Colorectal cancer is one of the leading causes of morbidity and mortality today. Knowledge of its pathogenesis has made it possible to advance the development of different therapeutic strategies. However, the appearance of drug resistance constitutes one of the main causes of treatment failure. Bioactive compounds of vegetable origin are being studied as a new strategy to improve antitumor treatment, due to their ability to regulate the pathways involved in the development of carcinogenesis or processes that are decisive in its evolution, including multidrug resistance. In vitro and in vivo studies of these substances in combination with cytotoxic drugs have shown that they reduce resistance and increase therapeutic efficacy. The objective of this review is to summarize the knowledge that is described in the scientific literature on the antitumor and chemo-sensitizing capacity of vegetable-derived biomolecules such as polyphenols, flavonoids, and terpenes. These compounds may hold a promising future in improving the treatment of colorectal cancer.
2. Colorectal Cancer: Resistant Mechanisms
Despite the discovery of new drugs against CRC, the emergence of resistance to these agents is inevitable. Drug resistance can be innate (dysregulations of tumor cells before treatment) or acquired (resistance after treatment cycles) [
25,
26]. Drug efflux mediated by transmembrane transporters, specifically those of the ATP binding cassette superfamily (ABC), is one of most relevant mechanisms in CRC. These proteins are capable of expelling toxic substances from the inside of cells including different anticancer agents [
27]. Specifically, p-GP, encoded by the ABCB1 gene, was overexpressed in different CRC cell lines, conferring resistance to treatment. In addition, resistant CRC cells overexpressed CD133, a protein that regulates p-GP expression through the AKT/NF-κB/MDR1 axis [
28,
29]. Other members of the ABC family, such as MRP1 and BCRP, are also overexpressed in some CRC cell lines, leading to multiple resistance to chemotherapeutic drugs as 5-FU, doxorubicin, irinotecan, vincristine, among others [
30,
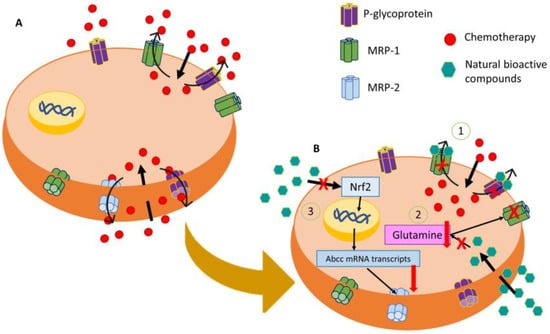
31], while MRP2-mediated resistance to oxaliplatin and vincristine in CRC, being Nrf2, signaling is critical for its expression (
Figure 1) [
32]. Drug resistance in CRC can also arise when there are alterations in antitumor drug targets, such as mutations or changes in expression due to epigenetic variations [
33]. Finally, an increase in the expression of repair protein-DNA, such as MGMT, was detected in some 5-FU-resistant CRC lines [
34].
Figure 1. Representative image of modulation of the MDR mechanism of resistance by natural bioactive compounds. (A) Main membrane transporters that are involved in chemoresistance by expelling them into the extracellular medium. (B) Action of natural bioactive compounds in the regulation of MDR by (1) MRP-2 downregulation through the inhibition of the Nrf2/MRP2 pathway, (2) P-glycoprotein and MRP-1 blocking by natural bioactive compounds, and (3) inhibition of glutamine cell intake producing an MRP-1 disfunction.
Apoptosis evasion promotes carcinogenesis and tumor progression, leading to the appearance of pharmacological resistance, especially to drugs that induce this pathway such as doxorubicin and cisplatin. Several apoptosis-resistant tumors were associated with the increased or decreased expression of antiapoptotic (BCL-2, MCL-1, and BCL-XL) and proapoptotic (p53, BAX, and BIM) genes, respectively [
35]. In addition, modulation of DNA methylation, histones and chromatin remodeling can alter the expression of genes that are involved in the metabolism and activity of chemotherapeutic drugs, inducing resistance [
33]. Moreover, tumor heterogeneity also plays a role in this phenomenon, as it makes treatment more difficult because of the presence of cancer stem cells which are more resistant to drugs. These cells have a self-healing and differentiation capacity and are associated with greater tumorigenicity. These cells are also capable of acquiring mesenchymal characteristics, which is related to the cell migration process and metastasis and a worse prognosis in patients [
36,
37]. On the other hand, it is known that the most resistant cells within the tumor sinus can transfer small miRNAs to their environment, inducing resistance in neighboring cells [
38]. Finally, another important factor to highlight is the tumor microenvironment, including the extracellular matrix, blood vessels, fibroblast, and immune system cells. This microenvironment will be an additional layer of protection against drugs, making the entry of chemotherapeutics into the tumor sinus more limited [
39].
2. Antitumor Potential of Natural Products for Colorectal Cancer
Chemotherapeutic treatment of colon cancer has been compromised mainly by the appearance of resistance, which reduces therapeutic efficacy and leads to a lower cure rate and worse prognosis. These can be produced by the existence of previous mutations in genes that are involved in resistance, by the activation of cellular pathways that are involved in cellular detoxification, and the existence of transmembrane transporters that expel the drugs to the exterior (such as p-glycoprotein) [
128]. In addition to this protein, other members of the transmembrane protein family such as MRP1 and BCRP are also overexpressed in CRC, and a relationship has been observed between their expression and resistance phenomena against drugs that are frequently used in this type of tumor, such as 5-FU and doxorubicin [
30,
31]. It has long been shown that plant compounds can be used as a therapy for different types of cancer. Thus, a plant-derived compound such as taxol has been used for years as a chemotherapeutic agent and is currently used as a therapy in non-small cell lung cancer (NSCLC), breast, pancreatic, and cervical cancer [
129]. These types of compounds can exert their actions at the molecular level through processes such as the regulation of oxidative stress or epigenetic modification in cells [
130].
It has been shown that the most studied compounds of plant origin in CRC are polyphenols, specifically resveratrol and curcumin (
Table 1). Resveratrol belongs to the stilbenes group and has been shown to possess high antioxidant and antitumor activity, causing cell death through the induction of apoptosis and autophagy. Similar to resveratrol, the other polyphenols that were studied induce death through these pathways [
48,
67,
73,
83].
Table 1. Summary of the in vitro and in vivo effects that were exerted by the bioactive natural compounds that were analyzed.
Polyphenols have been classified as chemopreventives and chemotherapeutics, although their sensitizing effect has also been observed in in vitro and in vivo models. These compounds can modulate drug resistance by increasing drug internalization into the cell, decreasing enzymes that are responsible for drug degradation (such as glutathione-S-transferases and cytochromes) and reducing the expression of transmembrane detoxifying proteins in the cell. In addition, they can induce apoptosis, oxidative stress damage, and inhibit metastasis-triggering processes such as EMT [
131]. The alteration of all these resistance mechanisms implies that these polyphenols have been shown to be effective in combination with traditional drugs such as 5-FU or oxaliplatin [
56,
73,
75,
87,
88,
89]. This effect is also observed in foods with high polyphenol contents such as the strawberry tree honey, that chemosensitizes the drug 5-FU in colon cancer lines such as HCT116 and LoVo [
132]. In addition to this, it has been observed that curcumin and resveratrol had the capacity to inhibit tumor proliferation in in vivo models and prevented the formation of tumor precursor lesions, with an increase in apoptosis of induced tumor tissues [
47,
53,
76]. Pharmacokinetic studies have been carried out in humans, where low bioavailability has been observed [
47,
52,
54,
71,
75]. Given the low bioavailability of these compounds, the use of nanotechnology for their encapsulation could help stabilize the compound and prevent its degradation in blood. In addition, the use of nanotechnology allows specific targeting of tumor cells through their functionalization with antibodies or peptides [
14,
15].
On the other hand, it has been observed that flavonoid compounds also have anticarcinogenic potential. Among them, the most investigated in CRC have been quercetin and EGCG. These compounds exerted their cytotoxic effect by producing cycle arrest, inhibiting key pathways in tumor development such as PI3K/AKT/mTOR and the MAPK pathway, and inhibiting processes that are linked to tumor progression such as cell migration [
93,
99,
100]. In addition, they showed a great chemosensitizing capacity in combination with traditional drugs such as doxorubicin, irinotecan, 5-FU, cisplatin, and oxaliplatin under in vitro and in vivo conditions. A study that was conducted by Hassanein et al. [
133] showed the chemopreventive effect of EGCG administration together with sulindac, a non-steroidal anti-inflammatory drug, showing that it was able to decrease the production of neoplastic lesions in in vivo models of CRC. As polyphenols, these compounds have a low bioavailability, which is a limitation for their use in humans [
18,
24,
87,
88,
89,
97,
101,
102,
103,
105]. In this context, the synthesis of gold NPs encapsulating EGCG has been shown to be an effective therapy against tumor cells while it has been shown that the co-encapsulation of EGCG and 5-FU in NPs allows an increase in the effect of both compounds separately, producing an anti-angiogenic and pro-apoptotic effect [
106,
134]. The encapsulation of this compound in nanoformulations would increase the half-life of the compound in serum, increasing its bioavailability and increasing its antitumor effect [
135].
The bibliographic analysis showed that terpenes are the least studied bioactive compounds as chemosensitizers in this type of cancer. Among this family, geraniol and ginsenosides have been the most studied compounds with sensitizing properties in CRC [
114,
117,
118]. Due to the small number of studies that have been conducted on these compounds, it is complicated for these results to be transferred to clinical studies at present. These compounds exert their antiproliferative activity by producing cell cycle arrest and inducing apoptotic pathways. In addition, it has been observed that they suppress pathways essential for tumor development such as PI3K/AKT/mTOR [
115,
119,
120]. Results in in vivo models showed that geraniol sensitized tumors that were induced in mice from a 5-FU-resistant CRC line to the drug, while the major ginsenoside (Rg3) showed synergy with 5-FU in tumors that were generated from the SW620 and LoVo cell lines. However, it has been shown that these compounds have clear preventive and therapeutic properties in CRC [
115,
116,
123].
This entry is adapted from the peer-reviewed paper 10.3390/app13042667