Thyroid cancer (TC), the most frequent malignancy of the endocrine system, has recorded an increasing incidence in the last decades. The etiology of TC remains at least partly unknown and, among modifiable risk factors, the gut microbiota and dietary nutrients (vitamins, essential microelements, polyphenols, probiotics) have been recognized to not only influence thyroid function, but exert critical effects on TC development and progression. Recent discoveries on the existence of tumor microbiota also in the TC microenvironment provide further evidence for the essential role of tumor microorganisms in TC etiology and severity, as well as acting as prognostic markers and as a potential target of adjuvant care in the treatment of TC patients.
- antioxidant nutrients
- gut–thyroid axis
- microbiota
- oxidative stress
1. Introduction
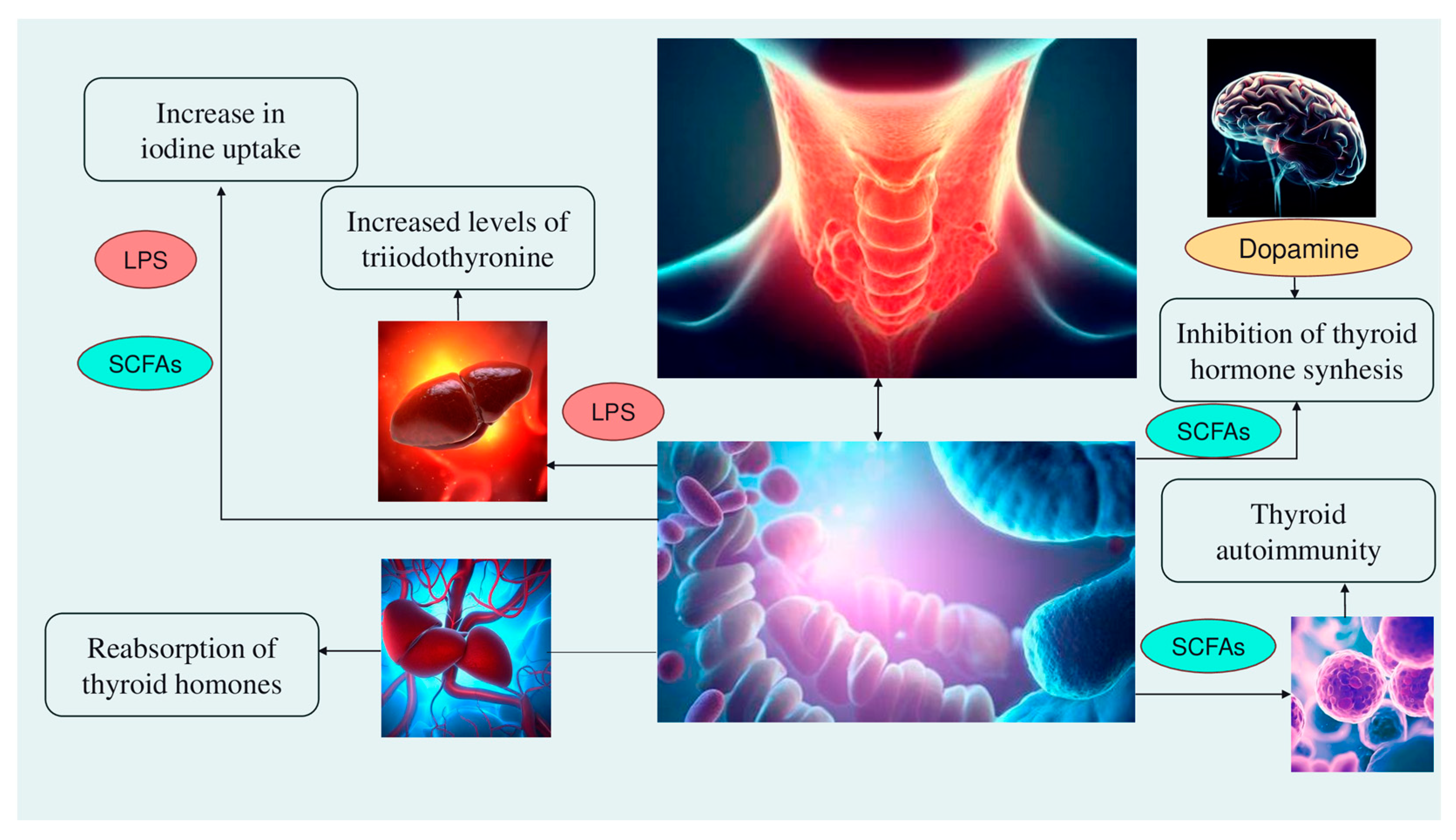
2. The Microbiota and Thyroid Axis
-
Alteration of iodine uptake, the main rate-limiting step in thyroid hormonogenesis, affecting the activity of sodium iodide symporter (NIS) through two processes: (a) The binding of the Gram-negative bacterial endotoxin lipopolysaccharide (LPS), released by the gut microbiota, to the thyroid cell toll-like receptor 4 (TLR-4). TLR-4 in turn activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), which subsequently promotes NIS transcription through paired box 8 (PAX8) [38]. (b) Alternatively, enhancement of NIS expression may also occur through histone deacetylase (HDAC) inhibition by an important metabolite of the gut microbiota, butyrate [39][40];
-
Modulation of activities of iodothyronine deiodinases, enzymes responsible for the conversion of thyroxine (T4) to its active form T3 by type 1 and type 2 deiodinases (D1, expressed mostly in the liver, kidney, thyroid, and pituitary, and D2, expressed primarily in the thyroid, central nervous system, pituitary, developing cochlea, brown adipose tissue, and skeletal muscle [41]) or to reverse T3, its inactive form, by type 3 deiodinase—D3 [42]. This occurs through a complex thyroid–gut axis pathway involving LPS capable of inducing the decrease in D1 activity in the liver [43] and, at the same time, activating D2 in the mediobasal hypothalamus, ultimately promoting the conversion of T4 to T3 [44];
-
Modulation of T3 and T4 bioavailability through the deconjugation of sulfoconjugated and glucuroconjugated iodothyronines by bacterial sulfate esterase or β-glucuronidase, respectively, thus inducing the reabsorption of thyroid hormones in the enterohepatic circulation. In humans, a recycling mechanism has been described for steroids hormones, biliary acids, and vitamins, while as for thyroid hormones, direct proof has been only established in animal models [31];
-
Regulation of the SCFAs-mediated balance between T helper 17 (Th17) cells and regulatory T cells (Treg), two subtypes of CD4+ lymphocytes exerting opposite effects (release of pro-inflammatory cytokines, i.e., interleukin—IL-17 or anti-inflammatory IL-10, respectively), in autoimmune inflammatory diseases and immune tolerance [35][45]. All these immune cells play a role in the pathogenesis of autoimmune thyroid disease (AITD), like Hashimoto disease (HD) and Graves’ disease. For instance, Prevotella is correlated with reduced proinflammatory Th17 polarization and increased differentiation of anti-inflammatory Treg; therefore, it has been speculated that the Th17/Treg homeostasis regulation might be a potential pathogenic pathway for Prevotella in HT patients [32][46];
-
Involvement of the microbiota–gut–brain signaling in dopamine release, synthesis, and bioavailability. Certain species up-/downregulate the system dopamine transporter/dopamine binding efficiency, while others are positively or negatively correlated with the activity of tyrosine hydroxylase, an enzyme involved in dopamine synthesis [47][48]. Furthermore, butyrate’s intrinsic HDAC inhibitor activity influences neurotransmitter levels [47]. Since dopamine inhibits synthesis and secretion of the thyroid-stimulating hormone (TSH), thyroid function may be affected [35] (Figure 1).
3. The Association between Microbiota and Thyroid Cancer
Microbial Communities in Thyroid Cancer Tissues
This entry is adapted from the peer-reviewed paper 10.3390/antiox12101898
References
- Liu, C.J.; Chen, S.Q.; Zhang, S.Y.; Wang, J.L.; Tang, X.D.; Yang, K.X.; Li, X.R. The comparison of microbial communities in thyroid tissues from thyroid carcinoma patients. J. Microbiol. 2021, 59, 988–1001.
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297.
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272.
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348.
- Olson, E.; Wintheiser, G.; Wolfe, K.M.; Droessler, J.; Silberstein, P.T. Epidemiology of Thyroid Cancer: A Review of the National Cancer Database, 2000–2013. Cureus 2019, 11, e4127.
- Kitahara, C.M.; Sosa, J.A.; Shiels, M.S. Influence of Nomenclature Changes on Trends in Papillary Thyroid Cancer Incidence in the United States, 2000 to 2017. J. Clin. Endocrinol. Metab. 2020, 105, e4823–e4830.
- Chung, R.; Guan, H.; Ponchiardi, C.; Cerda, S.; Marwaha, N.; Yilmaz, O.H.; Pinjic, E.; McAneny, D.; Lee, S.L.; Drake, F.T. Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: Epidemiology and Long-Term Outcomes in a Strictly Defined Cohort. Thyroid 2021, 31, 68–75.
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795.
- Zhao, H.; Liu, C.H.; Cao, Y.; Zhang, L.Y.; Zhao, Y.; Liu, Y.W.; Liu, H.F.; Lin, Y.S.; Li, X.Y. Survival prognostic factors for differentiated thyroid cancer patients with pulmonary metastases: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 990154.
- Fiore, M.; Cristaldi, A.; Okatyeva, V.; Lo Bianco, S.; Oliveri Conti, G.; Zuccarello, P.; Copat, C.; Caltabiano, R.; Cannizzaro, M.; Ferrante, M. Dietary habits and thyroid cancer risk: A hospital-based case-control study in Sicily (South Italy). Food Chem. Toxicol. 2020, 146, 111778.
- Barrea, L.; Gallo, M.; Ruggeri, R.M.; Giacinto, P.D.; Sesti, F.; Prinzi, N.; Adinolfi, V.; Barucca, V.; Renzelli, V.; Muscogiuri, G.; et al. Nutritional status and follicular-derived thyroid cancer: An update. Crit. Rev. Food Sci. Nutr. 2021, 61, 25–59.
- Dai, D.; Yang, Y.; Yang, Y.; Dang, T.; Xiao, J.; Wang, W.; Teng, L.; Xu, J.; Ye, J.; Jiang, H. Alterations of thyroid microbiota across different thyroid microhabitats in patients with thyroid carcinoma. J. Transl. Med. 2021, 19, 488.
- Kun, Y.; Wei, X.; Wang, H.; Nie, X.; Dai, Q. Exploring the oral-gut microbiota during thyroid cancer: Factors affecting the thyroid functions and cancer development. Food Sci. Nutr. 2023, 11, 5657–5674.
- Zhang, X.; Zhang, F.; Li, Q.; Feng, C.; Teng, W. Iodine nutrition and papillary thyroid cancer. Front. Nutr. 2022, 9, 1022650.
- Macvanin, M.T.; Gluvic, Z.; Zafirovic, S.; Gao, X.; Essack, M.; Isenovic, E.R. The protective role of nutritional antioxidants against oxidative stress in thyroid disorders. Front. Endocrinol. 2023, 13, 1092837.
- Heydarzadeh, S.; Kia, S.K.; Zarkesh, M.; Pakizehkar, S.; Hosseinzadeh, S.; Hedayati, M. The Cross-Talk between Polyphenols and the Target Enzymes Related to Oxidative Stress-Induced Thyroid Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 2724324.
- Muzza, M.; Pogliaghi, G.; Colombo, C.; Carbone, E.; Cirello, V.; Palazzo, S.; Frattini, F.; Gentilini, D.; Gazzano, G.; Persani, L.; et al. Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers 2022, 14, 5857.
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135.
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563.
- Feng, J.; Zhao, F.; Sun, J.; Lin, B.; Zhao, L.; Liu, Y.; Jin, Y.; Li, S.; Li, A.; Wei, Y. Alterations in the gut microbiota and metabolite profiles of thyroid carcinoma patients. Int. J. Cancer 2019, 144, 2728–2745.
- Liu, S.; An, Y.; Cao, B.; Sun, R.; Ke, J.; Zhao, D. The Composition of Gut Microbiota in Patients Bearing Hashimoto’s Thyroiditis with Euthyroidism and Hypothyroidism. Int. J. Endocrinol. 2020, 2020, 5036959.
- Su, X.; Zhao, Y.; Li, Y.; Ma, S.; Wang, Z. Gut dysbiosis is associated with primary hypothyroidism with interaction on gut-thyroid axis. Clin. Sci. 2020, 134, 1521–1535.
- Jiang, W.; Yu, X.; Kosik, R.O.; Song, Y.; Qiao, T.; Tong, J.; Liu, S.; Fan, S.; Luo, Q.; Chai, L.; et al. Gut Microbiota May Play a Significant Role in the Pathogenesis of Graves’ Disease. Thyroid 2021, 31, 810–820.
- Liu, J.; Qin, X.; Lin, B.; Cui, J.; Liao, J.; Zhang, F.; Lin, Q. Analysis of gut microbiota diversity in Hashimoto’s thyroiditis patients. BMC Microbiol. 2022, 22, 318.
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Díaz-Urbina, D.; Chirino, Y.I.; Pedraza-Chaverri, J. Food additives containing nanoparticles induce gastrotoxicity, hepatotoxicity and alterations in animal behavior: The unknown role of oxidative stress. Food Chem. Toxicol. 2020, 146, 111814.
- Naliyadhara, N.; Kumar, A.; Gangwar, S.K.; Devanarayanan, T.N.; Hegde, M.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakara, A. Interplay of dietary antioxidants and gut microbiome in human health: What has been learnt thus far? J. Funct. Foods. 2023, 100, 105365.
- Chen, Y.; Wu, F.H.; Wu, P.Q.; Xing, H.Y.; Ma, T. The Role of The Tumor Microbiome in Tumor Development and Its Treatment. Front. Immunol. 2022, 13, 935846.
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor microbiome—An integral part of the tumor microenvironment. Front. Oncol. 2022, 12, 1063100.
- Stone, T.W.; Darlington, L.G. Microbial carcinogenic toxins and dietary anti-cancer protectants. Cell. Mol. Life Sci. 2017, 74, 2627–2643.
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552.
- Virili, C.; Centanni, M. “With a little help from my friends”—The role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Mol. Cell Endocrinol. 2017, 458, 39–43.
- Jiang, W.; Lu, G.; Gao, D.; Lv, Z.; Li, D. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front. Endocrinol. 2022, 13, 943408.
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587.
- Liu, Q.; Sun, W.; Zhang, H. Interaction of Gut Microbiota with Endocrine Homeostasis and Thyroid Cancer. Cancers 2022, 14, 2656.
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769.
- Sirakov, M.; Plateroti, M. The thyroid hormones and their nuclear receptors in the gut: From developmental biology to cancer. Biochim. Biophys. Acta. 2011, 1812, 938–946.
- Fenneman, A.C.; Bruinstroop, E.; Nieuwdorp, M.; van der Spek, A.H.; Boelen, A.A. Comprehensive Review of Thyroid Hormone Metabolism in the Gut and Its Clinical Implications. Thyroid 2023, 33, 32–44.
- Nicola, J.P.; Nazar, M.; Mascanfroni, I.D.; Pellizas, C.G.; Masini-Repiso, A.M. NF-kappaB p65 subunit mediates lipopolysaccharide-induced Na(+)/I(-) symporter gene expression by involving functional interaction with the paired domain transcription factor Pax8. Mol. Endocrinol. 2010, 24, 1846–1862.
- Puppin, C.; D’Aurizio, F.; D’Elia, A.V.; Cesaratto, L.; Tell, G.; Russo, D.; Filetti, S.; Ferretti, E.; Tosi, E.; Mattei, T.; et al. Effects of histone acetylation on sodium iodide symporter promoter and expression of thyroid-specific transcription factors. Endocrinology 2005, 146, 3967–3974.
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940.
- Luongo, C.; Trivisano, L.; Alfano, F.; Salvatore, D. Type 3 deiodinase and consumptive hypothyroidism: A common mechanism for a rare disease. Front. Endocrinol. 2013, 4, 115.
- Fröhlich, E.; Wahl, R. Microbiota and thyroid interaction in health and disease. Trends Endocrinol. Metab. 2019, 30, 479–490.
- Boelen, A.; Kwakkel, J.; Alkemade, A.; Renckens, R.; Kaptein, E.; Kuiper, G.; Wiersinga, W.M.; Visser, T.J. Induction of type 3 deiodinase activity in inflammatory cells of mice with chronic local inflammation. Endocrinology 2005, 146, 5128–5134.
- Fekete, C.; Gereben, B.; Doleschall, M.; Harney, J.W.; Dora, J.M.; Bianco, A.C.; Sarkar, S.; Liposits, Z.; Rand, W.; Emerson, C.; et al. Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: Implications for the nonthyroidal illness syndrome. Endocrinology 2004, 145, 1649–1655.
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cells in vitro. Immunol. Invest. 2016, 45, 205–222.
- Li, J.; Sung, C.Y.; Lee, N.; Ni, Y.; Pihlajamaki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315.
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436.
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011, 508, 1–12.
- Azevedo, M.M.; Pina-Vaz, C.; Baltazar, F. Microbes and Cancer: Friends or Faux? Int. J. Mol. Sci. 2020, 21, 3115.
- Veziant, J.; Villéger, R.; Barnich, N.; Bonnet, M. Gut Microbiota as Potential Biomarker and/or Therapeutic Target to Improve the Management of Cancer: Focus on Colibactin-Producing Escherichia coli in Colorectal Cancer. Cancers 2021, 13, 2215.
- Lucas, C.; Barnich, N.; Nguyen, H.T.T. Microbiota, Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 1310.
- Wang, X.; Yang, Y.; Huycke, M.M. Microbiome-driven carcinogenesis in colorectal cancer: Models and mechanisms. Free Radic. Biol. Med. 2017, 105, 3–15.
- Wang, L.; Yu, K.C.; Hou, Y.Q.; Guo, M.; Yao, F.; Chen, Z.X. Gut microbiome in tumorigenesis and therapy of colorectal cancer. J. Cell. Physiol. 2023, 238, 94–108.
- Yasunaga, J.I.; Matsuoka, M. Oncogenic spiral by infectious pathogens: Cooperation of multiple factors in cancer development. Cancer Sci. 2018, 109, 24–32.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–441.
- Locey, K.J.; Lennon, J.T. Scaling laws predict global microbial diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 5970–5975.
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580.
- Ni, J.; Huang, R.; Zhou, H.; Xu, X.; Li, Y.; Cao, P.; Zhong, K.; Ge, M.; Chen, X.; Hou, B.; et al. Analysis of the Relationship Between the Degree of Dysbiosis in Gut Microbiota and Prognosis at Different Stages of Primary Hepatocellular Carcinoma. Front. Microbiol. 2019, 10, 1458.
- Zhang, J.; Zhang, F.; Zhao, C.; Xu, Q.; Liang, C.; Yang, Y.; Wang, H.; Shang, Y.; Wang, Y.; Mu, X.; et al. Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine 2019, 64, 564–574.
- Gantuya, B.; El-Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Oyuntsetseg, K.; Azzaya, D.; Uchida, T.; Yamaoka, Y. Gastric Microbiota in Helicobacter pylori-Negative and -Positive Gastritis Among High Incidence of Gastric Cancer Area. Cancers 2019, 11, 504.
- Sohn, S.H.; Kim, N.; Jo, H.J.; Kim, J.; Park, J.H.; Nam, R.H.; Seok, Y.J.; Kim, Y.R.; Lee, D.H. Analysis of Gastric Body Microbiota by Pyrosequencing: Possible Role of Bacteria Other Than Helicobacter pylori in the Gastric Carcinogenesis. J. Cancer Prev. 2017, 22, 115–125.
- Kumar, R.; Herold, J.L.; Schady, D.; Davis, J.; Kopetz, S.; Martinez-Moczygemba, M.; Murray, B.E.; Han, F.; Li, Y.; Callaway, E.; et al. Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development. PLoS Pathog. 2017, 13, e1006440.
- Zhao, J.; Nian, L.; Kwok, L.Y.; Sun, T.; Zhao, J. Reduction in fecal microbiota diversity and short-chain fatty acid producers in Methicillin-resistant Staphylococcus aureus infected individuals as revealed by PacBio single molecule, real-time sequencing technology. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1463–1472.
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25.
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105.
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892.
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86.
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66.
- Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An Element of Life Essential for Thyroid Function. Molecules 2021, 26, 7084.
- Li, J.; Xie, H.; Li, A.; Cheng, J.; Yang, K.; Wang, J.; Wang, W.; Zhang, F.; Li, Z.; Dhillon, H.S.; et al. Distinct plasma lipids profiles of recurrent ovarian cancer by liquid chromatography-mass spectrometry. Oncotarget 2017, 8, 46834–46845.
- Shen, S.; Yang, L.; Li, L.; Bai, Y.; Cai, C.; Liu, H. A plasma lipidomics strategy reveals perturbed lipid metabolic pathways and potential lipid biomarkers of human colorectal cancer. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1068–1069, 41–48.
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2020, 2, 27–59.
- Simsir, Y.I.; Cetinkalp, S.; Kabalak, T. Review of Factors Contributing to Nodular Goiter and Thyroid Carcinoma. Med. Princ. Pract. 2020, 29, 1–5.
- Azuma, H.; Inamoto, T.; Sakamoto, T.; Kiyama, S.; Ubai, T.; Shinohara, Y.; Maemura, K.; Tsuji, M.; Segawa, N.; Masuda, H.; et al. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res. 2003, 63, 8090–8096.
- Roberts, S.S.; Mendonça-Torres, M.C.; Jensen, K.; Francis, G.L.; Vasko, V. GABA receptor expression in benign and malignant thyroid tumors. Pathol. Oncol. Res. 2009, 15, 645–650.
- Huang, D.; Wang, Y.; Thompson, J.W.; Yin, T.; Alexander, P.B.; Qin, D.; Mudgal, P.; Wu, H.; Liang, Y.; Tan, L.; et al. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat. Cell Biol. 2022, 24, 230–241.
- Lau, A.; Tymianski, M. Excitotoxicity. In Comprehensive Toxicology, 2nd ed.; Elsevier: McQueen, CA, USA, 2010; Volume 13, pp. 515–535.
- Qian, B.; Shen, S.; Zhang, J.; Jing, P. Effects of Vitamin B6 Deficiency on the Composition and Functional Potential of T Cell Populations. J. Immunol. Res. 2017, 2017, 2197975.
- Yu, X.; Jiang, W.; Kosik, R.O.; Song, Y.; Luo, Q.; Qiao, T.; Tong, J.; Liu, S.; Deng, C.; Qin, S.; et al. Gut microbiota changes and its potential relations with thyroid carcinoma. J. Adv. Res. 2021, 35, 61–70.
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715.
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes Nutr. 2022, 17, 2.
- Yuan, L.; Yang, P.; Wei, G.; Hu, X.; Chen, S.; Lu, J.; Yang, L.; He, X.; Bao, G. Tumor microbiome diversity influences papillary thyroid cancer invasion. Commun. Biol. 2022, 5, 864.
- Xue, C.; Chu, Q.; Zheng, Q.; Yuan, X.; Su, Y.; Bao, Z.; Lu, J.; Li, L. Current understanding of the intratumoral microbiome in various tumors. Cell Rep. Med. 2023, 4, 100884.
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980.
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59.
- Rossi, T.; Vergara, D.; Fanini, F.; Maffia, M.; Bravaccini, S.; Pirini, F. Microbiota-Derived Metabolites in Tumor Progression and Metastasis. Int. J. Mol. Sci. 2020, 21, 5786.
- Scales, B.S.; Dickson, R.P.; LiPuma, J.J.; Huffnagle, G.B. Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin. Microbiol. Rev. 2014, 27, 927–948.
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131.
- Taglialegna, A. Pseudomonas against cancer. Nat. Rev. Microbiol. 2023, 21, 131.
- Chaudhary, P.P.; Conway, P.L.; Schlundt, J. Methanogens in humans: Potentially beneficial or harmful for health. Appl. Microbiol. Biotechnol. 2018, 102, 3095–3104.
- Liu, Y.; O’Brien, J.L.; Ajami, N.J.; Scheurer, M.E.; Amirian, E.S.; Armstrong, G.; Tsavachidis, S.; Thrift, A.P.; Jiao, L.; Wong, M.C.; et al. Lung tissue microbial profile in lung cancer is distinct from emphysema. Am. J. Cancer Res. 2018, 8, 1775–1787.
- Liu, X.; Shao, L.; Liu, X.; Ji, F.; Mei, Y.; Cheng, Y.; Liu, F.; Yan, C.; Li, L.; Ling, Z. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine 2019, 40, 336–348.
- Higuchi, R.; Goto, T.; Hirotsu, Y.; Otake, S.; Oyama, T.; Amemiya, K.; Ohyama, H.; Mochizuki, H.; Omata, M. Sphingomonas and Phenylobacterium as Major Microbiota in Thymic Epithelial Tumors. J. Pers. Med. 2021, 11, 1092.
- Richard, M.L.; Liguori, G.; Lamas, B.; Brandi, G.; da Costa, G.; Hoffmann, T.W.; Di Simone, P.M.; Calabrese, C.; Poggioli, G.; Langella, P.; et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes 2018, 9, 131–142.
- Tanaka, T.; Matsuno, Y.; Torisu, T.; Shibata, H.; Hirano, A.; Umeno, J.; Kawasaki, K.; Fujioka, S.; Fuyuno, Y.; Moriyama, T.; et al. Gastric microbiota in patients with Helicobacter pylori-negative gastric MALT lymphoma. Medicine 2021, 100, e27287.
- Jeong, J.Y.; Kim, T.B.; Kim, J.; Choi, H.W.; Kim, E.J.; Yoo, H.J.; Lee, S.; Jun, H.R.; Yoo, W.; Kim, S.; et al. Diversity in the Extracellular Vesicle-Derived Microbiome of Tissues According to Tumor Progression in Pancreatic Cancer. Cancers 2020, 12, 2346.
- Gnanasekar, A.; Castaneda, G.; Iyangar, A.; Magesh, S.; Perez, D.; Chakladar, J.; Li, W.T.; Bouvet, M.; Chang, E.Y.; Ongkeko, W.M. The intratumor microbiome predicts prognosis across gender and subtypes in papillary thyroid carcinoma. Comput. Struct. Biotechnol. J. 2021, 19, 1986–1997.
- Ianniello, N.M.; Andrade, D.C.; Ivancic, S.; Eckardt, P.A.; Lemos Ramirez, J.C. Native valve infective endocarditis due to Micrococcus luteus in a non-Hodgkin’s lymphoma patient. IDCases 2019, 18, e00657.
- Martín Guerra, J.M.; Martín Asenjo, M.; Rodríguez Martín, C. Bacteraemia by Micrococcus luteus in an inmunocompromised patient. Med. Clin. 2019, 152, 469–470.
- Quach, J.U.; Diaz, M.J.; Huda, T.I.; Kinskey, J.C.; Zaman, S.; Desantis, J.E.; Cios, K.J.; Blanck, G. Bacterial Sequencing Reads in Blood Exome Files from Melanoma and Cervical Cancer Patients are Associated with Cancer Recurrence. Mol. Biotechnol. 2023, 65, 1476–1484.
- Ding, J.; Wu, W.; Fang, J.; Zhao, J.; Jiang, L. Male sex is associated with aggressive behaviour and poor prognosis in Chinese papillary thyroid carcinoma. Sci. Rep. 2020, 10, 4141.
- Kiseleva, E.P.; Mikhailopulo, K.I.; Sviridov, O.V.; Novik, G.I.; Knirel, Y.A.; Szwajcer Dey, E. The role of components of Bifidobacterium and Lactobacillus in pathogenesis and serologic diagnosis of autoimmune thyroid diseases. Benef. Microbes 2011, 2, 139–154.
