Euzebya and other haloalkaliphilic bacteria can thrive under harsh conditions, such as high concentrations of sodium and/or calcium, high electric conductivity and alkaline pH, highly variable temperatures, and water fluctuations. These conditions are quasi-extreme in the studied terrestrial environments.
- biofilms
- caves
- soils
- Euzebya
1. Introduction
2. Metagenomic Detection of Euzebya in the Environment: Caves
The data revealed that Euzebyaceae were abundant in Covadura Cave white biofilms collected in 2010, but their relative abundance was drastically reduced in the 2022 sampling. This could be associated with the severe droughts, the last of which occurred between 2017 and 2018, and which continue until now. In the yellow biofilms, the decrease in abundance of Euzebyaceae was lower.
3. Euzebyales in Extreme Environments
Deserts, cover around 33% of the planet’s surface. The use of molecular tools (NGS) revealed dominant members of the extremophilic microbial communities that have not been yet isolated. They included Euzebya, both in cold environments (Antarctica) and hot deserts (Atacama, Sahara, Colorado Plateau, etc.) [54][55][56][57][58][59][60][61][62][63][64][65].
4. Euzebyales in Soils and Other Diverse Environments
5. Relationship of Euzebyales with Other Members of Microbial Communities in Diverse Environments
6. Culture Media for the Isolation of Euzebya in Terrestrial Environments
7. Attempts to Isolate Euzebya from Pindal Cave
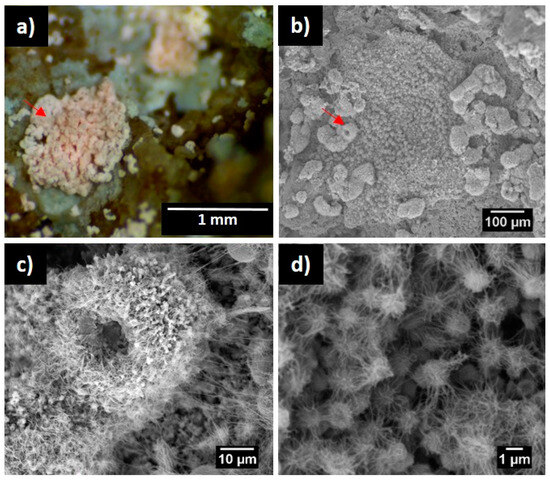
Culture media reproducing the environmental conditions reported in terrestrial ecosystems, e.g., SN medium and marine agar (including 1/10 dilutions of these media), pH 9–10, and sodium chloride concentrations around 3% or more, could allow the isolation of terrestrial Euzebya and other haloalkaliphilic genera. Marine agar and SN medium [116] have been used for the isolation of marine Euzebya [14][15][16]. Alternatively, for maintaining a high pH, the medium Z8-NK, as described by Flores et al. [117], R2A, and/or other media with the addition of trace elements, amino acids, vitamins, and simple carbon sources to a minimal culture medium should be explored.
8. Concluding Remarks
The data indicate that Euzebya is present across the entire biosphere. The question is whether their species were dispersed from marine sources to the terrestrial environment or if they are truly terrestrial, not yet described, species.
Here, it is shown that Euzebya and other bacteria can thrive under harsh conditions, such as high concentrations of sodium and/or calcium, high electric conductivity, alkaline pH, and highly variable temperature and water fluctuations. These ecological conditions in the studied terrestrial environments are quasi-extreme.
Unfortunately, the culture media used so far for the isolation of Euzebya failed to reproduce the original conditions of these harsh terrestrial ecosystems and this could be the reason why strains of Euzebya and other bacteria that inhabit the same niche were not isolated.
Some of the pitfalls and limitations of commonly used culture media and possible solutions to challenges faced in isolating terrestrial Euzebya strains are presented. The importance of combining high-throughput sequencing and cultivation techniques is of the utmost interest for this task. Data on the physicochemical and environmental parameters of the terrestrial ecosystems where Euzebya thrives should be taken into account when designing appropriate culture media.
It is expected that the interest in the biogeochemical role and geographical distribution of Euzebya will promote the optimization of culture media, and in this way, researchers will be able to isolate novel Euzebya species from different terrestrial environments.
This entry is adapted from the peer-reviewed paper 10.3390/app13179644
References
- Herndl, G.J.; Bayer, B.; Baltar, F.; Reinthaler, T. Prokaryotic life in the deep ocean’s water column. Annu. Rev. Mar. Sci. 2023, 15, 461–483.
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563.
- Eom, S.-H.; Kim, Y.-M.; Kim, S.-K. Marine bacteria: Potential sources for compounds to overcome antibiotic resistance. Appl. Microbiol. Biotechnol. 2013, 97, 4763–4773.
- Andryukov, B.; Mikhailov, V.; Besednova, N. The biotechnological potential of secondary metabolites from marine bacteria. J. Mar. Sci. Eng. 2019, 7, 176.
- Sun, W.; Wu, W.; Liu, X.; Zaleta-Pinet, D.A.; Clark, B.R. Bioactive compounds isolated from marine-derived microbes in China: 2009–2018. Mar. Drugs 2019, 17, 339.
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M. Marine natural products. Nat. Prod. Rep. 2023, 40, 275.
- Siro, G.; Donald, L.; Pipite, A. The diversity of deep-sea actinobacteria and their natural products: An epitome of curiosity and drug discovery. Diversity 2023, 15, 30.
- Wibowo, J.T.; Bayu, A.; Aryati, W.D.; Fernandes, C.; Yanuar, A.; Kijjoa, A.; Putra, M.Y. Secondary metabolites from marine-derived bacteria with antibiotic and antibiofilm activities against drug-resistant pathogens. Mar. Drugs 2023, 21, 50.
- Subramani, R.; Aalbersberg, W. Culturable rare Actinomycetes: Diversity, isolation and marine natural product discovery. Appl. Microbiol. Biotechnol. 2013, 97, 9291–9321.
- Dhakal, D.; Pokhrel, A.R.; Shrestha, B.; Sohng, J.K. Marine rare Actinobacteria: Isolation, characterization, and strategies for harnessing bioactive compounds. Front. Microbiol. 2017, 8, 1106.
- Subramani, R.; Sipkema, D. Marine rare actinomycetes: A promising source of structurally diverse and unique novel natural products. Mar. Drugs 2019, 17, 249.
- Gattoni, G.; de la Haba, R.R.; Martín, J.; Reyes, F.; Sánchez-Porro, C.; Feola, A.; Zuchegna, C.; Guerrero-Flores, S.; Varcamonti, M.; Ricca, E.; et al. Genomic study and lipidomic bioassay of Leeuwenhoekiella parthenopeia: A novel rare biosphere marine bacterium that inhibits tumor cell viability. Front. Microbiol. 2023, 13, 1090197.
- Wei, B.; Du, A.-Q.; Ying, T.-T.; Hu, G.-A.; Zhou, Z.-Y.; Yu, W.-C.; He, J.; Yu, Y.-L.; Wang, H.; Xu, X.-W. Secondary metabolic potential of Kutzneria. J. Nat. Prod. 2023, 86, 1120–1127.
- Kurahashi, M.; Fukunaga, Y.; Sakiyama, Y.; Harayama, S.; Yokota, A. Euzebya tangerina gen. nov., sp. nov., a deeply branching marine actinobacterium isolated from the sea cucumber Holothuria edulis, and proposal of Euzebyaceae fam. nov., Euzebyales ord. nov. and Nitriliruptoridae subclassis nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 2314–2319.
- Yin, Q.; Zhang, L.; Song, Z.M.; Wu, Y.; Hu, Z.-L.; Zhang, X.-H.; Zhang, Y.; Yu, M.; Xu, Y. Euzebya rosea sp. nov., a rare actinobacterium isolated from the East China Sea and analysis of two genome sequences in the genus Euzebya. Int. J. Syst. Evol. Microbiol. 2018, 68, 2900–2905.
- Jian, S.-L.; Xu, L.; Meng, F.-X.; Sun, C.; Xu, X.-W. Euzebya pacifica sp. nov., a novel member of the class Nitriliruptoria. Int. J. Syst. Evol. Microbiol. 2021, 71, 004864.
- Xu, L.; Sun, C.; Huang, M.M.; Wu, Y.-H.; Yuan, C.-Q.; Dai, W.-H.; Ye, K.; Han, B.; Xu, X.-W. Complete genome sequence of Euzebya sp. DY32-46, a marine Actinobacteria isolated from the Pacific Ocean. Mar. Genom. 2019, 44, 65–69.
- Cuezva, S.; Fernandez-Cortes, A.; Porca, E.; Pasic, L.; Jurado, V.; Hernandez-Marine, M.; Serrano-Ortiz, P.; Cañaveras, J.C.; Sanchez-Moral, S.; Saiz-Jimenez, C. The biogeochemical role of Actinobacteria in Altamira Cave, Spain. FEMS Microbiol. Ecol. 2012, 81, 281–290.
- Riquelme, C.; Hathaway, J.J.M.; Dapkevicius, M.L.N.E.; Miller, A.Z.; Kooser, A.; Northup, D.E.; Jurado, V.; Fernandez, O.; Saiz-Jimenez, C.; Cheeptham, N. Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front. Microbiol. 2015, 6, 1342.
- Spilde, M.N.; Northup, D.E.; Caimi, N.A.; Boston, P.J.; Stone, F.D.; Smith, S. Microbial Mat Communities in Hawaiian Lava Caves. In International Symposium on Vulcanospeleology 2016; 2016. Available online: http://www.cavepics.com/IVS17/SPILDE.pdf (accessed on 1 June 2023).
- Yun, Y.; Wang, H.; Man, B.; Xiang, X.; Zhou, J.; Qiu, X.; Duan, Y.; Engel, A.S. The relationship between pH and bacterial communities in a single karst ecosystem and its implication for soil acidification. Front. Microbiol. 2016, 7, 1955.
- Lepinay, C.; Mihajlovski, A.; Seyer, D.; Touron, S.; Bousta, F.; Di Martino, P. Biofilm communities survey at the areas of salt crystallization on the walls of a decorated shelter listed at UNESCO World cultural Heritage. Int. Biodeter. Biodegr. 2017, 122, 116–127.
- Itcus, C.; Pascu, M.D.; Lavin, P.; Persoiu, A.; Iancu, L.; Purcarea, C. Bacterial and archaeal community structures in perennial cave ice. Sci. Rep. 2018, 8, 15671.
- Gonzalez-Pimentel, J.L.; Miller, A.Z.; Jurado, V.; Laiz, L.; Pereira, M.F.C.; Saiz-Jimenez, C. Yellow colored mats from lava tube of La Palma (Canary Islands, Spain) are dominated by metabolically active Actinobacteria. Sci. Rep. 2018, 8, 1944.
- De, A.K.; Muthiyan, R.; Sunder, J.; Bhattacharya, B.; Kundu, A.; Roy, S.D. Profiling bacterial diversity of B2 cave, a limestone cave of Baratang, Andaman and Nicobar Islands, India. Proc. Indian Nat. Sci. Acad. 2019, 85, 853–862.
- González-Pimentel, J.L. Microorganismos de las Cuevas Volcánicas de La Palma (Islas Canarias): Diversidad y Potencial uso Biotecnológico. Ph.D. Thesis, Universidad Pablo de Olavide, Sevilla, Spain, 2019.
- Li, M.; Fang, C.; Kawasaki, S.; Huang, M.; Achal, V. Bio-consolidation of cracks in masonry cement mortars by Acinetobacter sp. SC4 isolated from a karst cave. Int. Biodeter. Biodegr. 2019, 141, 94–100.
- Luis-Vargas, M.N.; López-Martínez, R.A.; Vilchis-Nestor, A.R.; Daza, R.; Alcántara-Hernández, R.J. Bacterial insights into the formation of opaline stromatolites from the Chimalacatepec lava tube system, Mexico. Geomicrobiol. J. 2019, 36, 694–704.
- Frazier, V.E. Carbon Metabolism in Cave Subaerial Biofilms. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2020.
- Miller, A.Z.; García-Sánchez, A.M.; Coutinho, M.L.; Pereira, M.F.C.; Gázquez, F.; Calaforra, J.M.; Forti, P.; Martínez-Frías, J.; Toulkeridis, T.; Caldeira, A.T.; et al. Colored microbial coatings in show caves from the Galapagos Islands (Ecuador): First microbiological approach. Coatings 2020, 10, 1134.
- Iquebal, M.A.; Passari, A.K.; Jagannadham, J.; Ahmad, F.; Leo, V.V.; Singh, G.; Jaiswal, S.; Rai, A.; Kumar, D.; Singh, B.P. Microbiome of Pukzing Cave in India shows high antimicrobial activity against plant and animal pathogens. Genomics 2021, 113, 4098–4108.
- González-Riancho Fernández, C. Análisis Descriptivo y Funcional de las Colonias Microbianas Visibles que Crecen en la Cueva de Altamira, Enfocado al Diseño de Medidas de Control. Ph.D. Thesis, Universidad de Cantabria, Santander, Spain, 2021.
- Ma, L.; Huang, X.; Wang, H.; Yun, Y.; Cheng, X.; Liu, D.; Lu, X.; Qiu, X. Microbial interactions drive distinct taxonomic and potential metabolic responses to habitats in karst cave ecosystem. Microbiol. Spectr. 2021, 9, e01152-21.
- Weng, M.M.; Zaikova, E.; Millan, M.; Williams, A.J.; McAdam, A.C.; Knudson, C.A.; Fuqua, S.R.; Wagner, N.Y.; Craft, K.; Nawotniak, S.K.; et al. Life underground: Investigating microbial communities and their biomarkers in Mars-analog lava tubes at Craters of the Moon National Monument and Preserve. J. Geophys. Res. Planets 2022, 127, e2022JE007268.
- Yi, Y.-J.; Kim, S.-I.; Ahn, U.-S.; Lee, K.C.; Lee, M.-K.; Lee, J.-S.; Kim, D.-S.; Kim, J.-S. Hex code-based geological cross-sections describing landscape dynamics in the Jeju Geomunoreum lava tube system. Korean J. Environ. Agric. 2022, 41, 65–70.
- Martín Pozas, T. Papel de los Microorganismos en Procesos de Captación y Emisión de Gases de Efecto Invernadero en Ambientes Subterráneos. Ph.D. Thesis, Universidad Complutense, Madrid, Spain, 2023.
- Paul, V.G.; Mormile, M.R. A case for the protection of saline and hypersaline environments: A microbiological perspective. FEMS Microbiol. Ecol. 2017, 93, fix091.
- Castro-Silva, C.; Ruíz-Valdiviezo, V.M.; Valenzuela-Encinas, C.; Alcántara-Hernández, R.J.; Navarro-Noya, Y.E.; Vázquez-Núñez, E.; Luna-Guido, M.; Marsch, R.; Dendooven, L. The bacterial community structure in an alkaline saline soil spiked with anthracene. Electron. J. Biotechnol. 2013, 16, 10.
- Yan, H.; Hu, J.; Long, X.; Liu, Z.; Rengel, Z. Salinity altered root distribution and increased diversity of bacterial communities in the rhizosphere soil of Jerusalem artichoke. Sci. Rep. 2016, 6, 20687.
- De León-Lorenzana, A.S.; Delgado-Balbuena, L.; Domínguez-Mendoza, C.A.; Navarro-Noya, Y.E.; Luna-Guido, M.; Dendooven, L. Soil salinity controls relative abundance of specific bacterial groups involved in the decomposition of maize plant residues. Front. Ecol. Evol. 2018, 6, 51.
- Mukhtar, S.; Mirza, B.S.; Mehnaz, S.; Mirza, M.S.; Mclean, J.; Malik, K.A. Impact of soil salinity on the microbial structure of halophyte rhizosphere microbiome. World J. Microbiol. Biotechnol. 2018, 34, 136.
- Martínez-Olivas, M.A.; Jiménez-Bueno, N.G.; Hernández-García, J.A.; Fusaro, C.; Luna-Guido, M.; Navarro-Noya, Y.E.; Dendooven, L. Bacterial and archaeal spatial distribution and its environmental drivers in an extremely haloalkaline soil at the landscape scale. PeerJ 2019, 7, e6127.
- Sánchez-Sánchez, J.; Cerca, M.; Alcántara-Hernández, R.J.; Lozano-Flores, C.; Carreón-Freyre, D.; Levresse, G.; Vega, M.; Varela-Echavarría, A.; Aranda-Gómez, J.J. Extant microbial communities in the partially desiccated Rincon de Parangueo maar crater lake in Mexico. FEMS Microbiol. Ecol. 2019, 95, fiz051.
- Ibarra-Sánchez, C.L.; Pince, L.; Aguirre-Noyola, J.L.; Sánchez-Cerda, K.E.; Navaro-Noya, Y.E.; Luna-Guido, M.; Conde-Barajas, E.; Dendooven, L.; Gomez-Acata, E.S. The microbial community in an alkaline saline sediment of a former maar lake bed. J. Soils Sediments 2020, 20, 542–555.
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Microenvironment and microbial community in the rhizosphere of dioecious Populus cathayana at Chaka Salt Lake. J. Soils Sediments 2019, 19, 2740–2751.
- Baeshen, M.N.; Moussa, T.A.A.; Ahmed, F.; Abulfaraj, A.A.; Jalal, R.S.; Majaeed, M.A.; Baeshen, N.A.; Huelsenbeck, J.P. Diversity profiling of associated bacteria from the soils of stress tolerant plants from seacoast of Jeddah, Saudi Arabia. Appl. Ecol. Environ. Res. 2020, 18, 8217–8231.
- Camacho-Sanchez, M.; Barcia-Piedras, J.M.; Redondo-Gómez, S.; Camacho, M. Mediterranean seasonality and the halophyte Arthrocnemum macrostachyum determine the bacterial community in salt marsh soils in Southwest Spain. Appl. Soil Ecol. 2020, 151, 103532.
- Gao, W.; Xu, J.; Zhao, J.; Zhang, H.; Ni, Y.; Zhao, B.; Tebbe, C.C.; Zhang, J.; Jia, Z. Prokaryotic community assembly after 40 years of soda solonetz restoration by natural grassland and reclaimed farmland. Eur. J. Soil Biol. 2020, 100, 103213.
- Xu, J.; Gao, W.; Zhao, B.; Chen, M.; Ma, L.; Jia, Z.; Zhang, J. Bacterial community composition and assembly along a natural sodicity/salinity gradient in surface and subsurface soils. Appl. Soil Ecol. 2021, 157, 103731.
- Chang, C.; Tian, L.; Tian, Z.; McLaughlin, N.; Tian, C. Change of soil microorganism communities under saline-sodic land degradation on the Songnen Plain in northeast China. J. Plant Nutr. Soil Sci. 2022, 185, 297–307.
- Du, X.; Wang, S.; Huang, H.; Zhang, Y.; Ren, X.; Hu, S. Fermenting straw reduced salt damage and improved the stability of the bacterial community in a saline–sodic soil. J. Agric. Sci. Agrotechnol. 2022, 1, 1–18.
- Peng, M.; Wang, C.; Wang, Z.; Huang, X.; Zhou, F.; Yan, S.; Liu, X. Differences between the effects of plant species and compartments on microbiome composition in two halophyte Suaeda species. Bioengineered 2022, 13, 12475–12488.
- Urana, R.; Yadav, J.; Panchal, S.; Sharma, P.; Singh, N. Phytoremediation of PAH compounds by microbial communities in sodic soil. Int. J. Phytoremediation 2023, 25, 1501–1509.
- Van Goethem, M.W.; Makhalanyane, T.P.; Valverde, A.; Cary, S.C.; Cowan, D.A. Characterization of bacterial communities in lithobionts and soil niches from Victoria Valley, Antarctica. FEMS Microbiol. Ecol. 2016, 92, fiw051.
- Meslier, V.; Casero, M.C.; Dailey, M.; Wierzchos, J.; Ascaso, C.; Artieda, O.; McCullough, P.R.; DiRuggiero, J. Fundamental drivers for endolithic microbial community assemblies in the hyperarid Atacama Desert. Environ. Microbiol. 2018, 20, 1765–1781.
- Lee, K.C.; Caruso, T.; Archer, S.D.J.; Gillman, L.N.; Lau, M.C.Y.; Cary, S.C.; Lee, C.K.; Pointing, S.B. Stochastic and deterministic effects of a moisture gradient on soil microbial communities in the McMurdo Dry Valleys of Antarctica. Front. Microbiol. 2018, 9, 2619.
- Rego, A.; Raio, F.; Martins, T.P.; Ribeiro, H.; Sousa, A.G.G.; Séneca, J.; Baptista, M.S.; Lee, C.K.; Cary, S.C.; Ramos, V.; et al. Actinobacteria and cyanobacteria diversity in terrestrial Antarctic microenvironments evaluated by culture-dependent and independent methods. Front. Microbiol. 2019, 10, 1018.
- Araujo, R.; Gupta, V.V.S.R.; Reith, F.; Bisset, A.; Mele, P.; Franco, C.M.M. Biogeography and emerging significance of Actinobacteria in Australia and Northern Antarctica soils. Soil Biol. Biochem. 2020, 146, 107805.
- Miralles, I.; Soria, R.; Lucas-Borja, M.E.; Soriano, M.; Ortega, R. Effect of biocrusts on bacterial community composition at different soil depths in Mediterranean semi-arid ecosystems. Sci. Total Environ. 2020, 733, 138613.
- Bona, E.; Massa, N.; Toumatia, O.; Novello, G.; Cesaro, P.; Todeschini, V.; Boatti, L.; Mignone, F.; Titouah, H.; Zitouni, A.; et al. Climatic zone and soil properties determine the biodiversity of the soil bacterial communities associated to native plants from desert areas of North-Central Algeria. Microorganisms 2021, 9, 1359.
- Khomutovska, N.; de los Ríos, A.; Syczewski, M.D.; Jasser, I. Connectivity of edaphic and endolithic microbial niches in cold mountain desert of Eastern Pamir (Tajikistan). Biology 2021, 10, 314.
- Ortiz, M.; Leung, P.M.; Shelley, G.; Jirapanjawat, T.; Nauer, P.A.; Van Goethem, M.W.; Bay, S.K.; Islam, Z.F.; Jordaan, K.; Vikram, S.; et al. Multiple energy sources and metabolic strategies sustain microbial diversity in Antarctic desert soils. Proc. Natl. Acad. Sci. USA 2021, 118, e2025322118.
- Osman, J.R.; Wang, Y.; Jaubert, C.; Nguyen, T.-N.; Fernandes, G.R.; DuBow, M.S. The bacterial communities of surface soils from desert sites in the eastern Utah (USA) portion of the Colorado Plateau. Microbiol. Res. 2021, 244, 126664.
- Sun, X.; Pei, J.; Zhao, L.; Ahmad, B.; Huang, L.-F. Fighting climate change: Soil bacteria communities and topography play a role in plant colonization of desert areas. Environ. Microbiol. 2021, 23, 6876–6894.
- Li, Y.; He, X.; Yuan, H.; Lv, G. Differed growth stage dynamics of root-associated bacterial and fungal community structure associated with halophytic plant Lycium ruthenicum. Microorganisms 2022, 10, 1644.
- Echeverría Molinar, A. Efecto de Factores Abióticos y Bióticos Sobre la Estructura de la Comunidad Microbiana del Suelo en un Ambiente Oligotrófico. Master’s Thesis, Instituto Potosino de Investigación Científica y Tecnológica, San Luis Potosí, Mexico, 2017.
- López-Lozano, N.E.; Echeverría Molinar, A.; Ortiz Durán, E.A.; Hernández Rosales, M.; Souza, V. Bacterial diversity and interaction networks of Agave lechuguilla rhizosphere differ significantly from bulk soil in the oligotrophic basin of Cuatro Cienegas. Front. Plant Sci. 2020, 11, 1028.
- Sun, J.; Zhang, Q.; Zhou, J.; Wei, Q. Pyrosequencing technology reveals the impact of different manure doses on the bacterial community in apple rhizosphere soil. Appl. Soil Ecol. 2014, 78, 28–36.
- Lee, H.-J.; Han, S.-I.; Whang, K.-S. Phylogenetic characteristics of actinobacterial population in bamboo (Sasa borealis) soil. Korean J. Microbiol. 2016, 52, 59–64.
- An, Z.; Guo, F.; Chen, Y.; Bai, G.; Chen, Z. Rhizosphere bacterial and fungal communities during the growth of Angelica sinensis seedlings cultivated in an Alpine uncultivated meadow soil. PeerJ 2020, 8, e8541.
- Liu, A.; Li, Y.; Wang, Q.; Zhang, X.; Xiong, J.; Li, Y.; Lei, Y.; Sun, Y. Analysis of microbial diversity and community structure of rhizosphere soil of Cistanche salsa from different host plants. Front. Microbiol. 2022, 13, 971228.
- Duan, M.; Wang, L.; Song, X.; Zhang, X.; Wang, Z.; Lei, J.; Yan, M. Assessment of the rhizosphere fungi and bacteria recruited by sugarcane during smut invasion. Braz. J. Microbiol. 2023, 54, 385–395.
- Cheng, Y.; Xie, X.; Wang, X.; Zhu, L.; Qiu, Q.-S.; Xu, X. Effects of the salt-tolerant gramineous forage Echinochloa frumentacea on biological improvement and crop productivity in saline–alkali land on the Hetao Ningxia Plain in China. Sustainability 2023, 15, 5319.
- Wang, D.; Ren, H. Microbial community in buckwheat rhizosphere with different nitrogen application rates. PeerJ 2023, 11, e15514.
- Jiménez Bueno, N.G. Efecto de las Diferentes Prácticas de Agricultura Sobre las Comunidades Bacterianas en Suelos del Valle del Yaqui. Ph.D. Thesis, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Mexico City, Mexico, 2016.
- Wolinska, A.; Görniak, D.; Zielenkiewicz, U.; Kuzniar, A.; Izak, D.; Banach, A.; Blaszczyk, M. Actinobacteria structure in autogenic, hydrogenic and lithogenic cultivated and non-cultivated soils: A culture-independent approach. Agronomy 2019, 9, 598.
- Liu, C.; Zhao, X.; Lin, Q.; Li, G. Decrease in diversity and shift in composition of the soil bacterial community were closely related to high available phosphorus in agricultural Fluvisols of North China. Acta Agric. Scand. Soil Plant Sci. 2019, 69, 618–630.
- Biderre-Petit, C.; Hochart, C.; Gardon, H.; Dugat-Bony, E.; Terrat, S.; Jouan-Dufournel, I.; Paris, R. Analysis of bacterial and archaeal communities associated with Fogo volcanic soils of different ages. FEMS Microbiol. Ecol. 2020, 96, fiaa104.
- Cui, Y.; Yong, L.; Rongli, M.; Wen, D.; Zhixian, Z.; Xingming, H. Metagenomics analysis of the effects of long-term stand age on beneficial soil bacterial community structure under Chinese ancient mulberry farming practice. Hortic. Environ. Biotechnol. 2020, 61, 1063–1071.
- Lopes, L.S.; Mendes, L.W.; Antunes, J.E.L.; Oliverira, L.M.S.; Melo, V.M.M.; Pereira, A.P.A.; Costa, A.F.; Oliveira, J.P.; Martínez, C.R.; Figueiredo, M.V.B.; et al. Distinct bacterial community structure and composition along different cowpea producing ecoregions in Northeastern Brazil. Sci. Rep. 2021, 11, 831.
- Hazzouri, K.M.; Sudalaimuthuasari, N.; Saeed, E.E.; Kundu, B.; Al-Maskari, R.S.; Nelson, D.; AlShehhi, A.A.; Aldhuhoori, M.A.; Almutawa, D.S.; Alshehhi, F.R.; et al. Salt flat microbial diversity and dynamics across salinity gradient. Sci. Rep. 2022, 12, 11293.
- Pino-Otín, M.R.; Ferrando, N.; Ballestero, D.; Langa, E.; Roig, F.J.; Terrado, E.M. Impact of eight widely consumed antibiotics on the growth and physiological profile of natural soil microbial communities. Chemosphere 2022, 305, 135473.
- Baldi, D.S.; Humphrey, C.E.; Kyndt, J.A.; Moore, T.C. Native plant gardens support more microbial diversity and higher relative abundance of potentially beneficial taxa compared to adjacent turf grass lawns. Urban Ecosyst. 2023, 26, 807–820.
- David, A.B.; Mwaikomo, K.S.; Midega, C.; Magingo, F.; Alsanius, B.W.; Drinkwater, L.E.; Dekker, T.; Lyantagaye, S. A comparative study on the impact of five Desmodium species on soil microbiome reveals enrichment of selected bacterial and fungal taxa. bioRxiv 2023.
- Manucharova, N.A.; Kovalenko, M.A.; Alekseeva, M.G.; Babenko, A.D.; Stepanov, A.L. Biotechnological potential of hydrolytic prokaryotic component in soils. Eurasian Soil Sci. 2023, 56, 558–572.
- Wang, Y.; Wang, Y.; Zhang, Q.; Fan, H.; Wang, X.; Wang, J.; Zhou, Y.; Chen, Z.; Sun, F.; Cui, X. Saline-alkali soil property improved by the synergistic effects of Priestia aryabhattai JL-5, Staphylococcus pseudoxylosus XW-4, Leymus chinensis and soil microbiota. Int. J. Mol. Sci. 2023, 24, 7737.
- Peng, M.; Zi, X.; Wang, Q. Bacterial community diversity of oil-contaminated soils assessed by high throughput sequencing of 16S rRNA genes. Int. J. Environ. Res. Public Health 2015, 12, 12002–12015.
- Kumar, V.; AlMomin, S.; Al-Aqeel, H.; Al-Salameen, F.; Nair, S.; Shajan, A. Metagenomic analysis of rhizosphere microflora of oil-contaminated soil planted with barley and alfalfa. PLoS ONE 2018, 13, e0202127.
- Ke, W.; Zhang, X.; Zhu, F.; Wu, H.; Zhang, Y.; Shi, Y.; Hartley, W.; Xue, S. Appropriate human intervention stimulates the development of microbial communities and soil formation at a long-term weathered bauxite residue disposal area. J. Hazard. Mater. 2021, 405, 124689.
- Rahman, K.M.J.; Diba, F.; Shuvo, M.S.R.; Siddique, M.A.; Hossain, M.A.; Sultana, M. Metagenomic investigation of bacterial community of arsenic-prone area in the northwest region of Bangladesh. Bangladesh J. Microbiol. 2022, 39, 31–38.
- Ossanna, L.Q.R.; Serrano, K.; Jennings, L.L.; Dillon, J.; Maier, R.M.; Neilson, J.W. Progressive belowground soil development associated with sustainable plant establishment during copper mine waste revegetation. Appl. Soil Ecol. 2023, 186, 104813.
- Feng, G.; Yong, J.; Liu, Q.; Chen, H.; Hu, Y.; Mao, P. Remedial effect and operating status of a decommissioned uranium mill tailings (UMT) repository: A micro-ecological perspective based on bacterial community. J. Environ. Manag. 2023, 340, 117993.
- Héry, M.; Rizoulis, A.; Sanguin, H.; Cooke, D.A.; Pancost, R.D.; Polya, D.A.; Lloyd, J.R. Microbial ecology of arsenic-mobilizing Cambodian sediments: Lithological controls uncovered by stable-isotope probing. Environ. Microbiol. 2014, 17, 1857–1869.
- Mirete, S.; Mora-Ruiz, M.R.; Lamprecht-Grandío, M.; de Figueras, C.G.; Rosselló-Móra, R.; González-Pastor, J.E. Salt resistance genes revealed by functional metagenomics from brines and moderate-salinity rhizosphere within a hypersaline environment. Front. Microbiol. 2015, 6, 1121.
- Ivanova, E.A.; Pershina, E.V.; Kutovaya, O.V.; Sergaliev, N.K.h.; Nagieva, A.G.; Zhiengaliev, A.T.; Provorov, N.A.; Andronov, E.E. Comparative analysis of microbial communities of contrasting soil types in different plant communities. Russ. J. Ecol. 2018, 49, 30–39.
- Truchado, P.; Gil, M.I.; Suslow, T.; Allende, A. Impact of chlorine dioxide disinfection of irrigation water on the epiphytic bacterial community of baby spinach and underlying soil. PLoS ONE 2018, 13, e0199291.
- West, N.J.; Parrot, D.; Fayet, C.; Grube, M.; Tomasi, S.; Suzuki, M.T. Marine cyanolichens from different littoral zones are associated with distinct bacterial communities. PeerJ 2018, 6, e5208.
- Feby, A.; Divya, B.; Nair, S. Bacterial diversity in demosponges from the coral reefs of Lakshadweep, India. Rom. J. Biol. Zool. 2021, 66, 85–100.
- Li, C.; Liu, J.; Chen, X.; Ren, H.; Su, B.; Ma, K.; Tu, Q. Determinism governs the succession of disturbed bacterioplankton communities in a coastal maricultural ecosystem. Sci. Total Environ. 2022, 828, 154457.
- Parab, A.S.; Manohar, C.S.; Ghose, M.P. Influence of seasonal variations in primary productivity on the bacterial community structure at Chlorophyll Maximum (C-Max) depths along the west coast of India. bioRxiv 2023.
- Povedano-Priego, C.; Jroundi, F.; Lopez-Fernandez, M.; Sánchez-Castro, I.; Martín-Sánchez, I.; Huertas, J.; Merroun, M.L. Shifts in bentonite bacterial community and mineralogy in response to uranium and glicerol-2-phosphate exposure. Sci. Total Environ. 2019, 692, 219–232.
- Ephraim, E.; Brockman, J.A.; Jewell, D.E. A diet supplemented with polyphenols, prebiotics and omega-3 fatty acids modulates the intestinal microbiota and improves the profile of metabolites linked with anxiety in dogs. Biology 2022, 11, 976.
- Marchywka, M. Clinical and Microbiological Improvement in Dog after Metal and Benzoate Containing Supplement Mix; Tech Report MJM-2022-013; Public Note: Austin, TX, USA, 2022.
- Niemiec, B.A.; Gawor, J.; Tang, S.; Prem, A.; Krumbeck, J.A. The bacteriome of the oral cavity in healthy dogs and dogs with periodontal disease. Am. J. Vet. Res. 2022, 83, 50–58.
- Richter, H.E.; Carnes, M.U.; Komesu, Y.M.; Lukacz, E.S.; Arya, L.; Bradley, M.; Rogers, R.G.; Sung, V.W.; Siddiqui, N.Y.; Carper, B.; et al. Association between the urogenital microbiome and surgical treatment response in women undergoing midurethral sling operation for mixed urinary incontinence. Am. J. Obstet. Gynecol. 2022, 226, 93.e1–93.e15.
- Chen, B.-Y.; Lin, W.-Z.; Li, Y.-L.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Shi, C.-J.; et al. Roles of oral microbiota and oral-gut microbial transmission in hypertension. J. Adv. Res. 2023, 43, 147–161.
- Fan, S.; He, X.; Zhu, Z.; Chen, L.; Zou, Y.; Chen, Z.; Yu, J.; Chen, W.; Guan, H.; Ma, J. Integrating host transcriptomic signatures for distinguishing autoimmune encephalitis in cerebrospinal fluid by metagenomic sequencing. Cell Biosci. 2023, 13, 111.
- Dyda, M.; Pyzik, A.; Wilkojc, E.; Kwiatkowska-Kopka, B.; Sklodowska, A. Bacterial and fungal diversity inside the medieval building constructed with sandstone plates and lime mortar as an example of the microbial colonization of a nutrient-limited extreme environment (Wawel Royal Castle, Krakow, Poland). Microorganisms 2019, 7, 416.
- Wang, L.; Peng, C.; Gong, B.; Yang, Z.; Song, J.; Li, L.; Xu, L.; Yue, T.; Wang, X.; Yang, M.; et al. Actinobacteria community and their antibacterial and cytotoxic activity on the Weizhou and Xieyang volcanic islands in the Beibu Gulf of China. Front. Microbiol. 2022, 13, 911408.
- Weels, S.S.L.; Welz, P.J.; Prins, A.; Le Roes-Hill, M. Impact of physicochemical parameters on the diversity and distribution of microbial communities associated with three South African peatlands. Microorganisms 2022, 10, 2103.
- Martin-Pozas, T.; Gonzalez-Pimentel, J.L.; Jurado, V.; Laiz, L.; Cañaveras, J.C.; Fernandez-Cortes, A.; Cuezva, S.; Sanchez-Moral, S.; Saiz-Jimenez, C. Crossiella, a rare Actinomycetota genus, abundant in the environment. Appl. Biosci. 2023, 2, 194–210.
- Martin-Pozas, T.; Fernandez-Cortes, A.; Cuezva, S.; Cañaveras, J.C.; Benavente, D.; Duarte, E.; Saiz-Jimenez, C.; Sanchez-Moral, S. New insights into the structure, microbial diversity and ecology of yellow biofilms in a Paleolithic rock art cave (Pindal Cave, Asturias, Spain). Sci. Total Environ. 2023, 897, 165218.
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 1973, 246, 527–528.
- Borodina, E.; Kelly, D.P.; Rainey, F.A.; Ward-Rainey, N.L.; Wood, A.P. Dimethylsulfone as a growth substrate for novel methylotrophic species of Hyphomicrobium and Arthrobacter. Arch. Microbiol. 2000, 173, 425–437.
- Laiz, L.; Miller, A.Z.; Jurado, V.; Akatova, E.; Sanchez-Moral, S.; Gonzalez, J.M.; Dionísio, A.; Macedo, M.F.; Saiz-Jimenez, C. Isolation of Rubrobacter strains from biodeteriorated monuments. Naturwissenschaften 2009, 96, 71–79.
- Kurahashi, M.; Fukunaga, Y.; Sakiyama, Y.; Harayama, S.; Yokota, A. Iamia majanohamensis gen. nov., sp. nov., an actinobacterium isolated from sea cucumber Holothuria edulis, and proposal of Iamiaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 869–873.
- Flores, N.; Hoyos, S.; Venegas, M.; Galetovic, A.; Zúñiga, L.M.; Fábrega, F.; Paredes, B.; Salazar-Ardiles, C.; Vilo, C.; Ascaso, C.; et al. Haloterrigena sp. strain SGH1, a bacterioruberin-rich, perchlorate-tolerant halophilic archaeon isolated from halite microbial communities, Atacama Desert, Chile. Front. Microbiol. 2020, 11, 324.
- Jiao, J.-Y.; Liu, L.; Hua, Z.-S.; Fang, B.-Z.; Zhou, E.-M.; Salam, N.; Hedlund, B.P.; Li, W.-J. Microbial dark matter coming to light: Challenges and opportunities. Nat. Sci. Rev. 2021, 8, nwaa280.
- Pascoal, F.; Costa, R.; Magalhaes, C. The microbial rare biosphere: Current concepts, methods and ecological principles. FEMS Microbiol. Ecol. 2021, 97, fiaa227.
