart Defects in the United States. Circulation 2016, 134, 101–109.
- Brown, M.A.; Rajamarthandan, S.; Francis, B.; O’Leary-Kelly, M.K.; Sinha, P. Update on stem cell technologies in congenital heart disease. J. Card. Surg. 2020, 35, 174–179.
- Chowdhury, D.; Johnson, J.N.; Baker-Smith, C.M.; Jaquiss, R.D.B.; Mahendran, A.K.; Curren, V.; Bhat, A.; Patel, A.; Marshall, A.C.; Fuller, S.; et al. Health Care Policy and Congenital Heart Disease: 2020 Focus on Our 2030 Future. J. Am. Heart Assoc. 2021, 10, e020605.
- Jenkins, K.J.; Correa, A.; Feinstein, J.A.; Botto, L.; Britt, A.E.; Daniels, S.R.; Elixson, M.; Warnes, C.A.; Webb, C.L. Noninherited Risk Factors and Congenital Cardiovascular Defects: Current Knowledge. Circulation 2007, 115, 2995–3014.
- Freud, L.R.; Tworetzky, W. Fetal interventions for congenital heart disease. Curr. Opin. Pediatr. 2016, 28, 156–162.
- Halder, V.; Ghosh, S.; Shrimanth, Y.S.; Manoj, R.K.; Mandal, B.; Thingnam, S.K.S.; Kumar, R. Fetal cardiac intervention and fetal cardiac surgery: Where are we in 21st century? Am. J. Cardiovasc. Dis. 2021, 11, 642–646.
- Friedman, K.G.; Tworetzky, W. Fetal cardiac interventions: Where do we stand? Arch. Cardiovasc. Dis. 2020, 113, 121–128.
- Gellis, L.; Tworetzky, W. The boundaries of fetal cardiac intervention: Expand or tighten? Semin. Fetal Neonatal Med. 2017, 22, 399–403.
- Huang, J.; Feng, Q.; Wang, L.; Zhou, B. Human Pluripotent Stem Cell-Derived Cardiac Cells: Application in Disease Modeling, Cell Therapy, and Drug Discovery. Front. Cell Dev. Biol. 2021, 9, 655161.
- Terashvili, M.; Bosnjak, Z.J. Stem Cell Therapies in Cardiovascular Disease. J. Cardiothorac. Vasc. Anesth. 2019, 33, 209–222.
- Tsilimigras, D.I.; Oikonomou, E.K.; Moris, D.; Schizas, D.; Economopoulos, K.P.; Mylonas, K.S. Stem Cell Therapy for Congenital Heart Disease. Circulation 2017, 136, 2373–2385.
- Oh, H. Cell Therapy Trials in Congenital Heart Disease. Circ. Res. 2017, 120, 1353–1366.
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277.
- Bragança, J.; Lopes, J.A.; Mendes-Silva, L.; Santos, J.M.A. Induced pluripotent stem cells, a giant leap for mankind therapeutic applications. World J. Stem Cells 2019, 11, 421–430.
- Correia, C.D.; Ferreira, A.; Fernandes, M.T.; Silva, B.M.; Esteves, F.; Leitão, H.S.; Bragança, J.; Calado, S.M. Human Stem Cells for Cardiac Disease Modeling and Pre-clinical and Clinical Applications—Are We on the Road to Success? Cells 2023, 12, 1727.
- Trac, D.; Maxwell, J.T.; Brown, M.E.; Xu, C.; Davis, M.E. Aggregation of Child Cardiac Progenitor Cells Into Spheres Activates Notch Signaling and Improves Treatment of Right Ventricular Heart Failure. Circ. Res. 2019, 124, 526–538.
- Zhong, J.; Wang, S.; Shen, W.-B.; Kaushal, S.; Yang, P. The current status and future of cardiac stem/progenitor cell therapy for congenital heart defects from diabetic pregnancy. Pediatr. Res. 2018, 83, 275–282.
- Chase, D.M.; Gallicchio, V.S. The effect of stem cells in congenital heart disease. Clin. Med. Investig. 2019, 4, 1–12.
- Femminò, S.; Bonelli, F.; Brizzi, M.F. Extracellular vesicles in cardiac repair and regeneration: Beyond stem-cell-based approaches. Front. Cell Dev. Biol. 2022, 10, 996887.
- de Abreu, R.C.; Fernandes, H.; da Costa Martins, P.A.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 2020, 17, 685–697.
- Ibrahim, A.; Marbán, E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu. Rev. Physiol. 2016, 78, 67–83.
- Sahoo, S.; Losordo, D.W. Exosomes and Cardiac Repair after Myocardial Infarction. Circ. Res. 2014, 114, 333–344.
- Andriolo, G.; Provasi, E.; Lo Cicero, V.; Brambilla, A.; Soncin, S.; Torre, T.; Milano, G.; Biemmi, V.; Vassalli, G.; Turchetto, L.; et al. Exosomes from Human Cardiac Progenitor Cells for Therapeutic Applications: Development of a GMP-Grade Manufacturing Method. Front. Physiol. 2018, 9, 1169.
- Zou, L.; Ma, X.; Lin, S.; Wu, B.; Chen, Y.; Peng, C. Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Exp. Ther. Med. 2019, 18, 2574–2582.
- Cai, C.; Guo, Y.; Teng, L.; Nong, Y.; Tan, M.; Book, M.J.; Zhu, X.; Wang, X.L.; Du, J.; Wu, W.J.; et al. Preconditioning Human Cardiac Stem Cells with an HO-1 Inducer Exerts Beneficial Effects After Cell Transplantation in the Infarcted Murine Heart. Stem Cells 2015, 33, 3596–3607.
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626.
- Li, T.; Gu, J.; Yang, O.; Wang, J.; Wang, Y.; Kong, J. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal miRNA-29c Decreases Cardiac Ischemia/Reperfusion Injury Through Inhibition of Excessive Autophagy via the PTEN/Akt/mTOR Signaling Pathway. Circ. J. Off. J. Jpn. Circ. Soc. 2020, 84, 1304–1311.
- Ibrahim, A.G.-E.; Cheng, K.; Marbán, E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Rep. 2014, 2, 606–619.
- Maleki, B.; Alani, B.; Tamehri Zadeh, S.S.; Saadat, S.; Rajabi, A.; Ayoubzadeh, S.M.J.; Verdi, J.; Farrokhian, A.; Ghanbarian, H.; Noureddini, M.; et al. MicroRNAs and exosomes: Cardiac stem cells in heart diseases. Pathol. Res. Pract. 2022, 229, 153701.
- Xia, W.; Chen, H.; Xie, C.; Hou, M. Long-noncoding RNA MALAT1 sponges microRNA-92a-3p to inhibit doxorubicin-induced cardiac senescence by targeting ATG4a. Aging 2020, 12, 8241–8260.
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522.
- Zhao, Y.; Sun, X.; Cao, W.; Ma, J.; Sun, L.; Qian, H.; Zhu, W.; Xu, W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury. Stem Cells Int. 2015, 2015, 761643.
- Zarà, M.; Amadio, P.; Campodonico, J.; Sandrini, L.; Barbieri, S.S. Exosomes in Cardiovascular Diseases. Diagnostics 2020, 10, 943.
- Tseliou, E.; Fouad, J.; Reich, H.; Slipczuk, L.; de Couto, G.; Aminzadeh, M.; Middleton, R.; Valle, J.; Weixin, L.; Marbán, E. Fibroblasts Rendered Antifibrotic, Antiapoptotic, and Angiogenic by Priming with Cardiosphere-Derived Extracellular Membrane Vesicles. J. Am. Coll. Cardiol. 2015, 66, 599–611.
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C.; et al. Embryonic Stem Cell–Derived Exosomes Promote Endogenous Repair Mechanisms and Enhance Cardiac Function Following Myocardial Infarction. Circ. Res. 2015, 117, 52–64.
- Chen, K.; Liang, J.; Qin, T.; Zhang, Y.; Chen, X.; Wang, Z. The Role of Extracellular Vesicles in Embryo Implantation. Front. Endocrinol. 2022, 13, 809596.
- Agarwal, U.; George, A.; Bhutani, S.; Ghosh-Choudhary, S.; Maxwell, J.T.; Brown, M.E.; Mehta, Y.; Platt, M.O.; Liang, Y.; Sahoo, S.; et al. Experimental, Systems, and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell–Derived Exosomes From Pediatric Patients. Circ. Res. 2017, 120, 701–712.
- Hoffman, J.R.; Park, H.-J.; Bheri, S.; Jayaraman, A.R.; Davis, M.E. Comparative computational RNA analysis of cardiac-derived progenitor cells and their extracellular vesicles. Genomics 2022, 114, 110349.
- Yin, X.; Jiang, L.-H. Extracellular vesicles: Targeting the heart. Front. Cardiovasc. Med. 2023, 9, 1041481.
- Bruneau, B.G. The developmental genetics of congenital heart disease. Nature 2008, 451, 943–948.
- Kalisch-Smith, J.I.; Ved, N.; Sparrow, D.B. Environmental Risk Factors for Congenital Heart Disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a037234.
- Lim, T.B.; Foo, S.Y.; Chen, C.K. The Role of Epigenetics in Congenital Heart Disease. Genes 2021, 12, 390.
- Zhang, W.; Spero, T.L.; Nolte, C.G.; Garcia, V.C.; Lin, Z.; Romitti, P.A.; Shaw, G.M.; Sheridan, S.C.; Feldkamp, M.L.; Woomert, A.; et al. Projected Changes in Maternal Heat Exposure During Early Pregnancy and the Associated Congenital Heart Defect Burden in the United States. J. Am. Heart Assoc. 2019, 8, e010995.
- Morton, S.U.; Quiat, D.; Seidman, J.G.; Seidman, C.E. Genomic frontiers in congenital heart disease. Nat. Rev. Cardiol. 2021, 19, 26–42.
- Pierpont Mary, E.; Brueckner, M.; Chung Wendy, K.; Garg, V.; Lacro Ronald, V.; McGuire Amy, L.; Mital, S.; Priest James, R.; Pu William, T.; Roberts, A.; et al. Genetic Basis for Congenital Heart Disease: Revisited: A Scientific Statement From the American Heart Association. Circulation 2018, 138, e653–e711.
- Blue, G.M.; Kirk, E.P.; Sholler, G.F.; Harvey, R.P.; Winlaw, D.S. Congenital heart disease: Current knowledge about causes and inheritance. Med. J. Aust. 2012, 197, 155–159.
- Courtney, J.A.; Cnota, J.F.; Jones, H.N. The Role of Abnormal Placentation in Congenital Heart Disease; Cause, Correlate, or Consequence? Front. Physiol. 2018, 9, 1045.
- Maslen, C.L. Recent Advances in Placenta–Heart Interactions. Front. Physiol. 2018, 9, 735.
- Cohen, J.A.; Rychik, J.; Savla, J.J. The placenta as the window to congenital heart disease. Curr. Opin. Cardiol. 2021, 36, 56–60.
- Gittenberger-de Groot, A.C.; Jongbloed, M.R.M.; Poelmann, R.E.; Bartelings, M.M. Structural Heart Disease: Embryology. In Fetal Therapy: Scientific Basis and Critical Appraisal of Clinical Benefits, 2nd ed.; Johnson, A., Oepkes, D., Kilby, M.D., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 110–122.
- Moreau, J.L.M.; Kesteven, S.; Martin, E.M.M.A.; Lau, K.S.; Yam, M.X.; O’Reilly, V.C.; del Monte-Nieto, G.; Baldini, A.; Feneley, M.P.; Moon, A.M.; et al. Gene-environment interaction impacts on heart development and embryo survival. Development 2019, 146, dev172957.
- Bentham, J.; Michell, A.C.; Lockstone, H.; Andrew, D.; Schneider, J.E.; Brown, N.A.; Bhattacharya, S. Maternal high-fat diet interacts with embryonic Cited2 genotype to reduce Pitx2c expression and enhance penetrance of left-right patterning defects. Hum. Mol. Genet. 2010, 9, 3394–3401.
- Olson, E.N. Gene regulatory networks in the evolution and development of the heart. Science 2006, 313, 1922–1927.
- Meilhac, S.M.; Buckingham, M.E. The deployment of cell lineages that form the mammalian heart. Nat. Rev. Cardiol. 2018, 15, 705–724.
- Sturzu, A.C.; Rajarajan, K.; Passer, D.; Plonowska, K.; Riley, A.; Tan, T.C.; Sharma, A.; Xu, A.F.; Engels, M.C.; Feistritzer, R.; et al. Fetal Mammalian Heart Generates a Robust Compensatory Response to Cell Loss. Circulation 2015, 132, 109–121.
- Sharma, B.; Ho, L.; Ford, G.H.; Chen, H.I.; Goldstone, A.B.; Woo, Y.J.; Quertermous, T.; Reversade, B.; Red-Horse, K. Alternative Progenitor Cells Compensate to Rebuild the Coronary Vasculature in Elabela- and Apj-Deficient Hearts. Dev. Cell 2017, 42, 655–666.
- Drenckhahn, J.-D.; Schwarz, Q.P.; Gray, S.; Laskowski, A.; Kiriazis, H.; Ming, Z.; Harvey, R.P.; Du, X.-J.; Thorburn, D.R.; Cox, T.C. Compensatory Growth of Healthy Cardiac Cells in the Presence of Diseased Cells Restores Tissue Homeostasis during Heart Development. Dev. Cell 2008, 15, 521–533.
- Founta, K.-M.; Papanayotou, C. In Vivo Generation of Organs by Blastocyst Complementation: Advances and Challenges. Int. J. Stem Cells 2022, 15, 113–121.
- Coppiello, G.; Barlabé, P.; Moya-Jódar, M.; Abizanda, G.; Barreda, C.; Iglesias, E.; Linares, J.; Arellano-Viera, E.; Ruiz-Villalba, A.; Larequi, E.; et al. In vivo generation of heart and vascular system by blastocyst complementation. bioRxiv 2022.
- Kitajima, S.; Takagi, A.; Inoue, T.; Saga, Y. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development 2000, 127, 3215–3226.
- Houyel, L.; Meilhac, S.M. Heart Development and Congenital Structural Heart Defects. Annu. Rev. Genom. Hum. Genet. 2021, 22, 257–284.
- Tyser, R.C.V.; Ibarra-Soria, X.; McDole, K.; Arcot Jayaram, S.; Godwin, J.; van den Brand, T.A.H.; Miranda, A.M.A.; Scialdone, A.; Keller, P.J.; Marioni, J.C.; et al. Characterization of a common progenitor pool of the epicardium and myocardium. Science 2021, 371, eabb2986.
- Swedlund, B.; Lescroart, F. Cardiopharyngeal Progenitor Specification: Multiple Roads to the Heart and Head Muscles. Cold Spring Harb. Perspect. Biol. 2020, 12, a036731.
- Kloesel, B.; DiNardo, J.A.; Body, S.C. Cardiac Embryology and Molecular Mechanisms of Congenital Heart Disease—A Primer for Anesthesiologists. Anesth. Analg. 2016, 123, 551–569.
- Lawson, K.A.; Meneses, J.J.; Pedersen, R.A. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 1991, 113, 891–911.
- Scialdone, A.; Tanaka, Y.; Jawaid, W.; Moignard, V.; Wilson, N.K.; Macaulay, I.C.; Marioni, J.C.; GÃttgens, B. Resolving early mesoderm diversification through single-cell expression profiling. Nature 2016, 535, 289.
- Hofbauer, P.; Jahnel, S.M.; Mendjan, S. In vitro models of the human heart. Development 2021, 148, dev199672.
- Martyn, I.; Kanno, T.Y.; Ruzo, A.; Siggia, E.D.; Brivanlou, A.H. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature 2018, 558, 132–135.
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254.
- Shahbazi, M.N.; Jedrusik, A.; Vuoristo, S.; Recher, G.; Hupalowska, A.; Bolton, V.; Fogarty, N.M.E.; Campbell, A.; Devito, L.G.; Ilic, D.; et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016, 18, 700–708.
- Kagawa, H.; Javali, A.; Khoei, H.H.; Sommer, T.M.; Sestini, G.; Novatchkova, M.; Scholte op Reimer, Y.; Castel, G.; Bruneau, A.; Maenhoudt, N.; et al. Human blastoids model blastocyst development and implantation. Nature 2022, 601, 600–605.
- Dunwoodie, S.L. The Role of Hypoxia in Development of the Mammalian Embryo. Dev. Cell 2009, 17, 755–773.
- Huppertz, B. The anatomy of the normal placenta. J. Clin. Pathol. 2008, 61, 1296–1302.
- Jaremek, A.; Jeyarajah, M.J.; Jaju Bhattad, G.; Renaud, S.J. Omics Approaches to Study Formation and Function of Human Placental Syncytiotrophoblast. Front. Cell Dev. Biol. 2021, 9, 674162.
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94.
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568.
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of Placental Development and Its Impact on Fetal Growth-New Insights From Mouse Models. Front. Endocrinol. 2018, 9, 570.
- Deshpande, S.S.; Balasinor, N.H. Placental Defects: An Epigenetic Perspective. Reprod. Sci. 2018, 25, 1143–1160.
- Perez-Garcia, V.; Fineberg, E.; Wilson, R.; Murray, A.; Mazzeo, C.I.; Tudor, C.; Sienerth, A.; White, J.K.; Tuck, E.; Ryder, E.J.; et al. Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature 2018, 555, 463–468.
- Liu, S.; Joseph, K.S.; Lisonkova, S.; Rouleau, J.; Hof, M.V.d.; Sauve, R.; Kramer, M.S. Association between Maternal Chronic Conditions and Congenital Heart Defects. Circulation 2013, 128, 583–589.
- Camm, E.J.; Botting, K.J.; Sferruzzi-Perri, A.N. Near to One’s Heart: The Intimate Relationship Between the Placenta and Fetal Heart. Front. Physiol. 2018, 9, 629.
- Hemberger, M.; Hanna, C.W.; Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 2020, 21, 27–43.
- Jansson, T.; Powell, T.L. Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin. Sci. 2007, 113, 1–13.
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761.
- Wang, W.; Xu, A.; Xu, H. The roles of vascular endothelial growth factor gene polymorphisms in congenital heart diseases: A meta-analysis. Growth Factors 2018, 36, 232–238.
- Aplin, J.D.; Myers, J.E.; Timms, K.; Westwood, M. Tracking placental development in health and disease. Nat. Rev. Endocrinol. 2020, 16, 479–494.
- Svensson-Arvelund, J.; Mehta, R.B.; Lindau, R.; Mirrasekhian, E.; Rodriguez-Martinez, H.; Berg, G.; Lash, G.E.; Jenmalm, M.C.; Ernerudh, J. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol. 2015, 194, 1534–1544.
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610.
- Zhang, Q.; Carlin, D.; Zhu, F.; Cattaneo, P.; Ideker, T.; Evans, S.M.; Bloomekatz, J.; Chi, N.C. Unveiling Complexity and Multipotentiality of Early Heart Fields. Circ. Res. 2021, 129, 474–487.
- Kelly, R.G. The heart field transcriptional landscape at single-cell resolution. Dev. Cell 2023, 58, 257–266.
- Lescroart, F.; Wang, X.; Lin, X.; Swedlund, B.; Gargouri, S.; Sànchez-Dànes, A.; Moignard, V.; Dubois, C.; Paulissen, C.; Kinston, S.; et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 2018, 359, 1177–1181.
- Ivanovitch, K.; Soro-Barrio, P.; Chakravarty, P.; Jones, R.A.; Bell, D.M.; Mousavy Gharavy, S.N.; Stamataki, D.; Delile, J.; Smith, J.C.; Briscoe, J. Ventricular, atrial, and outflow tract heart progenitors arise from spatially and molecularly distinct regions of the primitive streak. PLOS Biol. 2021, 19, e3001200.
- Laugwitz, K.-L.; Moretti, A.; Caron, L.; Nakano, A.; Chien, K.R. Islet1 cardiovascular progenitors: A single source for heart lineages? Development 2008, 135, 193–205.
- Vincent, S.D.; Buckingham, M.E.; Peter, K. How to Make a Heart: The Origin and Regulation of Cardiac Progenitor Cells. Curr. Top Dev. Biol. 2010, 90, 1–41.
- van Weerd, J.H.; Christoffels, V.M. The formation and function of the cardiac conduction system. Development 2016, 143, 197–210.
- Bhattacharyya, S.; Munshi, N.V. Development of the Cardiac Conduction System. Cold Spring Harb. Perspect. Biol. 2020, 12, a037408.
- Mjaatvedt, C.H.; Yamamura, H.; Wessels, A.; Ramsdell, A.; Turner, D.; Markwald, R.R.; Rosenthal, N. 10—Mechanisms of Segmentation, Septation, and Remodeling of the Tubular Heart: Endocardial Cushion Fate and Cardiac Looping A2—Harvey, Richard P. In Heart Development; Academic Press: San Diego, CA, USA, 1999; pp. 159–177.
- Srivastava, D. Making or Breaking the Heart: From Lineage Determination to Morphogenesis. Cell 2006, 126, 1037–1048.
- George, R.M.; Maldonado-Velez, G.; Firulli, A.B. The heart of the neural crest: Cardiac neural crest cells in development and regeneration. Development 2020, 147, dev188706.
- Bardot, E.; Calderon, D.; Santoriello, F.; Han, S.; Cheung, K.; Jadhav, B.; Burtscher, I.; Artap, S.; Jain, R.; Epstein, J.; et al. Foxa2 identifies a cardiac progenitor population with ventricular differentiation potential. Nat. Commun. 2017, 8, 14428.
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct Compartments of the Proepicardial Organ Give Rise to Coronary Vascular Endothelial Cells. Dev. Cell 2012, 22, 639–650.
- Motoike, T.; Markham, D.W.; Rossant, J.; Sato, T.N. Evidence for novel fate of Flk1+ progenitor: Contribution to muscle lineage. Genesis 2003, 35, 153–159.
- Gaber, N.; Gagliardi, M.; Patel, P.; Kinnear, C.; Zhang, C.; Chitayat, D.; Shannon, P.; Jaeggi, E.; Tabori, U.; Keller, G.; et al. Fetal Reprogramming and Senescence in Hypoplastic Left Heart Syndrome and in Human Pluripotent Stem Cells during Cardiac Differentiation. Am. J. Pathol. 2013, 183, 720–734.
- Harding, J.; Bauer, M.; Kimble, R. Antenatal therapy for intrauterine growth retardation. Acta Paediatr. Suppl. 1997, 86, 196–200.
- Laviola, L.; Perrini, S.; Belsanti, G.; Natalicchio, A.; Montrone, C.; Leonardini, A.; Vimercati, A.; Scioscia, M.; Selvaggi, L.; Giorgino, R.; et al. Intrauterine Growth Restriction in Humans Is Associated with Abnormalities in Placental Insulin-Like Growth Factor Signaling. Endocrinology 2005, 146, 1498–1505.
- Marbán, E. A mechanistic roadmap for the clinical application of cardiac cell therapies. Nat. Biomed. Eng. 2018, 2, 353–361.
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22.
- Lässer, C.; Eldh, M.; Lötvall, J. Isolation and Characterization of RNA-Containing Exosomes. JoVE 2012, 59, e3037.
- Rosso, G.; Cauda, V. Biomimicking Extracellular Vesicles with Fully Artificial Ones: A Rational Design of EV-BIOMIMETICS toward Effective Theranostic Tools in Nanomedicine. ACS Biomater. Sci. Eng. 2022.
- Gurung, S.; Greening, D.W.; Catt, S.; Salamonsen, L.; Evans, J. Exosomes and soluble secretome from hormone-treated endometrial epithelial cells direct embryo implantation. Mol. Hum. Reprod. 2020, 26, 510–520.
- Cao, S.; Wu, Y.; Albert Reece, E.; Xu, C.; Shen, W.-B.; Kaushal, S.; Yang, P. Functional cargos of exosomes derived from Flk-1+ vascular progenitors enable neurulation and ameliorate embryonic anomalies in diabetic pregnancy. Commun. Biol. 2022, 5, 648.
- Chen, X.; Luo, Q. Potential clinical applications of exosomes in the diagnosis, treatment, and prognosis of cardiovascular diseases: A narrative review. Ann. Transl. Med. 2022, 10, 372.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Zhao, Q.; Beck, A.; Vitale, J.M.; Schneider, J.S.; Terzic, A.; Fraidenraich, D. Rescue of Developmental Defects by Blastocyst Stem Cell Injection: Towards Elucidation of Neomorphic Corrective Pathways. J. Cardiovasc. Transl. Res. 2010, 3, 66–72.
- Fraidenraich, D.; Stillwell, E.; Romero, E.; Wilkes, D.; Manova, K.; Basson, C.; Benezra, R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science 2004, 306, 247–252.
- Vermot, J.; Messaddeq, N.; Niederreither, K.; Dierich, A.; Dollé, P. Rescue of morphogenetic defects and of retinoic acid signaling in retinaldehyde dehydrogenase 2 (Raldh2) mouse mutants by chimerism with wild-type cells. Differentiation 2006, 74, 661–668.
- Santos, J.M.A.; Mendes-Silva, L.; Afonso, V.; Martins, G.; Machado, R.S.R.; Lopes, J.A.; Cancela, L.; Futschik, M.E.; Sachinidis, A.; Gavaia, P.; et al. Exogenous Wnt5a and Wnt11 proteins rescue Cited2 dysfunction in mouse embryonic stem cells and zebrafish morphants. Cell Death Dis. 2019, 10, 582.
- Bragança, J.; Mendes-Silva, L.; Lopes, J.; SM, C. CITED proteins in the heart of pluripotent cells and in heart’s full potential. Regen Med. Front. 2019, 1, e190005.
- Pacheco-Leyva, I.; Matias, A.C.; Oliveira, D.V.; Santos, J.M.A.; Nascimento, R.; Guerreiro, E.; Michell, A.C.; van De Vrugt, A.M.; Machado-Oliveira, G.; Ferreira, G.; et al. CITED2 Cooperates with ISL1 and Promotes Cardiac Differentiation of Mouse Embryonic Stem Cells. Stem Cell Rep. 2016, 7, 1037–1049.
- MacDonald, S.T.; Bamforth, S.D.; Bragança, J.; Chen, C.-M.; Broadbent, C.; Schneider, J.E.; Schwartz, R.J.; Bhattacharya, S. A cell-autonomous role of Cited2 in controlling myocardial and coronary vascular development. Eur. Heart J. 2013, 34, 2557–2567.
- Chen, C.-m.; Bentham, J.; Cosgrove, C.; Braganca, J.; Cuenda, A.; Bamforth, S.D.; Schneider, J.E.; Watkins, H.; Keavney, B.; Davies, B.; et al. Functional Significance of SRJ Domain Mutations in CITED2. PLoS ONE 2012, 7, e46256.
- MacDonald, S.T.; Bamforth, S.D.; Chen, C.-M.; Farthing, C.R.; Franklyn, A.; Broadbent, C.; Schneider, J.E.; Saga, Y.; Lewandoski, M.; Bhattacharya, S. Epiblastic Cited2 deficiency results in cardiac phenotypic heterogeneity and provides a mechanism for haploinsufficiency. Cardiovasc. Res. 2008, 79, 448–457.
- Bamforth, S.D.; Bragança, J.; Farthing, C.R.; Schneider, J.E.; Broadbent, C.; Michell, A.C.; Clarke, K.; Neubauer, S.; Norris, D.; Brown, N.A.; et al. Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nat. Genet. 2004, 36, 1189–1196.
- Bamforth, S.D.; Bragança, J.; Eloranta, J.J.; Murdoch, J.N.; Marques, F.I.R.; Kranc, K.R.; Farza, H.; Henderson, D.J.; Hurst, H.C.; Bhattacharya, S. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 2001, 29, 469–474.
- Xu, B.; Doughman, Y.; Turakhia, M.; Jiang, W.; Landsettle, C.E.; Agani, F.H.; Semenza, G.L.; Watanabe, M.; Yang, Y.-C. Partial rescue of defects in Cited2-deficient embryos by HIF-1 heterozygosity. Dev. Biol. 2007, 301, 130.
- Kranc, K.R.; Oliveira, D.V.; Armesilla-Diaz, A.; Pacheco-Leyva, I.; Matias, A.C.; Escapa, A.L.; Subramani, C.; Wheadon, H.; Trindade, M.; Nichols, J.; et al. Acute loss of Cited2 impairs Nanog expression and decreases self-renewal of mouse embryonic stem cells. Stem Cells 2015, 33, 699–712.
- Webber, S.A.; Wargowski, D.S.; Chitayat, D.; Sandor, G.G.S. Congenital heart disease and Robinow syndrome: Coincidence or an additional component of the syndrome? Am. J. Med. Genet. 1990, 37, 519–521.
- Li, P.; Li, H.; Zheng, Y.; Qiao, B.; Duan, W.; Huang, L.; Liu, W.; Wang, H. Variants in the Regulatory Region of WNT5A Reduced Risk of Cardiac Conotruncal Malformations in the Chinese Population. Sci. Rep. 2015, 5, 13120.
- Škorić-Milosavljević, D.; Tadros, R.; Bosada, F.M.; Tessadori, F.; Weerd, J.H.v.; Woudstra, O.I.; Tjong, F.V.Y.; Lahrouchi, N.; Bajolle, F.; Cordell, H.J.; et al. Common Genetic Variants Contribute to Risk of Transposition of the Great Arteries. Circ. Res. 2022, 130, 166–180.
- Sinha, T.; Li, D.; Théveniau-Ruissy, M.; Hutson, M.R.; Kelly, R.G.; Wang, J. Loss of Wnt5a disrupts second heart field cell deployment and may contribute to OFT malformations in DiGeorge syndrome. Hum. Mol. Genet. 2015, 24, 1704–1716.
- Yu, H.; Ye, X.; Guo, N.; Nathans, J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: Evidence for a network of interacting genes. Development 2012, 139, 4383–4394.
- Schleiffarth, J.R.; Person, A.D.; Martinsen, B.J.; Sukovich, D.J.; Neumann, A.; Baker, C.V.H.; Lohr, J.L.; Cornfield, D.N.; Ekker, S.C.; Petryk, A. Wnt5a Is Required for Cardiac Outflow Tract Septation in Mice. Pediatr. Res. 2007, 61, 386.
- Zhao, Y.; Kang, X.; Gao, F.; Guzman, A.; Lau, R.P.; Biniwale, R.; Wadehra, M.; Reemtsen, B.; Garg, M.; Halnon, N.; et al. Gene-environment regulatory circuits of right ventricular pathology in tetralogy of fallot. J. Mol. Med. 2019, 97, 1711–1722.
- Hayashi, K.; Erikson, D.W.; Tilford, S.A.; Bany, B.M.; Maclean, I.I.J.A.; Rucker, I.I.I.E.B.; Johnson, G.A.; Spencer, T.E. Wnt Genes in the Mouse Uterus: Potential Regulation of Implantation. Biol. Reprod. 2009, 80, 989–1000.
- Meinhardt, G.; Saleh, L.; Otti, G.R.; Haider, S.; Velicky, P.; Fiala, C.; Pollheimer, J.r.; Knöfler, M. Wingless ligand 5a is a critical regulator of placental growth and survival. Sci. Rep. 2016, 6, 28127.
- Andre, P.; Song, H.; Kim, W.; Kispert, A.; Yang, Y. Wnt5a and Wnt11 regulate mammalian anterior-posterior axis elongation. Development 2015, 142, 1516–1527.
- Sinha, T.; Lin, L.; Li, D.; Davis, J.; Evans, S.; Wynshaw-Boris, A.; Wang, J. Mapping the dynamic expression of Wnt11 and the lineage contribution of Wnt11-expressing cells during early mouse development. Dev. Biol. 2015, 398, 177–192.
- Bisson, J.A.; Mills, B.; Paul Helt, J.-C.; Zwaka, T.P.; Cohen, E.D. Wnt5a and Wnt11 inhibit the canonical Wnt pathway and promote cardiac progenitor development via the Caspase-dependent degradation of AKT. Dev. Biol. 2015, 398, 80–96.
- Cohen, E.D.; Miller, M.F.; Wang, Z.; Moon, R.T.; Morrisey, E.E. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development 2012, 139, 1931–1940.
- Chen, M.; Qian, C.; Bi, L.-L.; Zhao, F.; Zhang, G.-Y.; Wang, Z.-Q.; Gan, X.-D.; Wang, Y.-G. Enrichment of cardiac differentiation by a large starting number of embryonic stem cells in embryoid bodies is mediated by the Wnt11-JNK pathway. Biotechnol. Lett. 2015, 37, 475–481.
- Hardy, K.M.; Garriock, R.J.; Yatskievych, T.A.; D’Agostino, S.L.; Antin, P.B.; Krieg, P.A. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev. Biol. 2008, 320, 391–401.
- Vijayaragavan, K.; Szabo, E.; Bossé, M.; Ramos-Mejia, V.; Moon, R.T.; Bhatia, M. Noncanonical Wnt Signaling Orchestrates Early Developmental Events toward Hematopoietic Cell Fate from Human Embryonic Stem Cells. Cell Stem Cell 2009, 4, 248–262.
- Mastelaro de Rezende, M.; Zenker Justo, G.; Julian Paredes-Gamero, E.; Gosens, R. Wnt-5A/B Signaling in Hematopoiesis throughout Life. Cells 2020, 9, 1801.
- Huang, L.; Xiao, A.; Choi, S.Y.; Kan, Q.; Zhou, W.; Chacon-Heszele, M.F.; Ryu, Y.K.; McKenna, S.; Zuo, X.; Kuruvilla, R.; et al. Wnt5a Is Necessary for Normal Kidney Development in Zebrafish and Mice. Nephron Exp. Nephrol. 2014, 128, 80–88.
- Ryu, Y.K.; Collins, S.E.; Ho, H.-Y.H.; Zhao, H.; Kuruvilla, R. An autocrine Wnt5a-Ror signaling loop mediates sympathetic target innervation. Dev. Biol. 2013, 377, 79–89.
- Yang, R.; Goedel, A.; Kang, Y.; Si, C.; Chu, C.; Zheng, Y.; Chen, Z.; Gruber, P.J.; Xiao, Y.; Zhou, C.; et al. Amnion signals are essential for mesoderm formation in primates. Nat. Commun. 2021, 12, 5126.
This entry is offline, you can click here to edit this entry!
