Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Sustainable water desalination and purification membrane processes require new practical pathways to improve their efficiency. To this end, the inclusion of two-dimensional materials in membrane structure has proven to have a significant impact in various applications.
- two-dimensional materials
- membrane distillation
- membrane crystallization
1. Introduction
Agricultural expansion, industrial development, population growth, and climate change have led to increased water withdrawals and water shortages in many regions of the world. The need to solve the water crisis is becoming so urgent that freshwater has taken the role of a strategic resource. Because oceans constitute nearly 97% of the world’s water, desalination has garnered significant attention as an effective method to extract freshwater from seawater, wastewater, or brackish water.
Commercial desalination technologies can be divided into two main categories: thermal distillation (multi-effect distillation (MED) and first multi-stage flash (MSF) distillation) and membrane separation (reverse osmosis (RO) and nanofiltration (NF)). The former involves phase change processes, whilst the latter involves semipermeable membrane systems [1]. RO technology is used in >90% of the current desalination plants and contributes to 69% of annual desalinated water production [2]. However, RO can recover max 50% due to the osmotic phenomena.
Recently, among the different emerging technologies, membrane distillation (MD) has received considerable attention both from an industrial and academic perspective. MD is a thermally driven separation process that uses a microporous hydrophobic membrane to separate dissolved molecules from a liquid stream [3]. Its advantages include a lower operating pressure compared to RO process which allows for the use of low-grade thermal energy and renewable energy for its operation. In addition, many efforts to further reduce MD energy consumption have been reported in recent years, including photothermal membrane distillation [4] and hybrid membrane distillation systems [5].
One of the most interesting and promising extension of MD concept is represented by the membrane crystallization (MCr) process which provides the possibility of recovering simultaneously pure water and high-quality salt products using the logic of zero liquid discharge (ZLD) [6].
Nevertheless, despite their great potential, MD and MCr have some drawbacks such as flux lowering due to poor hydrodynamics and inefficient module design, and distillate contamination due to membrane wetting; the latter is one of the main obstacles to the wider application of MD. Therefore, developing new advanced membrane materials with good performance and low cost has become essential to intensify the MD process. Ideal MD membranes are expected to exhibit features such as high liquid entry pressure (LEP), high permeability, low fouling rate, low thermal conductivity, excellent chemical and thermal stability and excellent mechanical strength.
Polymeric membranes have long been considered for MD applications due to their good processability, intrinsic hydrophobicity, and low cost.
However, they present disadvantages such as limited chemical stability, fouling, and short lifetime. In contrast, inorganic membranes (zeolite, silica, zeolite, carbon, and hybrid inorganic–organic one) exhibit higher chemical, thermal, and mechanical stabilities that make them potential candidates for MD applications. In particular, zeolites [7][8][9] and carbon nanotubes [10][11] were suitable for water desalination due to their subnanometer pores/channels. However, the high cost- and time-consuming process of preparing perfectly ordered subnanometer arrays of zeolites and carbon nanotubes hinder their commercialization [12].
Since the discovery of single-layer graphene, two-dimensional (2D) materials have garnered notable interest in many fields of science and technology including MD processes. In the last decade, research on the role of 2D materials in membrane science has grown as they have a high potential of providing new solutions to significant problems. Two-dimensional materials are suitable for the fabrication of high-performance membranes in terms of specificity, selectivity, high flux, chemical and mechanical resistance [13]. Members of the family of 2D materials include graphene-based materials, transition metal dichalcogenides (TMDs), 2D zeolites, metal organic frameworks (MOFs) and carbides (MXenes) [7][14][15][16][17].
1.1. The Advent of 2D Materials
Two-dimensional materials are crystalline solids with a high ratio between their lateral size (∼1–10,000 μm) and thickness (<1 nm) [18]. They consist of single- or few-layer atoms in which the in-plane interatomic interactions are much stronger than those along the staking direction [19]. Being ultrathin, 2D materials exhibit exceptional physical, electrical, chemical, and optical properties [20][21][22].
Two-dimensional materials have garnered great interest in seawater treatment applications because ion-sieving, either via in-plane pores or stacked nanosheet channels, can be achieved and both can be economically produced on a large scale. The hyper-thinness of 2D monolayers makes it possible to fabricate ultra-thin membranes with excellent mass transport and high transmembrane fluxes [23]. Furthermore, 2D materials can significantly improve the selectivity of solutes due to their structural and chemical homogeneity [24]. Currently, nanofiltration is the membrane process where 2D materials are most commonly used.
In 2004, graphene was the first 2D material discovered, becoming the most representative of 2D materials [25][26]. The possibility of cheaper synthesis of large-area graphene sheets makes this material very interesting for large-scale applications [27]. Furthermore, graphene has shown great potential as filler for composite materials, giving it outstanding mechanical and thermal properties [28][29].
The early successes of graphene have inspired much research on the use of 2D materials for next-generation membranes [13]. As a result, a variety of new 2D materials have been discovered. Examples include 2D boron nitride (BN), 2D transition metal dicalcogenides (e.g., molybdenum disulfide (MoS2), tungsten chalcogenides (WS2)), monoelemental 2D materials (such as borophene or phosphorene) and carbides or transition metal carbonitrides (MXenes) [4][30][31][32][33]. Some of them (MoS2, BN and MXenes) were already extensively studied in membrane technologies for water purification. Two-dimensional transition metal dichalcogenides (TMDCs), in recent years, were mainly investigated as an alternative route to replace the metal oxide-based photocatalyst, thanks to their suitable bandgap (1.6–2.03 eV) for the collection of visible light. Bidimensional TMDCs are endowed with high mobility of charge carriers which provide large active surface for dye degradation [34][35][36][37].
1.1.1. Graphene
Graphene is a single layer of carbon atoms arranged in a flat honeycomb lattice. Over the years, graphene and its derivatives have found application in many fields, from water treatment to electronic and optics, due to its peculiarity such as excellent electronic conductivity, hydrophobic and anti-wetting nature, anti-fouling properties, great mechanical strength, low density and selective absorption of water vapors [38]. The introduction of graphene in membrane science is a promising approach to support sustainable growth in industrial development and research [39]. Few-layers graphene membranes exhibit ideal characteristics for the membrane contactor process, such as narrow pore size, high performance in terms of permeability and selectivity [40][41][42]. Generally, graphene is produced via exfoliation of graphite sheets where the particles of uncontaminated or expanded graphite (obtained from the thermal expansion of graphite intercalation compounds) are first dispersed in a solvent and subsequently, through an applied driving force (ultrasound, electric field or cutting), the graphite exfoliates into high quality graphene sheets. Dispersion in a solvent is necessary to reduce the strength of the van der Waals attraction between the graphene layers. A drawback of this process is the necessity to remove the non-exfoliated graphite residue. On the contrary, the advantages of this process are very the high scalability and the low cost of liquid phase exfoliation, which make it suitable for the production of graphene in large quantities. The exfoliation technique is fundamental for the quality of graphene in terms of the number of layers, lateral dimension and thickness of the graphene flakes. Various techniques for graphene exfoliation are reported in the literature such as mechanical exfoliation [43][44], epitaxial graphene in silicon carbide (SiC) [45], direct growth of graphene on thin nickel [46], chemical vapor deposition (CVD) [47][48], etc. The most used method is liquid phase exfoliation (LPE), which (in a liquid environment) allows ultrasound or shear forces to break the van der Waals bond between the graphite layers, thereby causing the exfoliation of graphite [49]. In recent years, an innovative and advanced technique known as wet jet milling (MJW) was developed for high-quality graphene exfoliation. In particular, this technique allows to prepare grapheme films with well-controlled thickness and lateral size in an easy and scalable way [44].
When chemical oxidation occurs on the graphene, several oxygen-containing groups, such as carboxylic, hydroxyl, and epoxide functional groups are formed on the carbon surface. This is called graphene oxide (GO), which is usually prepared using the Hummers method [50]. This method consists of the addition of potassium permanganate to a solution of graphite, sodium nitrate, and sulfuric acid. GO is a nonstoichiometric compound that varies in compositions depending on the synthesis conditions (oxidizing agent, solvents, time, exfoliation, etc.). The exact composition and structure of chemically synthesized GO are still being discussed [51]. Li et al. [52] prepared graphene membranes to study the ripple of GO nanosheets and found that GO nanosheets could be successfully used in the nanofiltration process with a 67% rejection rate for direct yellow (i.e., a dye molecule that is often used as a model molecule in the characterization of separation membranes).
1.1.2. Transition Metal Dichalcogenides
Transition metal dichalcogenides are layered metal oxides classified as two-dimensional (2D) materials. Their intrinsic properties make them interesting materials for energy storage [13], catalysis [53], sensors [15] and biomedical applications [54]. Two-dimensional transition metal dichalcogenides include different materials such as molybdenum sulphide (MoS2), tungsten disulfide (WS2), MXene, boron nitride (BN), carbon nitride g-(C3N4), metal–organic framework (MOF) and covalent–organic framework (COF) [55]. Recently, single-layer or few-layer TMDs were used in the field of membranes due to their excellent molecular sieve [56] and high flux and antifouling proprieties in water treatment. They have strong covalent bonds in the plane and weak van der Waals bonds outside the plane [57]. TMD nanosheets, depending on the coordination environment of the transition metal and chalcogen, can be found in the two different polymorphic states. These two polymorphs are the stable 2H trigonal prism polymorph (semiconducting) and the 1T metastable octahedral polymorph (metallic) [58]. Lithium interception predominantly produces the 1T phase, but exfoliation into the liquid phase produces the 2H phase.
The use of 2D materials in membranes has shown promising results in gas separation processes, especially for MXene membranes [59], demonstrating high separation performance. In the case of layered TMD, there are limited reports on the use of MoS2 as an additive for the preparation of composite membrane for gas separation. For example, Coleman et al. [60] prepared MoS2 membranes with different thicknesses after exfoliation with butyllithium, which showed an H2/CO2 separation factor of 4.4–3.4 and an H2 permeability in the range of 2454–27,400 GPU [60]. Chari and Eswaramoorthy prepared MoS2 nanosheets [61] in 1T phase using butyllithium and converted them to 2H phase by heating the membrane [62]. The 2H phase showed better perselectivity than the 1T phase (H2/CO2 selectivity of 6.2 and 8.2 with an H2 permeability of 1740 and 1175 barrers, respectively).
1.2. Exfoliation of 2D Materials
Exfoliation consists of separating the individual layers more or less regularly to obtain materials with few layers. Nanofabrication methods can be classified into two groups: top-down and bottom-up approach [63][64] (Figure 1).
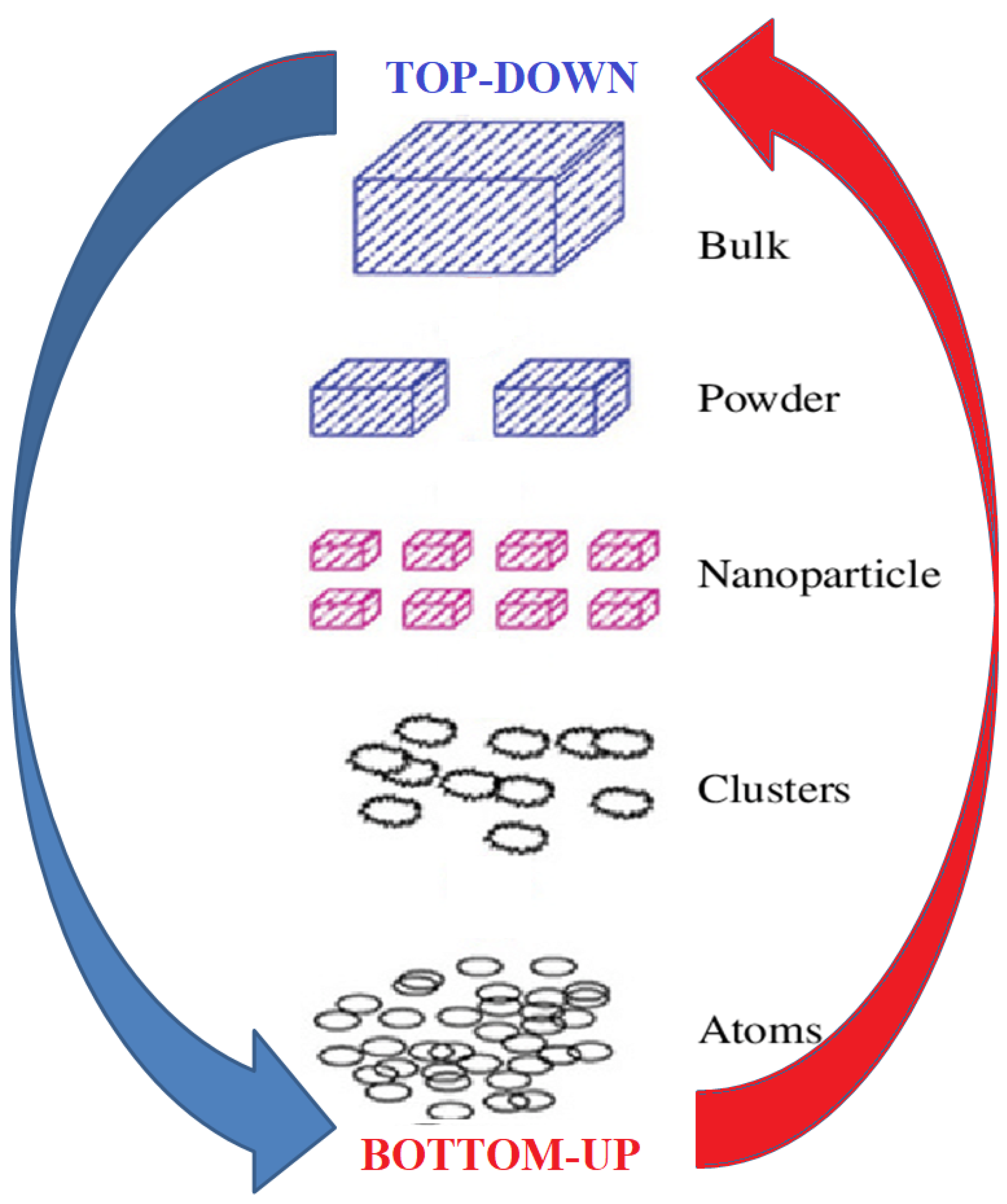
Figure 1. Top-down and bottom-up approaches for materials exfoliation.
The top-down method allows for the formation of 2D or nanometric materials starting from the raw material, reducing its size until the desired nanometric particle size is reached. Although top-down approaches are relatively simple to use, they are unsuitable for producing irregularly shaped and extremely small particles. The difficulty in obtaining the correct particle size and shape is the main drawback of this approach. The top-down method can be achieved through several techniques including tape exfoliation, metal exfoliation, intercalation and liquid-mediated exfoliation [65][66]. The exfoliation of layered bulk crystals into single- or few-layer sheets is generally achieved using chemical/physical driving forces to overcome the weak van der Waals interactions between the layers. Tape exfoliation of thin metal film is a very simple technique which mechanically strips loose crystals and produces single crystal flakes [44]. The single crystal flakes produced are often of high purity and clean lines, mainly suitable for the production of main devices and the characterization of new 2D systems. Regarding intercalation strategies, ionic species are interdispersed in the spaces between the layers. Intercalation creates expansion forces through the reaction and allows for the separation of layers in the liquid. Ultrasound, or liquid-mediated exfoliation, is used to produce a high local temperature and extreme pressure, which tends to break the bonds between the various layers [43]. Liquid phase preparations are considered more suitable for obtaining large production, although submicrometric scales with high defects and modified chemical or physical properties are often obtained. Monolayers prepared via fluid mediation are particularly useful for catalysis or other chemical applications [38].
The bottom-up approach is based on the direct construction of 2D nanomaterials with precursor atoms via the growth or self-assembly of atoms and molecules. The nanomaterials (NMs) produced using these techniques have a well-defined shape, size and chemical composition depending on the constituent elements. During deposition, nucleation at different local positions leads to continuous growth of individual domains, which subsequently coalesce into a continuous monolayer thin film constituting a polycrystalline film [67]. With tuned growth conditions, isolated single-crystalline flakes, down to a few hundred µm in the lateral dimension, can be obtained. Controlling the number of layers through targeted growth is particularly difficult due to the high barrier of nucleation [68].
Figure 2 illustrates the main exfoliation techniques. The illustrated techniques were utilized especially for graphene production. In some cases, several techniques were also utilized with other materials (such as carbon-based nanomaterials, silicate clays, TMDs, MOFs layered double hydroxides (LDHs), titanium dioxide (TiO2), Tetramethyl orthosilicate (TMOs), BN, black phosphorous (BP), etc.) [69][70]. High production costs are still the major barrier hindering commercial adoption. For example, in the case of graphene, on average, the cost is USD 550 per square centimeter; however, it could also drop down to between USD 100 and USD 200 when demand is expected to increase [71][72].
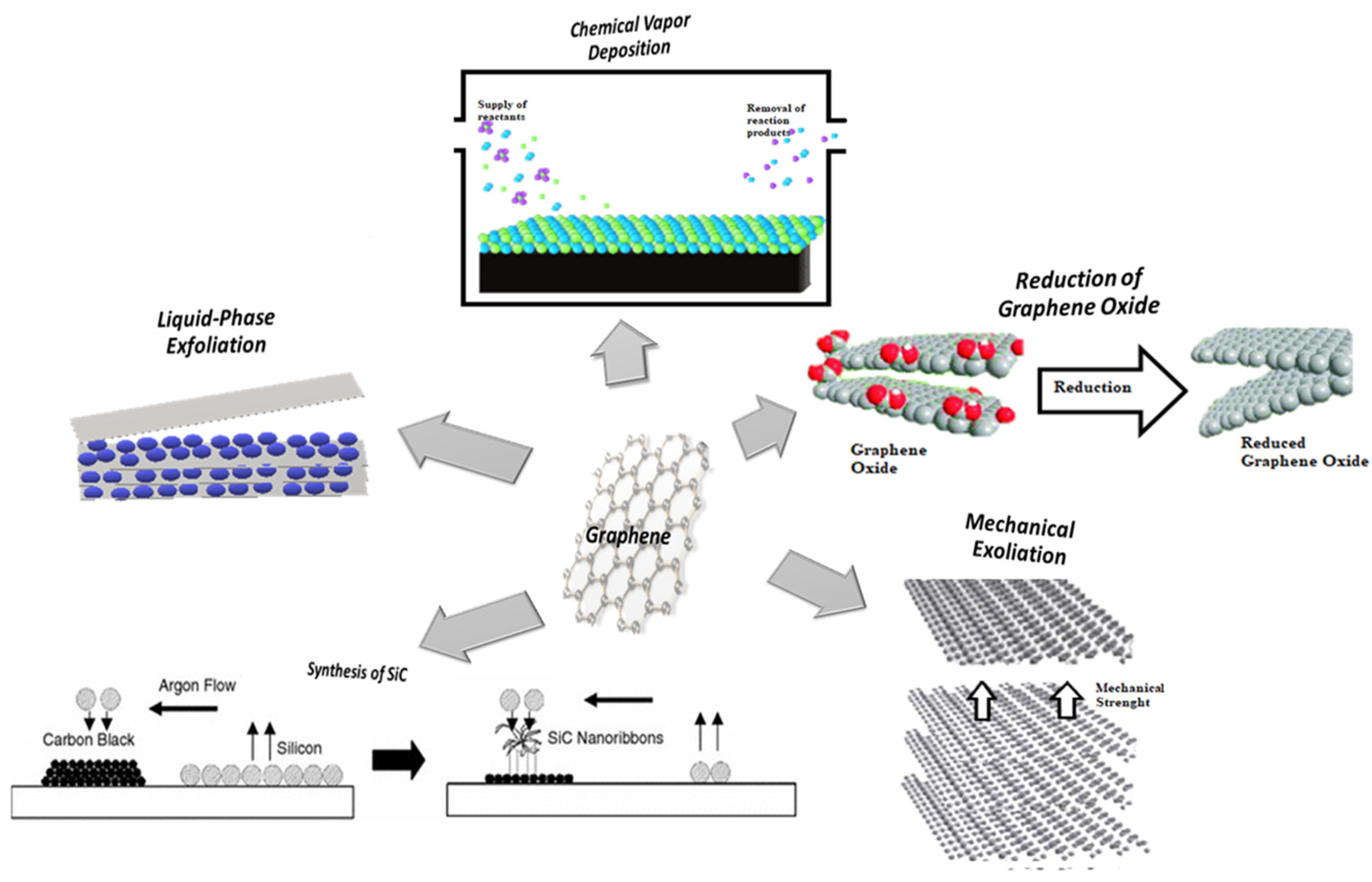
Figure 2. Main exfoliation methods used for the graphene production.
Due to low scalability and high manufacturing costs, methods such as mechanical exfoliation and SiC synthesis are no longer the forerunners to produce graphene for niche applications such as touch screens and high transistors frequency. Likewise, chemical vapor deposition of hydrocarbons, although an established technique in industry, appears to be generally unsuitable for mass production of graphene for electrochemical energy storage due to moderate product purity and rather low yields. Currently, the most popular production method for obtaining good quality graphene at reasonable cost is the chemical vapor deposition, in which the gaseous reactants form a graphene film on a metal substrate (usually a copper foil).
Reduced graphene oxide (rGO) is an alternative form of graphene oxide (GO) with a reduced amount of oxygen (as the latter makes GO unstable [40]). Typically, the reduction process can be achieved via electrochemical reduction, chemical reduction and thermal reduction [73][74][75][76]. rGO exhibits high conductivity, stability and also has the presence of defective sites that are chemically active, making it a potential candidate for application as an active material in biosensors [77]. rGO was commonly used to prepare composite membranes with various materials, such as metal and metal oxides, using different techniques to improve the performance of the supercapacitor.
Chemical vapor deposition (CVD) process is a gas-phase chemical methodology for the formation of thin layers (with thicknesses generally between 0.1 and 10 μm) of various materials [78][79]. The respective films or structures are formed as a result of the elimination of the volatile by-products produced during the thermally induced decomposition process. The operational requirements are as follows: controlled delivery of reagents in the gas phase, availability of a closed reaction chamber, gas discharge, regulation of the reaction pressure, supply of an energy source for chemical reactions, cleaning of the exhaust gases to achieve safe and non-toxic levels and control of the automation process to increase the stability of the deposition process [63][79].
Liquid phase exfoliation (LPE) is one of the main methods for producing two-dimensional (2D) materials in large quantities with a good balance between quality and cost. This technique is widely adopted by both the academic and industrial sectors. Liquid phase exfoliation can be applied in several ways. Based on the type of exfoliation used, different techniques can be distinguished. For example, a fragmentation or high-pressure mechanism can be used. The mechanisms involved in fragmentation and exfoliation are usually attributed to the interaction of ultrasound-induced forces with solvent molecules. The principal liquid phase exfoliation techniques are as follows [44]:
2. Preparation of Membranes Functionalized with 2D Materials
Several kinds of manufacturing techniques are proposed for the development of nanostructured membranes. Here, particular attention has been paid to the preparation techniques with a high degree of geometric order through two-dimensional (2D) and tridimensional (3D) space. Each approach has advantages and limitations, and endows certain characteristics to the membranes produced. Over the years, researchers have been trying to improve each technique in order to obtain increasingly efficient membranes [100].
The techniques based on traditional phase inversion allows to easily control the morphology of the membrane. These techniques require the polymer to be soluble in a suitable solvent or in a mixture of solvents. Two-dimensional materials are usually dispersed in the solvent or a mixture of solvent as described in Frappa et al. [23][101][102]. In principle, all the main phase inversion techniques can prepare membranes containing 2D materials (non-solvent-induced phase inversion (NIPS), vapor-induced phase separation (VIPS), thermally induced phase separation (TIPS) and evaporation of the solvent, (EIPS)). Phase inversion techniques have various advantages such as low formation of defects, possibility to use green solvents, high possibility to control the final morphology (pore size, thickness, etc.) and symmetric structures. At an industrial level, the membranes are commonly prepared with phase inversion techniques [103].
A second category of membranes is realized according to more sophisticated manufacturing strategies. These breakthrough methodologies include the following:
- (a)
-
Lithographic technique is proposed especially for creating polymeric surfaces with a regular structure and a precise geometric order. The main feature of lithography is the mechanical reproduction of certain images. At the nanometer level (lateral size between the size of a single atom and about 100 nm), lithography can be used in the fabrication of advanced semiconductor integrated circuits or nano electromechanical systems (NEMS). Lithography can also be performed using a support [104] on which the polymer solution is placed, making the final product exhibit certain structures and properties. Patterns are created with mechanical deformation of the impression strength and subsequent processes. The main disadvantages of this process are the high cost of making molds with dimensions of the nano order and the difficulty in obtaining a large resolution. On a larger scale, the creation of well-made surfaces turns out to be more efficient and easier.
- (b)
-
Phase separation micro-molding (PSμM) is a highly specialized manufacturing process that produces extremely small, high-precision thermoplastic parts and components with micron tolerances [105]. The process begins in a tooling department where a mold is created that has a cavity shaped like the desired part. Thermoplastic or resin is rapidly injected into the cavity, creating the component or part at high speed. The combination of micro-molding and phase separation techniques is another approach to create nanostructures with hierarchical ordered morphology. It can be seen as an evolution of the traditional phase reversal. A polymer is precipitated from a solution assuming a desired configuration similar to traditional phase inversion. The final configuration can be flat, cylindrical or spherical. The solidification process begins with the transition from a liquid to two liquid phases: one rich in polymer, the other rich in solvent. The first solidifies forming a solid network; the second generates pores or voids in the matrix [106].
- (c)
-
Colloidal templates use polymeric nanocapsules composed of a shell or membrane, which is mechanically very strong and separates the internal cavity from the outside medium, thus creating a barrier for various substances that can be encapsulated therein [107]. Scientists found that monodisperse colloidal spheres can self-assemble into arrays of periodic spheres, called colloidal crystals, in a hexagonal arrangement under well-controlled experimental conditions using drop coating, spin coating, dip coating, electrophoretic deposition and self-assembly at the liquid/gas interface. Moreover, in the large hollow spaces, substances such as drugs and biomolecules can be encapsulated and released in a controlled manner, which make the polymeric nanocapsules attractive devices for drug delivery, cancer and gene therapy, protecting enzymes, etc. [108]. The synthesis of monodisperse colloidal spheres offers an opportunity to extend their applications. Membranes with morphological features of high structural order at the nanometric scale may be achieved according to the colloidal template method. Colloid crystalline particles are three-dimensional close-packed crystals of sub-micrometer spheres working as imprinting agents, whose long-ranged ordered structure is replicated in a solid matrix, thus yielding materials with ordered pores. Colloidal crystal structures with this ordered architecture are of great interest for tissue engineering wherein the availability of arrays for cellular proliferation is requested to promote the optimum environment for a good adhesion and consequent cells proliferation [109].
- (d)
-
Self-assembly copolymers exploit the ability of some materials to spontaneously form ordered aggregates [110]. This allows to obtain nanostructures, even complex ones, depending on the intrinsic structure and chemistry of the molecules involved. The components most present in these types of assemblies are as follows:
-
Lipids, proteins, carbohydrates and nucleic acids;
-
Molecular crystals;
-
Liquid crystals;
-
Semi-crystalline and separate phase polymers.
-
In the case of membranes, the ability of polymers to self-assemble on a nanometric scale is exploited. Different techniques are used in these processes. The two main strategies that have been used by the researchers are as follows:
- -
-
The use of block polymers which give rise to a cylindrical morphology;
- -
-
The use of a block polymer with a bi-continuous morphology which obviates the need for alignment [111].
- (e)
-
Breath figure (BF) for bio-inspired high-defined membranes has been developed in the context of bio, innovative and bio processes in the preparation of micro-porous membranes [112]. This technique allows to obtain membranes with ordered pore geometry. The basic idea for using BF has been developed from the observation of the common phenomenon of fog formation which originates when water vapor comes into contact with a cold surface. During this event, the condensed water droplets tend to rearrange themselves into an ordered geometry that resembles honeycomb patterns. The condensation of water droplets on the surface of dilute polymeric solutions containing immiscible or partially miscible solvents also allows for an easier recovery of the solvent at the end of the process. Furthermore, water is a widely available non-toxic templating agent, so the general approach can be considered as an environmentally friendly production technology. Despite the simplicity with which droplets can be formed, the mechanism that controls the formation of BF geometry can be very complex and not perfectly unique. This may depend on the polymeric materials and solvents used, but also on changes in the surrounding experimental conditions, which make the management of water droplet dynamics somewhat difficult [113].
- (f)
-
Deposition for filtration is one of the most common and effective deposition methods. The vertical downtown force, supplied with pressure/vacuum filtration, drives the 2D nanosheets group in a layered interlocking structure on the substrate. The thickness is provided by the membrane that is supported, though it can slightly vary with deposition. Furthermore, other ions, molecules or nanoparticles can be easily mixed and interspersed in the strikers, providing additional flexibility on the tuning of the membrane structure [114].
- (g)
-
Coating: Coating is the creation of a thin layer on the surface of the membrane. Various coating methods have been reported to assemble 2D nanosheets on membranes, including drop coating, sterile coating, spin coating and casting, etc. The success of a uniform coating is based on the smoothness of the substrate, the surface tension of the coating solutions, as well as the process of evaporation applied. Among the methods, spin coating could provide centrifugal and cutting forces to control the assembly of nanosheets, producing a well-interloped ordered laminated structure. At present, this technique is also applied to prepare highly ordered labeled membranes [77][115].
- (h)
-
Layer-by-layer self-assembly (LBL) refers to the deposition process of different materials on the surface of the substrate. This approach is mainly based on the interactions between adjacent layers, including electrostatic bonding, hydrogen or even covalent interactions. The LBL method can accurately check the thickness of the selective layer by varying the number of deposition cycles and is useful for introducing the intercalary stabilization forces. Therefore, the resulting membranes can remain stable in aqueous or organic media. However, the implementation of this method requires the presence of material interactions and the preparation process takes time [116][117].
- (i)
-
Honeycomb membranes possess a specific distribution of the pores on the surface of the partition walls and an enlarged porosity in addition to subtle dividing walls of a prescribed value. The preparation of these particular structures is based on the preparation of lithographic precision membranes according to a BF biospirated process [113]. The condensation waterdrops act as the imprinting agents on the polymer surface and with the balance between solvent evaporation and humid air condensation in a 3D construction, bee nest membranes can be obtained in a single pass. After condensation, the dripping water drills grow and self-assemble in ordered arrays, producing a highly defined hexagonal geometry as a result of their imprinting action, different from what is observed for the separation techniques of conventional phases. The main limitation is using this process to produce commercially large-scale films is the lack of control over the long-range structural order through the surface of the films created [118].
This entry is adapted from the peer-reviewed paper 10.3390/chemistry5040148
References
- Mezher, T.; Fath, H.; Abbas, Z.; Khaled, A. Techno-economic assessment and environmental impacts of desalination technologies. Desalination 2011, 266, 263–273.
- Naseer, M.N.; Zaidi, A.A.; Khan, H.; Kumar, S.; Bin Owais, M.T.; Wahab, Y.A.; Dutta, K.; Jaafar, J.; Uzair, M.; Johan, M.R.; et al. Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis. Green Process. Synth. 2022, 11, 306–315.
- Naidu, G.; Tijing, L.; Johir, M.A.H.; Shon, H.; Vigneswaran, S. Hybrid membrane distillation: Resource, nutrient and energy recovery. J. Membr. Sci. 2020, 599, 117832.
- Tan, Y.Z.; Wang, H.; Han, L.; Tanis-Kanbur, M.B.; Pranav, M.V.; Chew, J.W. Photothermal-enhanced and fouling-resistant membrane for solar-assisted membrane distillation. J. Membr. Sci. 2018, 565, 254–265.
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. A Forward Osmosis–Membrane Distillation Hybrid Process for Direct Sewer Mining: System Performance and Limitations. Environ. Sci. Technol. 2013, 47, 13486–13493.
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total. Environ. 2021, 806, 150692.
- Ananthoji, R.; Eubank, J.F.; Nouar, F.; Mouttaki, H.; Eddaoudi, M.; Harmon, J.P. Symbiosis of zeolite-like metal–organic frameworks (rho-ZMOF) and hydrogels: Composites for controlled drug release. J. Mater. Chem. 2011, 21, 9587–9594.
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–506.
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17.
- Jhaveri, J.H.; Murthy, Z. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154.
- Daer, S.; Kharraz, J.; Giwa, A.; Hasan, S.W. Recent applications of nanomaterials in water desalination: A critical review and future opportunities. Desalination 2015, 367, 37–48.
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51.
- Xiong, P.; Ma, R.; Wang, G.; Sasaki, T. Progress and perspective on two-dimensional unilamellar metal oxide nanosheets and tailored nanostructures from them for electrochemical energy storage. Energy Storage Mater. 2018, 19, 281–298.
- Wu, X.; Ding, M.; Xu, H.; Yang, W.; Zhang, K.; Tian, H.; Wang, H.; Xie, Z. Scalable Ti3C2Tx MXene Interlayered Forward Osmosis Membranes for Enhanced Water Purification and Organic Solvent Recovery. ACS Nano 2020, 14, 9125–9135.
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712.
- Shafie, Z.M.H.M.; Ahmad, A.L. Juxtaposition of PES based hollow fiber membrane: Antifouling and antibacterial potential of LiCl mediated PVA–ZnO blend. J. Ind. Eng. Chem. 2018, 62, 273–283.
- Gude, V.G. Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Bio/Technol. 2017, 16, 591–609.
- Kumar, C.; Das, S.; Jit, S. Device physics and device integration of two-dimensional heterostructures. In 2D Nanoscale Heterostructured Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–214.
- Song, C.; Huang, S.; Wang, C.; Luo, J.; Yan, H. The optical properties of few-layer InSe. J. Appl. Phys. 2020, 128, 060901.
- Er, D.; Ghatak, K. Atomistic modeling by density functional theory of two-dimensional materials. In Synthesis, Modeling, and Characterization of 2D Materials, and Their Heterostructures; Elsevier: Amsterdam, The Netherlands, 2020; pp. 113–123.
- Senapati, S.; Maiti, P. Emerging bio-applications of two-dimensional nanoheterostructure materials. In 2D Nanoscale Heterostructured Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–255.
- Hasan, M.N.; Nafiujjaman, M.; Lee, Y.-K. 2D Nanomaterials for Gene Delivery. In Biomedical Applications of Graphene and 2D Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–104.
- Frappa, M.; Castillo, A.E.D.R.; Macedonio, F.; Politano, A.; Drioli, E.; Bonaccorso, F.; Pellegrini, V.; Gugliuzza, A. A few-layer graphene for advanced composite PVDF membranes dedicated to water desalination: A comparative study. Nanoscale Adv. 2020, 2, 4728–4739.
- Gugliuzza, A.; Politano, A.; Drioli, E. The advent of graphene and other two-dimensional materials in membrane science and technology. Curr. Opin. Chem. Eng. 2017, 16, 78–85.
- Zhao, L.; Lu, X.; Wu, C.; Zhang, Q. Flux enhancement in membrane distillation by incorporating AC particles into PVDF polymer matrix. J. Membr. Sci. 2016, 500, 46–54.
- Attia, H.; Osman, M.S.; Johnson, D.J.; Wright, C.; Hilal, N. Modelling of air gap membrane distillation and its application in heavy metals removal. Desalination 2017, 424, 27–36.
- Attia, H.; Alexander, S.; Wright, C.J.; Hilal, N. Superhydrophobic electrospun membrane for heavy metals removal by air gap membrane distillation (AGMD). Desalination 2017, 420, 318–329.
- Li, J.; Gunister, E.; Barsoum, I. Effect of graphene oxide as a filler material on the mechanical properties of LLDPE nanocomposites. J. Compos. Mater. 2019, 53, 2761–2773.
- Tang, B.; Hu, G.; Gao, H.; Hai, L. Application of graphene as filler to improve thermal transport property of epoxy resin for thermal interface materials. Int. J. Heat Mass Transf. 2015, 85, 420–429.
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942.
- Miró, P.; Audiffred, M.; Heine, T. An atlas of two-dimensional materials. Chem. Soc. Rev. 2014, 43, 6537–6554.
- Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanostructures for water purification: Graphene and beyond. Nanoscale 2016, 8, 15115–15131.
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30.
- Gusakova, J.; Wang, X.; Shiau, L.L.; Krivosheeva, A.; Shaposhnikov, V.; Borisenko, V.; Gusakov, V.; Tay, B.K. Electronic Properties of Bulk and Monolayer TMDs: Theoretical Study within DFT Framework (GVJ-2e Method). Phys. Status Solidi. 2017, 214, 1700218.
- Wang, J.; Wang, Q.; Zheng, Y.; Peng, T.; Yao, K.; Xie, S.; Zhang, X.; Xia, X.; Li, J.; Jiang, H. Development of a quantitative fluorescence-based lateral flow immunoassay for determination of chloramphenicol, thiamphenicol and florfenicol in milk. Food Agric. Immunol. 2018, 29, 56–66.
- Dai, H.; Feng, N.; Li, J.; Zhang, J.; Li, W. Chemiresistive humidity sensor based on chitosan/zinc oxide/single-walled carbon nanotube composite film. Sens. Actuators B Chem. 2019, 283, 786–792.
- Zhang, T.; Xiao, C.; Zhao, J.; Liu, X.; Ji, D.; Zhang, H. One-step facile fabrication of PVDF/graphene composite nanofibrous membrane with enhanced oil affinity for highly efficient gravity-driven emulsified oil/water separation and selective oil absorption. Sep. Purif. Technol. 2021, 254, 117576.
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275.
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187.
- Cranford, S.W.; Brommer, D.B.; Buehler, M.J. Extended graphynes: Simple scaling laws for stiffness, strength and fracture. Nanoscale 2012, 4, 7797–7809.
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397.
- Gugliuzza, A.; Macedonio, F.; Politano, A.; Drioli, E. Prospects of 2D materials-based membranes in water desalination. Chem. Eng. Trans. 2019, 73, 265–270.
- Kauling, A.P.; Seefeldt, A.T.; Pisoni, D.P.; Pradeep, R.C.; Bentini, R.; Oliveira, R.V.B.; Novoselov, K.S.; Neto, A.H.C. The Worldwide Graphene Flake Production. Adv. Mater. 2018, 30, e1803784.
- Castillo, A.E.D.R.; Pellegrini, V.; Ansaldo, A.; Ricciardella, F.; Sun, H.; Marasco, L.; Buha, J.; Dang, Z.; Gagliani, L.; Lago, E.; et al. High-yield production of 2D crystals by wet-jet milling. Mater. Horizons. 2018, 5, 890–904.
- Dawlaty, J.M.; Shivaraman, S.; Chandrashekhar, M.; Rana, F.; Spencer, M.G. Measurement of ultrafast carrier dynamics in epitaxial graphene. Appl. Phys. Lett. 2008, 92, 042116.
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710.
- Nikkho, S.; Mirzaei, M.; Sabet, J.K.; Moosavian, M.A.; Hedayat, S.M. Enhanced quality of transfer-free graphene membrane for He/CH4 separation. Sep. Purif. Technol. 2020, 232, 115972.
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314.
- Gu, X.; Zhao, Y.; Sun, K.; Vieira, C.L.; Jia, Z.; Cui, C.; Wang, Z.; Walsh, A.; Huang, S. Method of ultrasound-assisted liquid-phase exfoliation to prepare graphene. Ultrason. Sonochem. 2019, 58, 104630.
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene and Derivatives: Sample Handling. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018.
- Woo, Y.C.; Kim, S.-H.; Shon, H.K.; Tijing, L.D. Introduction: Membrane Desalination Today, Past, and Future. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2019; pp. xxv–xlvi.
- Qiu, L.; Zhang, X.; Yang, W.; Wang, Y.; Simon, G.P.; Li, D. Controllable corrugation of chemically converted graphene sheets in water and potential application for nanofiltration. Chem. Commun. 2011, 47, 5810–5812.
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191.
- Farrusseng, D. Metal-Organic Frameworks: Applications from Catalysis to Gas Storage; John Wiley & Sons: Hoboken, NJ, USA, 2011.
- Raza, A.; Hassan, J.Z.; Mahmood, A.; Nabgan, W.; Ikram, M. Recent advances in membrane-enabled water desalination by 2D frameworks: Graphene and beyond. Desalination 2022, 531, 115684.
- Macedonio, F.; Politano, A.; Drioli, E.; Gugliuzza, A. Bi2Se3-assisted membrane crystallization. Mater. Horizons 2018, 5, 912–919.
- Ajayan, P.; Kim, P.; Banerjee, K. Two-dimensional van der Waals materials. Phys. Today 2016, 69, 38–44.
- Zhang, X.; Lai, Z.; Ma, Q.; Zhang, H. Novel structured transition metal dichalcogenide nanosheets. Chem. Soc. Rev. 2018, 47, 3301–3338.
- Li, L.; Zhang, T.; Duan, Y.; Wei, Y.; Dong, C.; Ding, L.; Qiao, Z.; Wang, H. Selective gas diffusion in two-dimensional MXene lamellar membranes: Insights from molecular dynamics simulations. J. Mater. Chem. A 2018, 6, 11734–11742.
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571.
- Achari, A.; Sahana, S.; Eswaramoorthy, M. High performance MoS2 membranes: Effects of thermally driven phase transition on CO2 separation efficiency. Energy Environ. Sci. 2016, 9, 1224–1228.
- Keshebo, D.L.; Hu, C.-P.; Hu, C.-C.; Hung, W.-S.; Wang, C.-F.; Tsai, H.-C.; Lee, K.-R.; Lai, J.-Y. Effect of composition of few-layered transition metal dichalcogenide nanosheets on separation mechanism of hydrogen selective membranes. J. Membr. Sci. 2021, 634, 119419.
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597.
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mater. Today: Proc. 2019, 18, 2182–2190.
- Liu, F. Mechanical exfoliation of large area 2D materials from vdW crystals. Prog. Surf. Sci. 2021, 96, 100626.
- Castillo, A.E.D.R.; Reyes-Vazquez, C.D.; Rojas-Martinez, L.E.; Thorat, S.B.; Serri, M.; Martinez-Hernandez, A.L.; Velasco-Santos, C.; Pellegrini, V.; Bonaccorso, F. Single-step exfoliation and functionalization of few-layers black phosphorus and its application for polymer composites. FlatChem 2019, 18, 100131.
- Murphy, G.W. Desalination by Photoelectrodialysis. II. J. Electrochem. Soc. 1981, 128, 1819–1821.
- Peng, Y.-H.; Kashale, A.A.; Lai, Y.; Hsu, F.-C.; Chen, I.-W.P. Exfoliation of 2D materials by saponin in water: Aerogel adsorption/photodegradation organic dye. Chemosphere 2021, 274, 129795.
- Sakthivel, R.; Keerthi, M.; Chung, R.-J.; He, J.-H. Heterostructures of 2D materials and their applications in biosensing. Prog. Mater. Sci. 2023, 132, 101024.
- Foller, T.; Wang, H.; Joshi, R. Rise of 2D materials-based membranes for desalination. Desalination 2022, 536, 115851.
- Karagiannidis, P.G.; Hodge, S.A.; Lombardi, L.; Tomarchio, F.; Decorde, N.; Milana, S.; Goykhman, I.; Su, Y.; Mesite, S.V.; Johnstone, D.N.; et al. Microfluidization of Graphite and Formulation of Graphene-Based Conductive Inks. ACS Nano 2017, 11, 2742–2755.
- Elessawy, N.A.; Rafea, M.A.; Roushdy, N.; Youssef, M.E.; Gouda, M.H. Development and evaluation of cost-effective and green Bi-functional nickel oxide decorated graphene electrocatalysts for alkaline fuel cells. Results Eng. 2023, 17, 100871.
- Huang, H.-H.; Joshi, R.K.; De Silva, K.K.H.; Badam, R.; Yoshimura, M. Fabrication of reduced graphene oxide membranes for water desalination. J. Membr. Sci. 2019, 572, 12–19.
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154.
- Yuan, S.; Li, Y.; Xia, Y.; Kang, Y.; Yang, J.; Uddin, H.; Liu, H.; Selomulya, C.; Zhang, X. Minimizing Non-selective Nanowrinkles of Reduced Graphene Oxide Laminar Membranes for Enhanced NaCl Rejection. Environ. Sci. Technol. Lett. 2020, 7, 273–279.
- Buha, J.; Gaspari, R.; Castillo, A.E.D.R.; Bonaccorso, F.; Manna, L. Thermal Stability and Anisotropic Sublimation of Two-Dimensional Colloidal Bi2Te3 and Bi2Se3 Nanocrystals. Nano Lett. 2016, 16, 4217–4223.
- Huang, X.; Marsh, K.L.; McVerry, B.T.; Hoek, E.M.V.; Kaner, R.B. Low-Fouling Antibacterial Reverse Osmosis Membranes via Surface Grafting of Graphene Oxide. ACS Appl. Mater. Interfaces 2016, 8, 14334–14338.
- Zhou, Y.; Zhang, H.; Yan, Y. Catalytic oxidation of ethyl acetate over CuO/ZSM-5 zeolite membrane coated on stainless steel fibers by chemical vapor deposition. Chem. Eng. Res. Des. 2020, 157, 13–24.
- Lang, H.; Dietrich, S. Metals—Gas-Phase Deposition and Applications. In Comprehensive Inorganic Chemistry II.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 211–269.
- JOKOH. Available online: https://jokoh.com/en/ (accessed on 1 February 2011).
- Loh, Z.H.; Samanta, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 255–274.
- Del Rio-Castillo, A.E.; Merino, C.; Díez-Barra, E.; Vázquez, E. Selective suspension of single layer graphene mechanochemically exfoliated from carbon nanofibres. Nano Res. 2014, 7, 963–972.
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors. Science 2008, 319, 1229–1232.
- Fu, B.; Sun, J.; Wang, G.; Shang, C.; Ma, Y.; Ma, J.; Xu, L.; Scardaci, V. Solution-processed two-dimensional materials for ultrafast fiber lasers (invited). Nanophotonics 2020, 9, 2169–2189.
- Tyurnina, A.V.; Tzanakis, I.; Morton, J.; Mi, J.; Porfyrakis, K.; Maciejewska, B.M.; Grobert, N.; Eskin, D.G. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon 2020, 168, 737–747.
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329.
- Hu, J.; Hou, J.; Huang, S.; Zong, L.; Li, X.; Zhang, Z.; Duan, Y.; Zhang, J. One-pot preparation of zwitterionic graphene nanosheets with exceptional redispersibility and its application in pickering emulsions. Carbon 2020, 157, 448–456.
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316.
- Kumar, D.S.; Kumar, B.J.; Mahesh, H. Quantum Nanostructures (QDs): An Overview. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 59–88.
- Zhang, Y.; Ng, Y.L.; Goh, K.-L.; Chow, Y.; Wang, S.; Zivkovic, V. Fluidization of fungal pellets in a 3D-printed micro-fluidized bed. Chem. Eng. Sci. 2021, 236, 116466.
- Zivkovic, V.; Kashani, M.N.; Biggs, M.J. Experimental and theoretical study of a micro-fluidized bed. AIP Conf. Proc. 2013, 1542, 93–96.
- Maa, Y.-F.; Hsu, C.C. Performance of Sonication and Microfluidization for Liquid–Liquid Emulsification. Pharm. Dev. Technol. 1999, 4, 233–240.
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123.
- Yurdacan, H.M.; Sari, M.M. Functional green-based nanomaterials towards sustainable carbon capture and sequestration. In Sustainable Materials for Transitional and Alternative Energy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 125–177.
- Ansaldo, A.; Bondavalli, P.; Bellani, S.; Castillo, A.E.D.R.; Prato, M.; Pellegrini, V.; Pognon, G.; Bonaccorso, F. High-Power Graphene–Carbon Nanotube Hybrid Supercapacitors. Chemnanomat 2017, 3, 436–446.
- Magesa, F.; Wu, Y.; Tian, Y.; Vianney, J.-M.; Buza, J.; He, Q.; Tan, Y. Graphene and graphene like 2D graphitic carbon nitride: Electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem. 2019, 23, e00064.
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792.
- Saidin, N.; Zen, D.I.M.; Hamida, A.B.; Khan, S.; Ahmad, H.; Dimyati, K.; Harun, S.W. AQ-switched thulium-doped fiber laser with a graphene thin film based saturable absorber. Laser Phys. 2013, 23, 115102.
- Calabrò, V.; Basile, A. Economic analysis of membrane use in industrial applications. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2011.
- Asli, A.E.N.; Guo, J.; Lai, P.L.; Montazami, R.; Hashemi, N.N. High-Yield Production of Aqueous Graphene for Electrohydrodynamic Drop-on-Demand Printing of Biocompatible Conductive Patterns. Biosensors 2020, 10, 6.
- Frappa, M.; Castillo, A.D.R.; Macedonio, F.; Di Luca, G.; Drioli, E.; Gugliuzza, A. Exfoliated Bi2Te3-enabled membranes for new concept water desalination: Freshwater production meets new routes. Water Res. 2021, 203, 117503.
- Frappa, M.; Macedonio, F.; Gugliuzza, A.; Jin, W.; Drioli, E. Performance of PVDF Based Membranes with 2D Materials for Membrane Assisted-Crystallization Process. Membranes 2021, 11, 302.
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28.
- Lee, H.-J.; Yoon, T.-H.; Park, J.-H.; Perumal, J.; Kim, D.-P. Characterization and fabrication of polyvinylsilazane glass microfluidic channels via soft lithographic technique. J. Ind. Eng. Chem. 2008, 14, 45–51.
- Tüzün-Antepli, B.; Elçin, A.E.; Elçin, Y.M. Construction of micro-grooved PCL/nanohydroxyapatite membranes by non-solvent induced phase separation method and its evaluation for use as a substrate for human periodontal ligament fibroblasts. Chem. Eng. Sci. 2022, 248, 117120.
- Vogelaar, L.; Barsema, J.; van Rijn, C.; Nijdam, W.; Wessling, M. Phase Separation Micromolding—PSμM. Adv. Mater. 2003, 15, 1385–1389.
- Li, Y.; Koshizaki, N.; Cai, W. Periodic one-dimensional nanostructured arrays based on colloidal templates, applications, and devices. Co-ord. Chem. Rev. 2011, 255, 357–373.
- Haladjova, E.; Rangelov, S.; Tsvetanov, C.; Simon, P. Preparation of polymeric nanocapsules via nano-sized poly(methoxydiethyleneglycol methacrylate) colloidal templates. Polymer 2014, 55, 1621–1627.
- Velev, O.D.; Kaler, E.W. Structured Porous Materials via Colloidal Crystal Templating: From Inorganic Oxides to Metals. Adv. Mater. 2000, 12, 531–534.
- Marques, D.S.; Vainio, U.; Chaparro, N.M.; Calo, V.M.; Bezahd, A.R.; Pitera, J.W.; Peinemann, K.-V.; Nunes, S.P. Self-assembly in casting solutions of block copolymer membranes. Soft Matter 2013, 9, 5557–5564.
- Oss-Ronen, L.; Schmidt, J.; Abetz, V.; Radulescu, A.; Cohen, Y.; Talmon, Y. Characterization of Block Copolymer Self-Assembly: From Solution to Nanoporous Membranes. Macromolecules 2012, 45, 9631–9642.
- Pingitore, V.; Gugliuzza, A. Fabrication of Porous Semiconductor Interfaces by pH-Driven Assembly of Carbon Nanotubes on Honeycomb Structured Membranes. J. Phys. Chem. C 2013, 117, 26562–26572.
- Gugliuzza, A.; Perrotta, M.L.; Drioli, E. Controlled Bulk Properties of Composite Polymeric Solutions for Extensive Structural Order of Honeycomb Polysulfone Membranes. Membranes 2016, 6, 27.
- Ye, W.; Liu, H.; Lin, F.; Lin, J.; Zhao, S.; Yang, S.; Hou, J.; Zhou, S.; Van der Bruggen, B. High-flux nanofiltration membranes tailored by bio-inspired co-deposition of hydrophilic g-C3N4 nanosheets for enhanced selectivity towards organics and salts. Environ. Sci. Nano 2019, 6, 2958–2967.
- Kotoka, F.; Merino-Garcia, I.; Velizarov, S. Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review. Membranes 2020, 10, 160.
- Rahaman, S.; Thérien-Aubin, H.; Ben-Sasson, M.; Ober, C.K.; Nielsen, M.; Elimelech, M. Control of biofouling on reverse osmosis polyamide membranes modified with biocidal nanoparticles and antifouling polymer brushes. J. Mater. Chem. B 2014, 2, 1724–1732.
- Hung, W.-S.; Tsou, C.-H.; De Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-Linking with Diamine Monomers To Prepare Composite Graphene Oxide-Framework Membranes with Varying d-Spacing. Chem. Mater. 2014, 26, 2983–2990.
- Perrotta, M.; Saielli, G.; Casella, G.; Macedonio, F.; Giorno, L.; Drioli, E.; Gugliuzza, A. An ultrathin suspended hydrophobic porous membrane for high-efficiency water desalination. Appl. Mater. Today 2017, 9, 1–9.
This entry is offline, you can click here to edit this entry!
