Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Omega-3s are found in three main forms, namely, α-linolenic acid (ALA, 18C:3 n-3), eicosapentaenoic acid (EPA, 20C:5 n-3), and docosahexaenoic acid (DHA, 22C:6 n-3).
- chronic liver disease
- specialized pro-resolving mediators
- inflammation
- macrophages M1/M2 polarization
1. Introduction
Historically, high fat consumption in humans has been associated with metabolic problems, metabolic disease deterioration, overweight, and cardiovascular disease. However, since 1960, there has been increasing evidence of the health benefits and protective effects of omega-3 fatty acids (FAs) [1][2]. This has led to physiological resolution of inflammation and could have a therapeutic role in a wide range of pathologies, both acute and chronic, among the latter being chronic liver diseases (CLDs) [2][3]. According to the global burden of liver disease: 2023 update, CLD was the 11th cause of mortality and the 15th cause of disability-associated life-years worldwide [4][5]. In the context of CLD associated with metabolic disorders, the nonalcoholic fatty liver disease (NAFLD) has increased in the last years, the overall prevalence of NAFLD worldwide is 32.4%, and may appear in absence if obesity or metabolic syndrome clinical criteria’s, and may coexist with insulin resistance or cardiovascular risk [4]. According to the Center for Disease Control and Prevention Analysis (CDC), the NAFLD cases in man are expected to increase by 21% in the US for the 2015–2030 period, a pattern that will be followed by other occidental countries [6]. These records justify the search for new non-invasive treatments with a better cost/effectiveness ratio.
2. Omega-3 Fatty Acids and Their Role on M1/M2 Macrophage
The study of omega-3s dates back to the early 1960s. Danish researchers Bang and Dyerberg observed that Alaska Natives with a diet high in omega-3s, derived from eating seal meat and fish, had a lower incidence of heart disease [1][7]. This study began a series of investigations in which the importance and benefits of this type of fat in the diet were studied. Omega-3s are found in three main forms, namely, α-linolenic acid (ALA, 18C:3 n-3), eicosapentaenoic acid (EPA, 20C:5 n-3), and docosahexaenoic acid (DHA, 22C:6 n-3). In food, EPA and DHA are found in fish such as salmon and tuna, or algae, while vegetable oils, chia, and nuts are the main foods with a high content of ALA [8]. ALA is a precursor of EPA and DHA, and the synthesis of both fatty acids occurs through desaturation and elongation reactions mainly in the liver, and in brain, testicle, and kidney, [9]. Also, EPA and DHA synthesis is directly related with nutritional status and oxidative stress [10]. Several studies show the importance of maintaining omega-6/omega-3 consumption in a ratio between 1/1 and 4/1. It should be considered that the average Western diet, heavily based on the consumption of meat and animal fats, has an approximate ratio of 16/1 in what the consumption of omega-6 and omega-3 refers to [11]. It has been observed that this results in increased metabolism of omega-6 polyunsaturated fatty acids (PUFAs), generating a greater number of metabolic mediators that, as mentioned above, have proinflammatory and neoplastic properties [12].
EPA and DHA (at 100 mM) have been shown to reduce oxidative damage to endothelial cell DNA, reducing the concentration of H2O2 and other reactive oxygen species (ROS) at the intracellular level in a model of human aortic endothelial cells (HAECs). These findings suggest that omega-3s have protective effects at the genetic level through mechanisms that reduce damage to genetic material without promoting its repair [13]. Based on the above, it was reported that the consumption of omega-3 is related to an improvement in endothelial function associated with an increase in nitric oxide (NO) in human endothelial cells [14][15]. In addition, it has been observed that the administration of omega-3 has protective functions in the development of some relevant pathologies (see Figure 1). In different animals, clinical trials, and cellular models, the treatment with DHA and/or EPA promotes beneficial effects through the activation of immune cells such as PMNs or monocytes, decreasing inflammation [3][16][17][18][19][20][21][22]. For example, the addition of 2.4 g/d to type two diabetes (T2DM) patients for 8 weeks may modulate the activity of PPARγ nuclear receptors, protecting by this way the cardiovascular system against atherosclerotic lesion formation and exerting an anti-inflammatory role [19]. Following the same line, the administration of EPA+DHA at 2 g/d (for six months) can lower c-reactive protein (as marker of cardiovascular disease) in patients with end-stage renal disease, and 4 g/d (for one month in healthy volunteers) can ameliorate acute and chronic vascular inflammation, with a decrease in C–C motif chemokine ligand 2 (CCL2), a chemokine that enhances macrophage responses to pro-inflammatory stimuli [20][21]. In all the cases previously explained, the central positive actions of omega-3 are related to their ability to modify the inflammatory status.
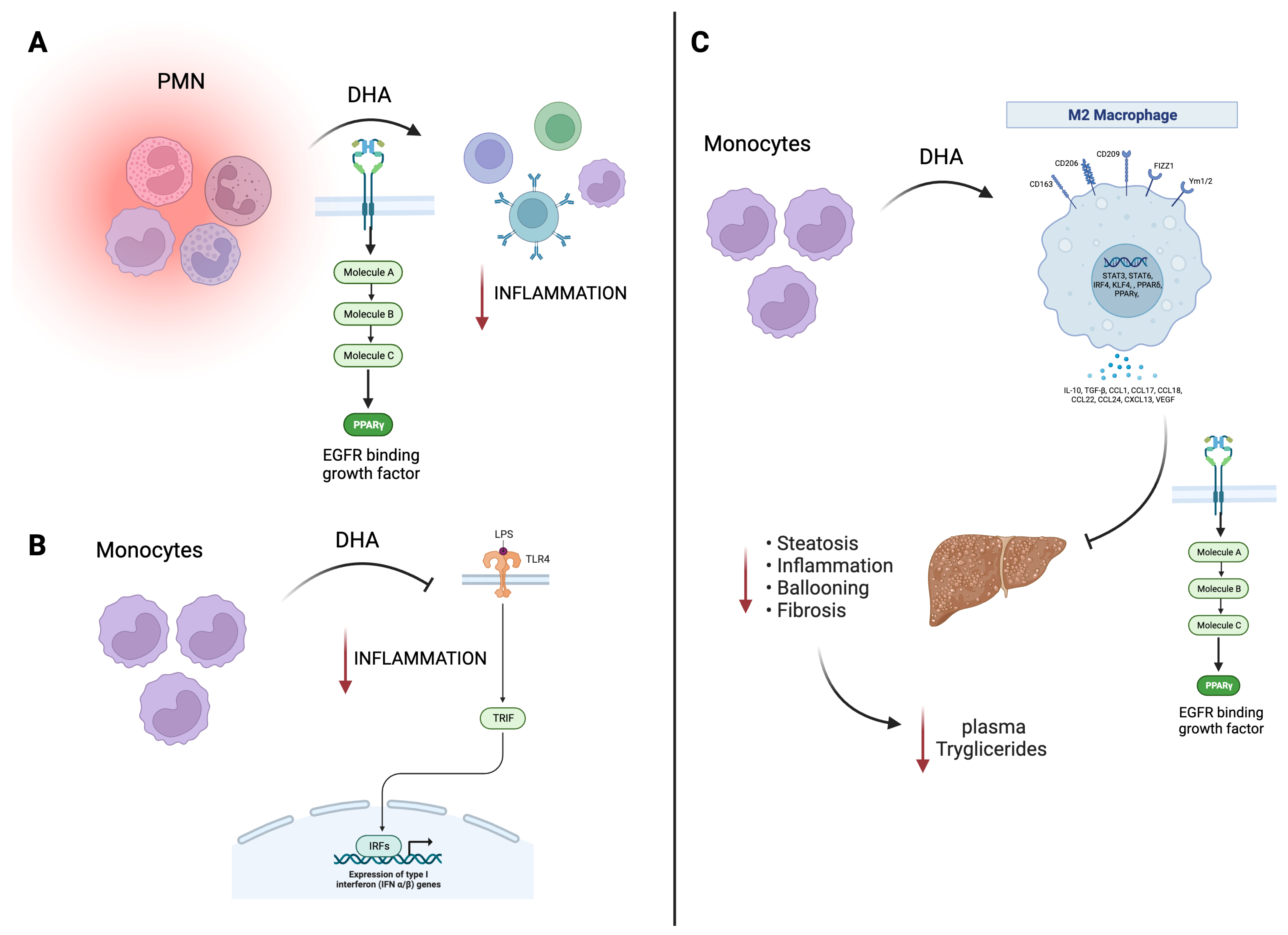
Figure 1. Beneficial effects of EPA/DHA on immune and cardiac models. (A) DHA treatment in PMN cells promotes PPAR-γ activation with a decrease in the inflammatory response. (B) In murine monocytes, DHA inhibits TLR4 signaling, also translating this to a decrease in inflammation. (C) In macrophages, DHA, through PPAR-γ, promotes an hepato-protecting effect decreasing inflammation, steatosis, and fibrosis, related to a decrease in plasma triglycerides. Black arrow informs a activation or inhibition of a pathway; Red arrow is related to negative effects. EGFR, endothelial growth factor receptor; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LPS, lipopolyssacharide; PMN, polymorphonuclear leukocytes; PPAR, peroxisome proliferator-activated receptors; TLR4, toll-like receptor-4; TRIF, TIR-domain-containing adapter-inducing interferon-β; IFN, interferon; STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor.
Moreover, the direct effect of these fatty acids can be seen on the different tissues, such as cardiac tissue (chosen due to its high relevance) where EPA has been reported to reduce heartbeat through a decrease in membrane potential [23]. On the other hand, in cardiac fibroblast, the combination of EPA and DHA (added to c57bl/6 mice for 8 weeks as 1% of total dietary energy) inhibit TGF-β pathways decreasing fibrosis [24], and in humans, the addition of 4 g/d of omega-3 ethyl esters (Omega-Remodell clinical trial) showed a significant reduction in left ventricular end-systolic volume index, and high dose of omega-3 is associated with significant reduction in inflammation and myocardial fibrosis in patients during convalescent phase of acute infarct healing [16][25]. Also, it was found, in clinical trial analysis, that EPA plus DHA can decrease lipogenesis and liver steatosis [26][27]. Additionally, EPA and DHA being both preventive and therapeutic protective factors related to immunity and particularly macrophages anti-inflammatory balances, as shown in Table 1 [28][29][30][31][32][33][34][35][36][37][38][39][40]. The Table 1 presents a comprehensive summary of the effects of EPA/DHA on different mechanisms and models (clinical trials and animal studies). In clinical trials, EPA/DHA supplementation demonstrated promising results, such as reducing T helper 2/T helper 1 chemokines in newborns from mothers with pregnancy-related depression, improving various metabolic parameters in T2DM, and modulating inflammatory pathways in obese individuals. Moreover, it enhanced specific macrophage markers and promoted anti-inflammatory cytokine production in children with low DHA intake, and it contributed to improved atherosclerotic plaque morphology in patients awaiting carotid endarterectomy. In animal models, EPA/DHA exhibited a range of effects, including attenuating atherosclerotic plaque development and suppressing atherogenesis, reducing aneurysm formation and macrophage infiltration, modulating skin inflammation in a psoriasis model, and influencing various signaling pathways. These findings underscore the potential health benefits of EPA/DHA in diverse contexts, from cardiovascular health to inflammation modulation.
Table 1. Protective functions of omega-3 related to inflammatory and macrophage modulation.
| Omega-3 | Mechanism | Effect | Model |
|---|---|---|---|
| Clinical Trials Analysis | |||
| A | Decrease in T helper 2/T helper 1 chemokines | Lower macrophage-derived chemokine/interferon-inducible protein | Cord plasma from newborns from pregnancy-related depressive mothers (prenatal supplementation [28]) |
| A | Decrease levels of sCD63 * | Decrease in triglycerides, waist to height ratio and waist circumference | Type 2 diabetic patients [29] |
| A | Modulation of Wnt/ beta-catenine pathways ** |
White adipose tissue downregulation of inflammatory pathways with less macrophage infiltration | Obese subjects [30] |
| A | Enhance of CD54 macrophages *** | Less T CD8+/T CD4+ after immune challenger and greater production of IL-10 (an anti-inflammatory cytokine) | Children (ages 5–7 years), who had low intakes of DHA [31] |
| A | Less macrophage in atherosclerotic plaques | Improvement of atherosclerotic plaque morphology (tin fibrous cap) |
Patients awaiting carotid endarterectomy [32] |
| Animals model | |||
| A | Attenuated the development and destabilization of atherosclerotic plaques and reduction in TLR4 | Suppressed atherogenesis | Apolipoprotein E-deficient (ApoE-/-) mice [33] |
| A | Decreased of TNF-α, MCP-1, TGF β, and arginase 2; this last one is a marker of pro-inflammatory macrophages |
Reduction in aneurism formation and macrophage infiltration | Abdominal aortic aneurysm (AAA) animal model [34] |
| C | increased levels of resolvin D5, protectin DX, and maresin 2 in the mouse skin | Decrease proinflammatory cytokines altered psoriasis macrophage phenotypes and lipid oxidation, modulating psoriasis skin inflammation | K14-Rac1V12 mouse model [35] |
| A | Induction of Nrf2 signaling | Decrease proinflammatory cytokines, iNOS and COX-2 | Nrf2 knockout (-/-; KO) mice [36] |
| B | Down-regulation of NF-κB activation and regulated genes | Inhibited tubule-interstitial injury and the infiltration of macrophages into tubule-interstitial lesions | Thy-1 nephritis model [37] |
| A | reduce TNFα and caspase-3, and could increase splenic GSH Bcl-2. Restoring macrophages, and B- and T- lymphocytes. | Decrease the Methotrexate-induced histopathological injury | Methotrexato-induced splenic suppression on Sprague-Dawley rats [38] |
| A | Reduced pro-inflammatory macrophages | Promoted wound closure by accelerating the resolution of inflammation | Wound healing in db/db mice [39] |
| A | Reduction in hepatic SREBP-1c and enhancement of PPARγ nuclear receptor | Lower concentration on plasma lipids, triglycerides, and liver lipid content. Enhance of endothelial function |
HFD in hamsters [40] |
A: EPA + DAH; B: EPA; C: EPA or DHA. * Macrophages activation marker; ** Pathway related to genes involved in adipogenesis; *** Co-stimulatory molecule on antigen-presenting cells that facilitates MHC-restricted immune response. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; TLR4, toll-like receptor-4; TNF-α, tumor necrosis factor alpha; MCP-1, monocyte chemoattractant protein-1; TGF-β, transforming growth factor beta; Nrf2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor-kappa B; GSH, glutathione; SREBP, sterol regulatory element binding proteins; PPAR, peroxisome proliferator-activated receptors; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; HFD, high fat diet.
The importance of reviewing data from both human and animal trials in Table 1 allows to make a critical comparison between the effects of EPA/DHA, where human clinical trials provide direct insights into the effects of EPA/DHA in human populations. The clinical trials focus on health conditions directly relevant to humans, such as pregnancy-related depression, T2DM and atherosclerosis in patients awaiting carotid endarterectomy. This relevance ensures that the observed effects have immediate implications for human health with high ethical standards to protect the rights and well-being of human participants. The wide range of health parameters studied, including chemokine levels, triglycerides, waist measurements, and atherosclerotic plaque morphology, underscores the potential multi-faceted benefits of EPA/DHA in human health. The phenomena observed in the clinical trial enable readily interpretations for medical practice and treatment strategies. For example, the reduction in triglyceride levels in T2DM patients suggests a potential therapeutic opportunity. Also, the results allow to understand that EPA/DHA can decrease inflammatory pathways mostly related to macrophages activity and infiltration, and T-cell cytokine production. Animal models, on the other hand, offer controlled environments for mechanistic studies and initial insights, but require cautious interpretation and translation to human health due to species differences in physiology and metabolism; however, preclinical studies allow elucidation of the underlying mechanisms of action, providing a deeper understanding of how EPA/DHA exert their effects at the cellular and molecular levels. For example, the Apolipoprotein E-deficient (ApoE-/-) mice model helps elucidate the role of EPA/DHA in atherosclerosis development and the induction of Nrf2 to promote the cytokines switch from pro- to anti-inflammatory response, offering valuable mechanistic insights into how EPA/DHA affects pathways, cellular processes, and disease development. For instance, the K14-Rac1V12 mouse model sheds light on EPA/DHA’s effects on psoriasis, due to an increase in the SPM in the skin. These findings serve as a starting point for further research in humans. Combining both types of research can yield a more comprehensive understanding of EPA/DHA’s effects, from basic mechanisms to clinical applications.
The effects of EPA and DHA are of great interest in the scientific community, both in the field of chronic diseases and in the polarization of the M1 and M2 macrophage phenotypes. In this sense, there are multiple studies on EPA and DHA that show their participation in the reduction in arachidonic acid (ARA)-derived lipid mediators and the modulation of inflammation. According to Allam-Ndoul et al. [41], a combination of 75 μM each of EPA and DHA in a 1:1 ratio modulates inflammation (dose-dependently) inhibiting polarization towards M1, in a human monocytic THP-1 cell line. EPA seems to have larger effects where inflammation is already established that it has resolving effects, while DHA and the EPA/DHA mixture presented higher effectiveness when administered at the same time as the proinflammatory inducer (LPS) [41]. Also, they found that DHA is more potent than EPA. In the same line, Kawano et al. [42] studied the effect of DHA on macrophage polarization, in cells of human monocytic lineage (U937 and THP-1). They showed a polarization towards M2 (CD23, CD206), with secretion of anti-inflammatory cytokines such as TGF-β and IL-10. DHA acts on a p38 MAPK-dependent pathway, a route that participates in the polarization and production of cytokines, and increases the transcription factor KLF4, an inhibitor of NF-κB activity [42]. In the context of metabolic disease, there are a few studies related to the role of omega-3 in macrophage polarization. Song et al. [43] found that macrophage infiltration into the liver and adipose tissue is diminished in a model of fat-1 high-fat diet (HFD)-induced obesity mice, where fat-1 is a gene modification able to convert omega-6 to omega-3 PUFAs in vivo. These investigators found that omega-3 suppresses proinflammatory M1, enhancing M2 polarization in adipose tissue macrophages, and causes anti-inflammatory and insulin-sensitizing effects {70]. More recently, Ontoria-Oviedo et al. reported that the administration of a commercial nutritional preparation of omega-3 (LIPINOVA®) could promote wound closure in a db/db model in the context of diabetes mellitus type-2 (DM2)-related ulcers [39]. The resolution of the wound was directly related to a decrease in the ratio M1/M2 in the area of the noxa, measured by the ratio F4/80+CD274+ (M1) /F4/80+/CD206+ (M2) [31]. Going further, Carpino et al. evaluated the macrophage polarization in 32 children with biopsy-proven NAFLD, 20 of whom received 250 mg/day DHA for 18 months [44]. DHA-treatment determined a significant reduction in liver steatosis, hepatocyte ballooning, and the number of portal CD68+ and total S100A9+ macrophages, concomitantly with the enhancement of the anti-inflammatories CD206+ and CD163+/Arginase1+ lobular macrophages. Also, DHA treatment caused an increased number of apoptotic macrophages [45]. Despite the slight information that exists at the hepatic level, there is clear evidence of factors that would be key in the modulation of the polarization of macrophages by omega-3s, and given their importance, the derivatives of these fatty acids will be described below.
3. Omega-3 Lipid Mediators
Studies in the last 15 years show the existence of specialized pro-resolving mediators (SPMs) derived from omega-3s with resolving and protecting properties in inflammatory processes, known as resolvins (Rvs), protectins (PDs/NPDs), and maresins (MaRs) [45][46][47]. These molecules were isolated for the first time from inflammatory exudates of murine models and are the product of a series of enzymatic reactions, where EPA and DHA are metabolized by the same enzymes, cyclooxygenase-2 (COX-2) and 15-lipoxygenase (15-LOX), that also participate in ARA metabolism. It should be noted that these enzymes have a higher affinity for ARA than omega-3s, which is why high concentrations of intracellular EPA and DHA are required for clinical benefits [48][49]. Among the Rvs, it is possible to find the E-series including RvE1 and RvE2. Their synthesis is initiated in the presence of aspirin (acetylsalicylic acid) which acetylates COX-2 that produces prostaglandins (PG) [50][51]. SPMs can also be generated independently of aspirin via cytochrome P450 mono-oxygenases that convert n-3 PUFAs into epoxy and hydroxy fatty acids [52][53]. The D-series of Rvs include RvD1/RvD2/RvD3/RvD4/RvD5/RvD6, and they can have or not forms derived from aspirin [54][55][56][57][58].
In relation to PD1 or Neuroprotectin (NPD1), their names change depending on where their biosynthesis occurs; the “neuro” suffix for NPD1 is added when it occurs in neural cells/neural ectoderm, while PD1 is mainly synthesized in immune cells [45][47][59]. In particular, PD1 (Table 2) has been related to a decrease in leukocyte infiltration in murine models of the immune, cardiovascular, and renal systems [53].
Maresin-1 and 2 were the last discovered SPMs. They are generated by the 12-LOX catalyzed epoxidation and enzymatic hydrolysis in macrophages and platelets [60][61][62][63]. In recent years, MaR1 has been studied in different tissues and organs, describing its resolution actions in inflammatory pathologies in rodent and human brain [64][65], heart [66][67], kidney [68][69], and liver [70][71][72] (Table 2). The effects of MaR1 would even exceed, in some cases, those produced by other pro-resolving molecules whose effects have already been proven, such as RvD1. This opens up the discussion of its efficacy in more complex and exhaustive models and its possible role, in the long term, as treatment of inflammatory diseases [63].
Table 2. Protective functions of n-3 PUFA derived specific proresolving mediators (SPMs; resolvins, protectins, and maresins) related to inflammatory and macrophage modulation.
| 1. General Functions | ||
| SPM | Doses | Effects |
| Resolvin E1 (RvE1) |
100 ng per mouse | Breaks off inflammatory infiltration by over 50% in a murine model of acute inflammation, promoting PMN macrophage ingestion, reducing inflammatory pain, and regulating the activity of leukocytes and platelets [53] |
| Resolvin D1 (RvD1) |
2 ug/kg | RvD1 reduces macrophages by >50% in adipose tissue (F4/80+CD11c+ in a male leptin receptor-deficient (db/db) mice) thought ALX/FPR2 lipoxin receptor [54]. |
| Resolvin D3 (RvD3) |
10 ng per mouse | Enhances human macrophage efferocytosis and reduces human platelet-PMN aggregation in mice’s model of E. coli peritonitis [55] |
| Resolvin D4 (RvD4) |
1 to 100 nM | Stimulates whole-blood neutrophil phagocytosis of Escherichia coli. RvD4 increased bone marrow macrophage efferocytosis of neutrophils [56] |
| Resolvin D5 (RvD5) |
0.1–1 μg per mouse | Reduce granulocyte infiltration preventing local and systemic inflammation in an intestinal ischemia/reperfusion model and decrease neutrophil counts in a zymosan peritonitis mice model [57]. |
| Resolvin D6 (RvD6) |
---- | Promotes corneal wound healing and restores corneal innervation after injury mice model and its present in mouse tears [58]. |
| Protectin D1 (PD1) |
10 ng | Reduces of PMN and leukocyte infiltration into the inflammatory exudate, and also limits the expression of cytokines of this type, such as IL-6 in a murine model of peritonitis. In addition, PD1 decreases cell damage and, in turn, promotes tissue recovery [50]. |
| Neuroprotectin D1 (NPD1) |
100 ng per mouse | PD1 has been studied in models of stroke-mediated brain damage, which is defined as brain lesions associated with an interruption of blood supply to this organ, and ocular damage where it has a powerful protective action on the retina and the brain, giving its neuro-protective character [53]. |
| Maresin 1 (MaR1) |
4 to 25 μ/Kg (mouse model) 1 and 10 nM (human in vitro) 1 μg per mouse |
In the in vivo and in vitro administration, a very low concentration of MaR1 was able to decrease PMN infiltration and increase phagocytosis of apoptotic PMNs (efferocytosis), which relate to a shortening of the resolution phase of inflammation and restoration of homeostasis, that also protects the remaining cells, that are less exposed to oxidative stress and, therefore, are able to maintain homeostasis [61]. Also, MaR1 increased the proportion of Tr1 cells (CD3+, CD4+, CD49b+, which are crucial in maintaining tolerance to self-antigens) and increased in ~ 50% the percentage of this lymphocyte subset producing IL-10 in a mice model of experimental autoimmune encephalomyelitis (EAE) [63]. |
| 2. Role in macrophage polarization | ||
| SPM | Doses | Effects |
| SPMs | Historically, SPMs have been characterized as molecules with anti-inflammatory and resolving characteristics through mechanisms such as reduced inflammatory infiltration, decreased trans-epithelial migration of PMNs, decreased expression of proinflammatory cytokines and increased efferocytosis. This has been related to an improvement in the resolution phase of inflammatory processes and restoration of general homeostasis; thus, SPMs likely act on the inflammatory environment by promoting macrophage polarization towards the anti-inflammatory M2 phenotype [61][63]. | |
| RvE1–RvD1 | 0.1, 1, 10, and 100 nM | In studies of lung inflammation induced by nanomaterials in a murine model, there is a temporal correlation between the endogenous RvD1 and RvE1 peaks and the polarization towards an M2 anti-inflammatory macrophage phenotype [73]. RvD1 administration increased phagocytic activity, attenuating ROS production, a process typically associated with the M1 pro-inflammatory phenotype [74]. |
| MaR1 | 10 nM 100 ng |
MaRs not only is produced by human macrophages, but it also shifted macrophage phenotype from a CD54+CD80+ M1 to a CD163+CD206+ M2 phenotype [75]. M1 macrophage differentiation while promoting M2 macrophage differentiation in LPS-stimulated mice, inhibiting an CD38+CD80+CD86+iNOS+ M1 macrophage differentiation promoting a CD36+CD163+CD206+Arg-1+ M2 phenotype [76]. |
| PD1 | 100 nM | In the context of PD1, it was not possible to find assays related to macrophage polarization activity, although Ma et al. demonstrated that PD1 inhibits inflammatory cell death or piroptosis in macrophages, improving survival in LPS-induced sepsis inflammatory model [77]. |
CD, cluster of differentiation; IFN-γ, interferon-γ; IL-1β, interleukin-1β; LPS, lipopolyssacharide; PMN, polymorphonuclear cells; TNF-α, tumor necrosis factor-α; ----, no doses informed.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242115528
References
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Mondaca, S.; Vane, J.R. Eicosaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119.
- Valenzuela, R.; Ortiz, M.; Hernández-Rodas, M.C.; Echeverría, F.; Videla, L.A. Targeting n-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease. Curr. Med. Chem. 2020, 27, 5250–5272.
- Vielma, F.H.; Valenzuela, R.; Videla, L.A.; Zúñiga-Hernández, J. N-3 polyunsaturated fatty acids and their lipid mediators as a potential immune–nutritional intervention: A molecular and clinical view in hepatic disease and other non-communicable illnesses. Nutrients 2021, 13, 3384.
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537.
- Cheemerla, S.; Balakrishnan, M. Global epidemiology of chronic liver disease. Clin. Liver Dis. 2021, 17, 365–370.
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133.
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in north Western Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661.
- Tur, J.A.; Bibiloni, M.M.; Sureda, A.; Pons, A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. Brit. J. Nutr. 2012, 107, S23–S52.
- Valenzuela, R.; Metherel, A.; Cisbani, G.; Smith, M.; Chouinard-Watkins, R.; Klievik, B.J.; Videla, L.A.; Bazinet, R.P. Protein concentrations and activities of fatty acid desaturase and elongase enzymes in liver, brain, testicle, and kidney from mice: Substrate dependency. Biofactors 2023.
- Videla, L.A.; Hernandez-Rodas, M.C.; Metherel, A.H.; Valenzuela, R. Influence of the nutritional status and oxidative stress in the desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids: Impact on non-alcoholic fatty liver disease. Prostaglandins Leukot. Essent. Fat. Acids 2022, 181, 102441.
- Dinicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low omega-6/omega-3 ratio for reducing inflammation. Open Heart 2018, 5, e000946.
- Johnson, A.M.; Kleczko, E.K.; Nemenoff, R.A. Eicosanoids in cancer: New roles in immunoregulation. Front. Pharmacol. 2020, 11, 595498.
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934.
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140.
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L.A. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200.
- Fujikura, K.; Heydari, B.; Ge, Y.; Kaneko, K.; Abdullah, S.; Harris, W.S.; Jeydari, B. Insulin resistance modifies the effects of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction (from the OMEGA-REMODEL Randomized Clinical Trial). Am. J. Cardiol. 2020, 125, 678–684.
- Zúñiga-Hernández, J.; Sambra, V.; Echeverría, F.; Videla, L.A.; Valenzuela, R. N-3 PUFAs and their specialized pro-resolving lipid mediators on airway inflammatory response: Beneficial effects in the prevention and treatment of respiratory diseases. Food Funct. 2022, 13, 4260–4272.
- Watanabe, Y.; Tatsuno, I. Prevention of cardiovascular events with omega-3 polyunsaturated fatty acids and the mechanism involved. J. Atheroscler. Thromb. 2020, 27, 183–198.
- Naeini, Z.; Toupchian, O.; Vatannejad, A.; Sotoudeh, G.; Teimouri, M.; Ghorbani, M.; Nasli-Esfahani, E.; Koohdani, F. Effects of DHA-enriched fish oil on gene expression levels of p53 and NF-kappa B and PPAR-gamma activity in PBMCs of patients with T2DM: A randomized, double-blind, clinical trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 441–447.
- Bowden, R.G.; Wilson, R.L.; Deike, E.; Gentile, M. Fish oil supplementation lowers C-reactive protein levels independent of triglyceride reduction in patients with end-stage renal disease. Nutr. Clin. Pract. 2009, 24, 508–512.
- Pisaniello, A.D.; Psaltis, P.J.; King, P.M.; Liu, G.; Gibson, R.A.; Tan, J.T.; Duong, M.; Nguyen, T.; Bursill, C.A.; Worthley, M.I.; et al. Omega-3 fatty acids ameliorate vascular inflammation: A rationale for their atheroprotective effects. Atherosclerosis 2021, 324, 27–37.
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Duong, M.; Nguyen, T.; Bursill, C.A.; Worthley, M.I.; et al. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003, 44, 479–486.
- Leaf, A.; Kang, J.X.; Xiao, Y.F.; Billman, G.E. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation 2003, 107, 2646–2652.
- Chen, J.; Shearer, G.C.; Chen, Q.; Healy, C.L.; Beyer, A.J.; Nareddy, V.B.; Gerdes, A.M.; Harris, W.S.; O’Connell, T.D.; Wang, D. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation 2011, 123, 584–593.
- Heydari, B.; Abdullah, S.; Pottala, J.V.; Shah, R.; Abbasi, S.; Mandry, D.; Francis, S.A.; Lumish, H.; Ghoshhajra, B.B.; Hoffmann, U.; et al. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: The OMEGA-REMODEL Randomized Clinical Trial. Circulation 2016, 134, 378–391.
- Okada, L.S.D.R.R.; Oliveira, C.P.; Stefano, J.T.; Nogueira, M.A.; da Silva, I.D.C.G.D.; Cordeiro, F.B.; Alves, V.A.F.; Torrinhas, R.S.; Carrilho, F.J.; Puri, P.; et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH—Proteomic and lipidomic insight. Clin. Nutr. 2018, 37, 1474–1484.
- Song, L.; Zhao, X.G.; Ouyang, P.L.; Guan, Q.; Yang, L.; Peng, F.; Du, H.; Yin, F.; Yan, W.; Yu, W.J.; et al. Combined effect of n-3 fatty acids and phytosterol esters on alleviating hepatic steatosis in non-alcoholic fatty liver disease subjects: A double-blind placebo-controlled clinical trial. Br. J. Nutr. 2020, 123, 1148–1158.
- Romero, V.C.; Somers, E.C.; Stolberg, V.; Clinton, C.; Chensue, S.; Djuric, Z.; Berman, D.R.; Treadwell, M.C.; Vahratian, A.M.; Mozurkewich, E.; et al. Developmental programming for allergy: A secondary analysis of the Mothers, Omega-3, and Mental Health Study. Am. J. Obstet. Gynecol. 2013, 208, 316.e1–316.e6.
- Toupchian, O.; Sotoudeh, G.; Mansoori, A.; Nasli-Esfahani, E.; Djalali, M.; Keshavarz, S.A.; Koohdani, F. Effects of DHA-enriched fish oil on monocyte/macrophage activation marker sCD163, asymmetric dimethyl arginine, and insulin resistance in type 2 diabetic patients. J. Clin. Lipidol. 2016, 10, 798–807.
- Fisk, H.L.; Childs, C.E.; Miles, E.A.; Ayres, R.; Noakes, P.S.; Paras-Chavez, C.; Antoun, E.; Lillycrop, K.A.; Calder, P.C. Dysregulation of subcutaneous white adipose tissue inflammatory environment modelling in non-insulin resistant obesity and responses to omega-3 fatty acids—A double blind, randomised clinical trial. Front. Immunol. 2022, 13, 922654.
- Mazurak, V.C.; Lien, V.; Field, C.J.; Goruk, S.D.; Pramuk, K.; Clandinin, M.T. Long-chain polyunsaturated fat supplementation in children with low docosahexaenoic acid intakes alters immune phenotypes compared with placebo. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 570–579.
- Thies, F.; Garry, J.M.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485.
- Takashima, A.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Nishimoto, S. Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis 2016, 254, 142–150.
- Yoshihara, T.; Shimada, K.; Fukao, K.; Sai, E.; Sato-Okabayashi, Y.; Matsumori, R. Omega 3 polyunsaturated fatty acids suppress the development of aortic aneurysms through the inhibition of macrophage-mediated inflammation. Circ. J. 2015, 79, 1470–1478.
- Sorokin, A.V.; Arnardottir, H.; Svirydava, M.; Ng, Q.; Baumer, Y.; Berg, A. Comparison of the dietary omega-3 fatty acids impact on murine psoriasis-like skin inflammation and associated lipid dysfunction. J. Nutr. Biochem. 2023, 117, 109348.
- Wang, H.; Khor, T.O.; Saw, C.L.; Lin, W.; Wu, T. Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Mol. Pharm. 2010, 7, 2185–2193.
- Takase, O.; Hishikawa, K.; Kamiura, N.; Nakakuki, M.; Kawano, H.; Mizuguchi, K.; Fujita, T. Eicosapentaenoic acid regulates IκBα and prevents tubulointerstitial injury in kidney. Eur. J. Pharmacol. 2011, 669, 128–135.
- Elsayed, H.R.H.; Anbar, H.S.; Rabei, M.R.; Adel, M.; El-Gamal, R. Eicosapentaenoic and docosahexaenoic acids attenuate methotrexate-induced apoptosis and suppression of splenic T, B-Lymphocytes and macrophages with modulation of expression of CD3, CD20 and CD68. Tissue Cell 2021, 72, 101533.
- Ontoria-Oviedo, I.; Amaro-Prellezo, E.; Castellano, D.; Venegas-Venegas, E.; González-Santos, F.; Ruiz-Saurí, A.; Pelacho, B.; Prósper, F.; Pérez del Caz, M.D.; Sepúlveda, P. Topical Administration of a marine oil rich in pro-resolving lipid mediators accelerates wound healing in diabetic db/db mice through angiogenesis and macrophage polarization. Int. J. Mol. Sci. 2022, 23, 9918.
- Chadli, F.K.; Andre, A.; Prieur, X.; Loirand, G.; Meynier, A.; Krempf, M. n-3 PUFA prevent metabolic disturbances associated with obesity and improve endothelial function in golden Syrian hamsters fed with a high-fat diet. Br. J. Nutr. 2012, 107, 1305–1315.
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.C. Effect of different concentrations of omega-3 fatty acids on stimulated THP-1 macrophages. Genes Nutr. 2017, 12, 7.
- Kawano, A.; Ariyoshi, W.; Yoshioka, Y.; Hikiji, H.; Nishihara, T.; Okinaga, T. Docosahexaenoic acid enhances M2 macrophage polarization via the p38 signaling pathway and autophagy. J. Cell. Biochem. 2019, 120, 12604–12617.
- Song, M.Y.; Wang, J.; Lee, Y.; Lee, J.; Kwon, K.S.; Bae, E.J.; Park, B.H. Enhanced M2 macrophage polarization in high n-3 polyunsaturated fatty acid transgenic mice fed a high-fat diet. Mol. Nutr. Food Res. 2016, 60, 2481–2492.
- Carpino, G.; Nobili, V.; Renzi, A.; De Stefanis, C.; Stronati, L.; Franchitto, A.; Alisi, A.; Onori, P.; De Vito, R.; Alpini, G.; et al. Macrophage activation in pediatric nonalcoholic fatty liver disease (NAFLD) correlates with hepatic progenitor cell response via wnt3a pathway. PLoS ONE 2016, 11, e0157246.
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48.
- Mariqueo, T.A.; Zúñiga-Hernández, J. Omega-3 derivatives, specialized pro-resolving mediators: Promising therapeutic tools for the treatment of pain in chronic liver disease. Prostaglandins Leukot. Essent. Fat. Acids 2020, 158, 102095.
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 2011, 18, 629–647.
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Progr. Lipid Res. 2022, 86, 101165.
- Cabo-Garcia, L.; Achon-Tunon, M.; Gonzalez-Gonzalez, M.P. The influence of the polyunsaturated fatty acids in the prevention and promotion of cancer. Nutr. Hosp. 2015, 32, 41–49.
- Sommer, C.; Birklein, F. Resolvins and inflammatory pain. F1000 Med. Rep. 2011, 3, 19.
- Kwon, Y. Immuno-resolving ability of resolvins, protectins, and maresins derived from omega-3 fatty acids in metabolic syndrome. Mol. Nutr. Food Res. 2020, 64, e1900824.
- Divanovic, S.; Dalli, J.; Jorge-Nebert, L.F.; Flick, L.M.; Gálvez-Peralta, M.; Boespflug, N.D.; Stankiewicz, T.E.; Fitzgerald, J.M.; Somarathna, M.; Karp, C.L.; et al. Contributions of the three CYP1 monooxygenases to pro-inflammatory and inflammation-resolution lipid-mediator pathways. J. Immunol. 2013, 191, 3347–3357.
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943.
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413.
- Norris, P.C.; Arnardottir, H.; Sanger, J.M.; Fichtner, D.; Keyes, G.S.; Serhan, C.N. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 81–89.
- Libreros, S.; Nshimiyimana, R.; Lee, B.; Serhan, C.N. Infectious neutrophil deployment is regulated by resolving D4. Blood 2023, 142, 586–606.
- Gobbetti, T.; Dalli, J.; Colas, R.A.; Canova, D.F.; Aursnes, M.; Bonnet, D.; Alric, L.; Vergnolle, N.; Deraison, C.; Hansen, T.V.; et al. Protectin D1n-3 DPA and resolvin D5n-3 DPA are effectors of intestinal protection. Proc. Natl. Acad. Sci. USA 2017, 114, 3963–3968.
- Pham, T.L.; Kakazu, A.H.; He, J.; Jun, B.; Bazan, N.G.; Bazan, H.E.P. Novel RvD6 stereoisomer induces corneal nerve regeneration and wound healing post-injury by modulating trigeminal transcriptomic signature. Sci. Rep. 2020, 10, 4582.
- Marcheselli, V.L.; Mukherjee, P.K.; Arita, M.; Hong, S.; Antony, R.; Kristopher Sheets, K.; Winkler, J.W.; Petasis, N.A.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 27–34.
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23.
- Liu, W.C.; Yang, Y.H.; Wang, Y.C.; Chang, W.M.; Wang, C.W. Maresin: Macrophage Mediator for Resolving Inflammation and Bridging Tissue Regeneration—A System-Based Preclinical Systematic Review. Int. J. Mol. Sci. 2023, 24, 101012.
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.K.; Xu, Z.Z.; Ji, R.R.; Zhu, M.; Petasis, N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765.
- Sánchez-Fernández, A.; Zandee, S.; Mastrogiovanni, M.; Charabati, M.; Rubbo, H.; Prat, A.; López-Vales, R. Administration of Maresin-1 ameliorates the physiopathology of experimental autoimmune encephalomyelitis. J. Neuroinflam. 2022, 19, 27.
- Xian, W.; Li, T.; Li, L.; Hu, L.; Cao, J. Maresin 1 attenuates the inflammatory response and mitochondrial damage in mice with cerebral ischemia/reperfusion in a SIRT1-dependent manner. Brain Res. 2019, 1711, 83–90.
- Wahyuni, T.; Kobayashi, A.; Tanaka, S.; Miyake, Y.; Yamamoto, A.; Bahtiar, A.; Mori, S.; Kametani, Y.; Tomimatsu, M.; Matsumoto, K.; et al. Maresin-1 induces cardiomyocyte hypertrophy through IGF-1 paracrine pathway. Am. J. Physiol. Cell Physiol. 2021, 321, C82–C93.
- Yang, Y.; Li, X.Y.; Li, L.C.; Xiao, J.; Zhu, Y.M.; Tian, Y.; Sheng, Y.M.; Chen, Y.; Wang, J.G.; Jin, S.W. γδ T/Interleukin-17A contributes to the effect of maresin conjugates in tissue regeneration 1 on lipopolysaccharide-induced cardiac injury. Front. Immunol. 2021, 12, 674542.
- Li, J.; Zhang, Z.; Wang, L.; Jiang, L.; Qin, Z.; Yuliang Zhao, Y.; Su, B. Maresin 1 attenuates lipopolysaccharide-induced acute kidney injury via inhibiting NOX4/ROS/NF-κB pathway. Front. Pharmacol. 2021, 12, 782660.
- Li, X.; Xu, B.; Wu, J.; Pu, Y.; Wan, S.; Zeng, Y.; Wang, M.; Luo, L.; Zhang, F.; Jiang, Z.; et al. Maresin 1 alleviates diabetic kidney disease via LGR6-mediated cAMP-SOD2-ROS pathway. Oxid. Med. Cell. Longev. 2022, 2022, 7177889.
- Soto, G.; Rodríguez, M.J.; Fuentealba, R.; Treuer, A.V.; Castillo, I.; González, D.R.; Zúñiga-Hernández, J. Maresin 1, a proresolving lipid mediator, ameliorates liver ischemia-reperfusion injury and stimulates hepatocyte proliferation in Sprague-Dawley rats. Int. J. Mol. Sci. 2020, 21, 540.
- Rodríguez, M.J.; Sabaj, M.; Tolosa, G.; Herrera Vielma, F.; Zúñiga, M.J.; González, D.R.; Zúñiga-Hernández, J. Maresin-1 prevents liver fibrosis by targeting Nrf2 and NF-κB, reducing oxidative stress and inflammation. Cells 2021, 10, 3406.
- Tang, D.; Fu, G.; Li, W.; Sun, P.; Loughran, P.A.; Deng, M.; Scott, M.J.; Billiar, T.R. Maresin 1 protects the liver against ischemia/reperfusion injury via the ALXR/Akt signaling pathway. Mol. Med. 2021, 27, 18.
- Dalli, J.; Serhan, C.N. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012, 120, e60–e72.
- Lim, C.S.; Porter, D.W.; Orandle, M.S.; Green, B.J.; Barnes, M.; Croston, T.L.; Wolfarth, M.G.; Battelli, L.A.; Andrew, M.E.; Beezhold, D.H.; et al. Resolution of pulmonary inflammation induced by carbon nanotubes and fullerenes in mice: Role of macrophage polarization. Front. Immunol. 2020, 11, 1186.
- Titos, E.; Rius, B.; González-Périz, A.; López-Vicario, C.; Morán-Salvador, E.; Martínez-Clemente, M.; Arroyo, V.; Clària, J. Resolvin D1 and Its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011, 187, 5408–5418.
- Dalli, J.; Zhu, M.; Vlasenko, N.A.; Deng, B.; Haeggström, J.Z.; Petasis, N.A.; Serhan, C.N. The novel 13S,14S-epoxy-maresinis converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013, 27, 2573–2583.
- Li, D.; Wang, M.; Ye, J.; Zhang, J.; Xu, Y.; Wang, Z.; Zhao, M.; Ye, D.; Wan, J. Maresin 1 alleviates the inflammatory response, reduces oxidative stress and protects against cardiac injury in LPS-induced mice. Life Sci. 2021, 277, 119467.
- Ma, M.Q.; Zheng, S.S.; Chen, H.L.; Xu, H.B.; Zhang, D.L.; Zhang, Y.A.; Xiang, S.Y.; Cheng, B.H.; Jin, S.W.; Fu, P.H. Protectin conjugates in tissue regeneration 1 inhibits macrophage pyroptosis by restricting NLRP3 inflammasome assembly to mitigate sepsis via the cAMP-PKA pathway. Lab. Investig. 2023, 103, 100028.
This entry is offline, you can click here to edit this entry!
