Spermatogenesis is a very complex process with an intricate transcriptional regulation. The transition from the diploid to the haploid state requires the involvement of specialized genes in meiosis, among other specific functions for the formation of the spermatozoon. The transcription factor cAMP-response element modulator (CREM) is a key modulator that triggers the differentiation of the germ cell into the spermatozoon through the modification of gene expression. CREM has multiple repressor and activator isoforms whose expression is tissue-cell-type specific and tightly regulated by various factors at the transcriptional, post-transcriptional and post-translational level. The activator isoform CREMτ controls the expression of several relevant genes in post-meiotic stages of spermatogenesis. In addition, exposure to xenobiotics negatively affects CREMτ expression, which is linked to male infertility. On the other hand, antioxidants could have a positive effect on CREMτ expression and improve sperm parameters in idiopathically infertile men.
- CREM
- spermatogenesis
- male fertility
- gene regulation
- CREM isoforms
1. Introduction
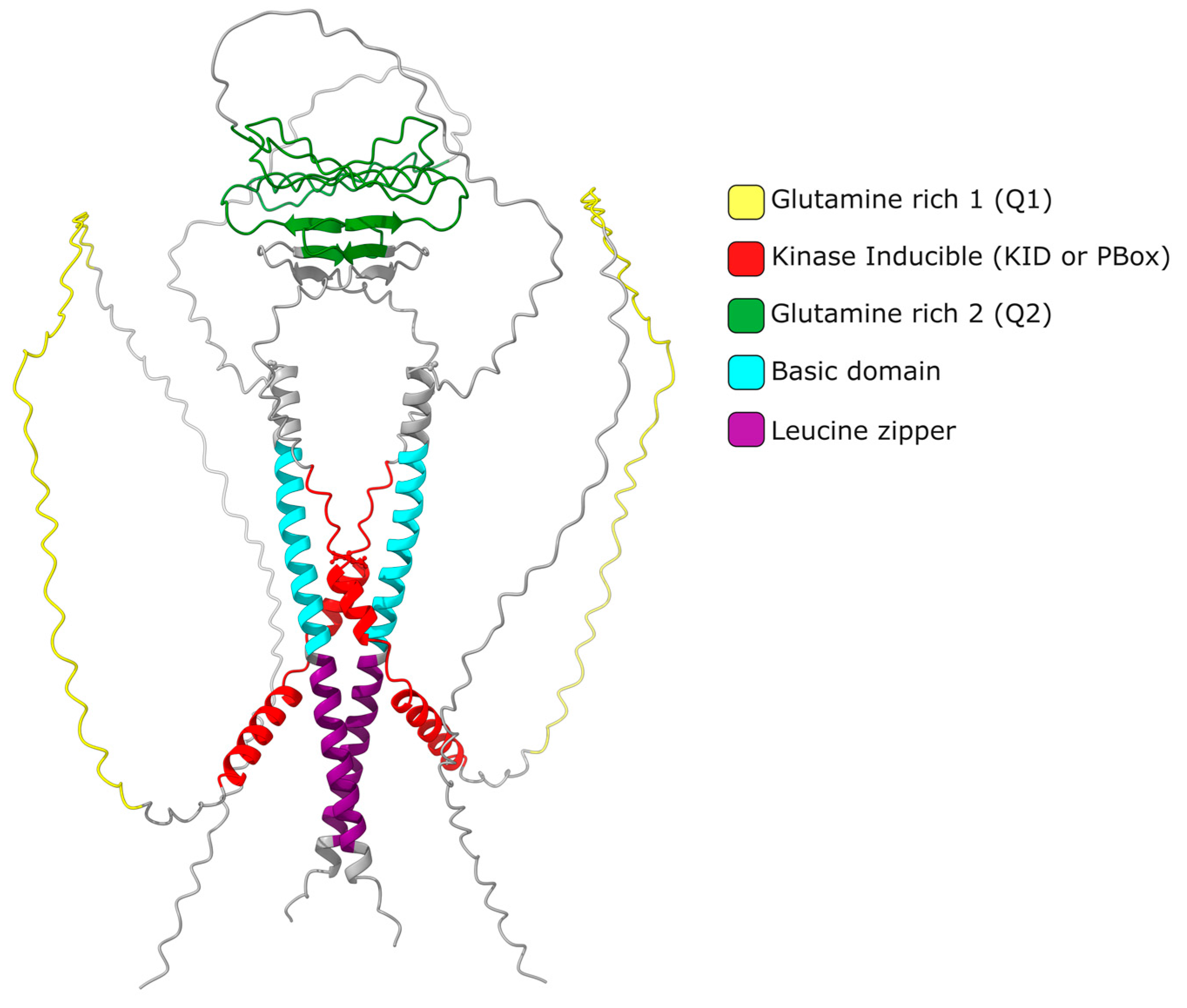
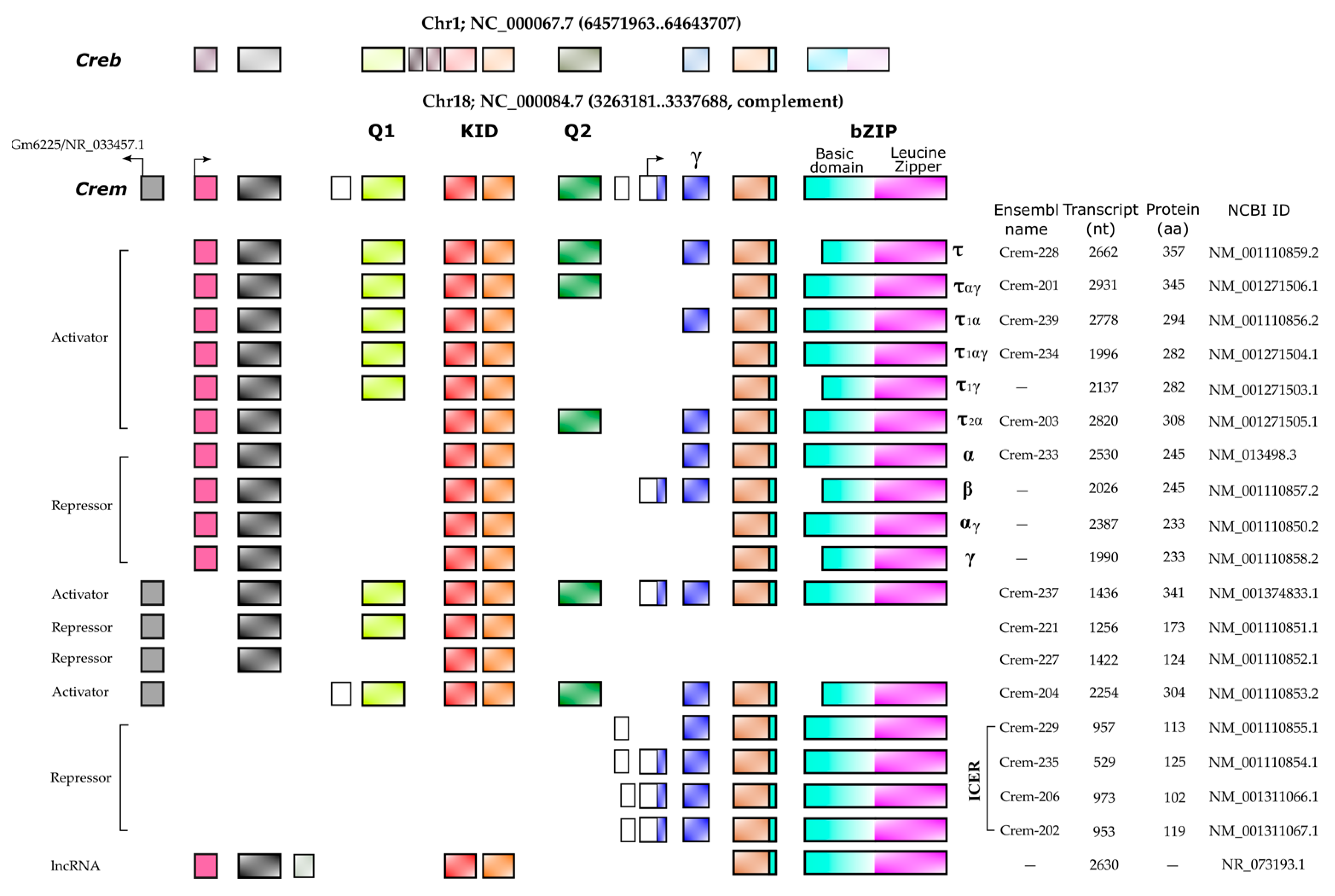
2. CREMτ Expression
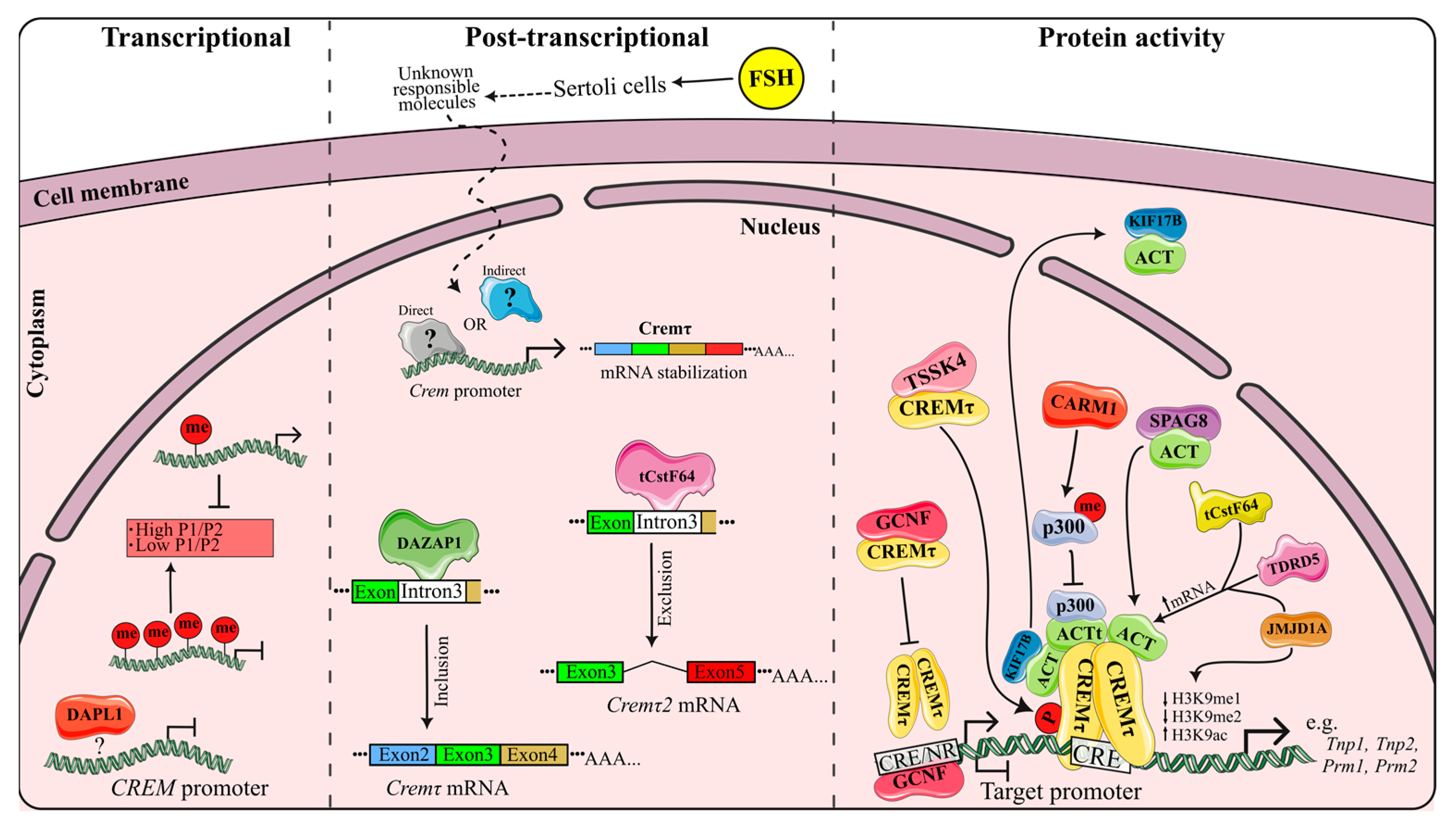
3. CREM Regulation
4. Genes Regulated by CREM
5. Male Fertility and CREM
6. Impact of Xenobiotics in CREMτ Regulation
6.1. CREM Disruption by ROS Production Xenobiotics
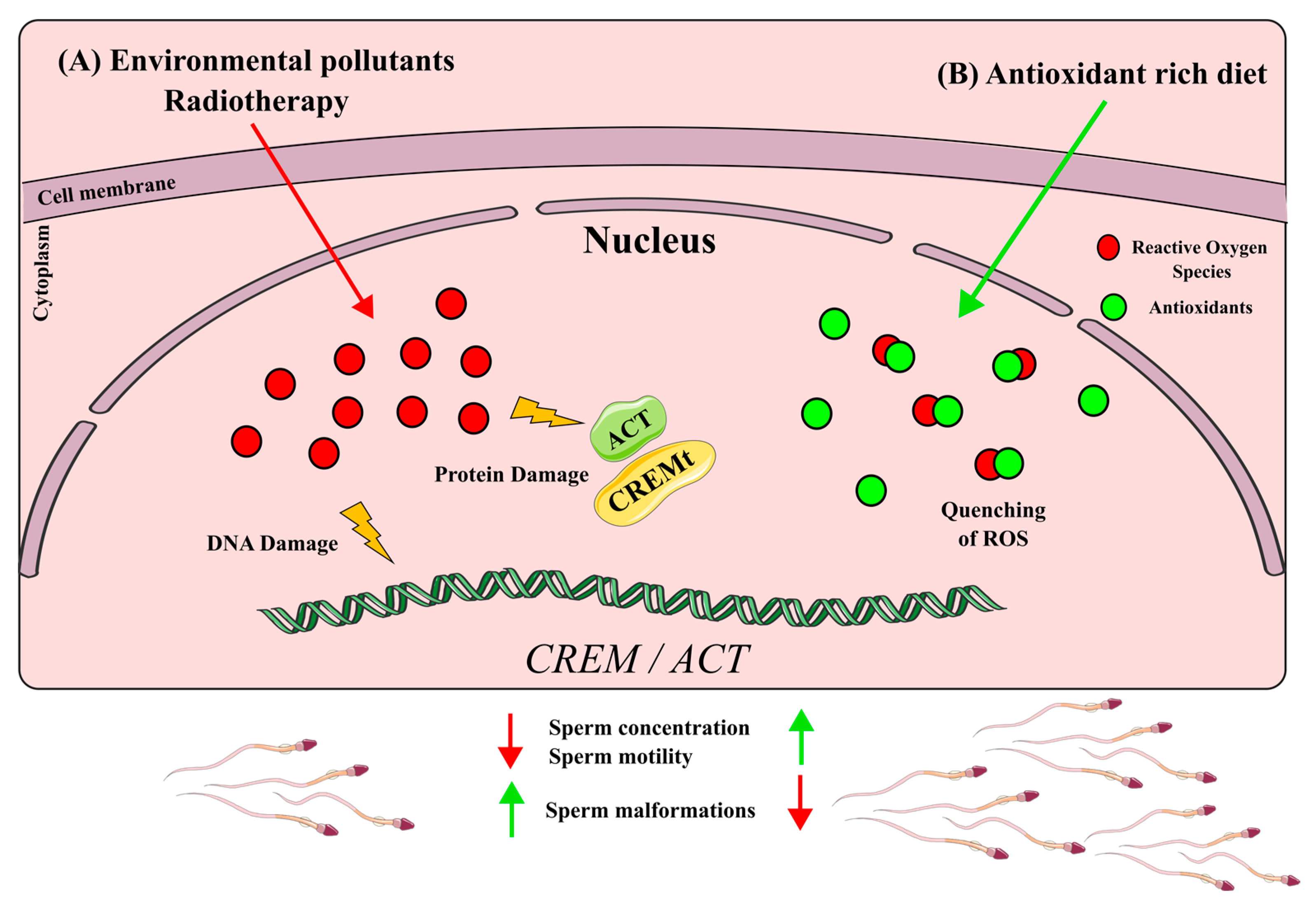
6.2. CREM Up-Regulation by Antioxidants
7. Other CREM Implications in Health and Disease
This entry is adapted from the peer-reviewed paper 10.3390/ijms241612558
References
- Kendall, S.K.; Samuelson, L.C.; Saunders, T.L.; et al.; Targeted disruption of the pituitary glycoprotein hormone alpha-subunit produces hypogonadal and hypothyroid mice. . Genes Dev. 1995, 9(16), 2007-2019, .
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; et al.; Spermatogenesis.. Hum. Reprod. 1998, 13(suppl.1), 1-8, .
- Griswold, M.D.; Cellular and molecular basis for the action of retinoic acid in spermatogenesis. . J. Mol. Endocrinol. 2022, 69(4), T51-T57, .
- Schultz, N.; Hamra, F.K.; Garbers, D.L.; A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. . Proc. Natl. Acad. Sci. USA 2003, 100(21), 12201-12206, .
- Shima, J.E.; McLean, D.J.; McCarrey, J.R.; et al.; The murine testicular transcriptome: Characterizing gene expression in the testis during the progression of spermatogenesis. . Biol. Reprod. 2004, 71(1), 319-330, .
- Foulkes, N.S.; Mellström, B.; Benusiglio, E.; et al.; Developmental switch of CREM function during spermatogenesis: From antagonist to activator.. Nature 1992, 355(6355), 80-84, .
- Don, J.; Stelzer, G.; The expanding family of CREB/CREM transcription factors that are involved with spermatogenesis.. Mol. Cell. Endocrinol. 2002, 187(1-2), 115-124, .
- Foulkes, N.S.; Schlotter, F.; Pévet, P.; et al.; Pituitary hormone FSH directs the CREM functional switch during spermatogenesis.. Nature 1993, 362(6417), 264-267, .
- Foulkes, N.S.; Borrelli, E.; Sassone-Corsi, P.; CREM gene: Use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell 1991, 64(4), 739-749, .
- Behr, R.; Weinbauer, G.F.; cAMP response element modulator (CREM): An essential factor for spermatogenesis in primates? . Int. J. Androl 2001, 24(3), 126-135, .
- De Cesare, D.; Fimia, G.M.; Sassone-Corsi, P.; Signaling routes to CREM and CREB: Plasticity in transcriptional activation. . Trends. Biochem. Sci. 1999, 24(7), 281-285, .
- Fimia, G.M.; De Cesare, D.; Sassone-Corsi, P.; CBP-independent activation of CREM and CREB by the LIM-only protein ACT. Nature 1999, 398(6723), 165-169, .
- Fimia, G.M.; Morlon, A.; Macho, B.; et al.; Transcriptional cascades during spermatogenesis: Pivotal role of CREM and ACT. . Mol. Cell. Endocrinol. 2001, 179(1-2), 17-23, .
- Macho, B.; Brancorsini, S.; Fimia, G.M.; et al.; CREM-dependent transcription in male germ cells controlled by a kinesin. . Science 2002, 298(5602), 2388-2390, .
- Kotaja, N.; De Cesare, D.; Macho, B.; et al; Abnormal sperm in mice with targeted deletion of the act (activator of cAMP-responsive element modulator in testis) gene. . Proc. Natl. Acad. Sci. USA 2004, 101(29), 10620-10625, .
- Hogeveen, K.N.; Sassone-Corsi, P.; Regulation of gene expression in post-meiotic male germ cells: CREM-signalling pathways and male fertility. . Hum. Fertil. 2006, 9(2), 73-79, .
- Monaco, L.; Foulkes, N.S.; Sassone-Corsi, P.; Pituitary follicle-stimulating hormone (FSH) induces CREM gene expression in Sertoli cells: Involvement in long-term desensitization of the FSH receptor. . Proc. Natl. Acad. Sci. USA 1995, 92(23), 10673-10677, .
- Delmas, V.; Sassone-Corsi, P.; The key role of CREM in the cAMP signaling pathway in the testis. . Mol. Cell. Endocrinol. 1994, 100(1-2), 121-124, .
- Ruppert, S.; Cole, T.J.; Boshart, M.; Multiple mRNA isoforms of the transcription activator protein CREB: Generation by alternative splicing and specific expression in primary spermatocytes. . EMBO J. 1992, 11(4), 1503-1512, .
- Walker, W.H.; Sanborn, B.M.; Habener, J.F.; An isoform of transcription factor CREM expressed during spermatogenesis lacks the phosphorylation domain and represses cAMP-induced transcription. . Proc. Natl. Acad. Sci. USA 1994, 91(26), 12423-12427, .
- Gene ID 1390 . Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004. Retrieved 2023-10-13
- GENE ID 12916 . Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004. Retrieved 2023-10-13
- Peri, A.; Krausz, C.; Cioppi, F.; et al; Cyclic adenosine 3’,5’-monophosphate-responsive element modulator gene expression in germ cells of normo- and oligoazoospermic men. J. Clin. Endocrinol. Metab 1998, 83(10), 3722-3726, .
- Noda, T.; Shidara, O.; Harayama, H.; et al; Detection of the activator cAMP responsive element modulator (CREM) isoform ortholog proteins in porcine spermatids and sperm. Theriogenology 2012, 77(7), 1360-1368, .
- Jazireian, P.; Favaedi, R.; Sadighi Gilani, M.A.; et al; Dynamic Expression and Chromatin Incorporation of ACT and CREM Transcription Factors in Testis Tissues of Infertile Men. . Cell J 2021, 23(7), 736-741, .
- Kaprio, H.; Heuser, V.D.; Orte, K.; et al; Expression of Transcription Factor CREM in Human Tissues. J. Histochem. Cytochem 2021, 69(8), 495-509, .
- Kumar, T. R; Low, M. J.; Matzuk, M. M.; Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology 1998, 139(7), 3289-3295, .
- Nantel, F.; Monaco, L.; Foulkes, N.S.; et al; Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature 1996, 380(6570), 159-162, .
- Martianov, I.; Choukrallah, M.A.; Krebs, A.; et al; Cell-specific occupancy of an extended repertoire of CREM and CREB binding loci in male germ cells. BMC Genom 2010, 11, 530, .
- Christensen, G.L.; Wooding, S.P.; Ivanov, I.P.; et al; Sequencing and haplotype analysis of the activator of CREM in the testis (ACT) gene in populations of fertile and infertile males. Mol. Hum. Reprod 2006, 12(4), 257-262, .
- Kotaja, N.; Macho, B.; Sassone-Corsi, P.; Microtubule-independent and protein kinase A-mediated function of kinesin KIF17b controls the intracellular transport of activator of CREM in testis (ACT). . J. Biol. Chem 2005, 280(36), 31739-31745, .
- Wu, H.; Chen, Y.; Miao, S.; Zhang, C.; et al; Sperm associated antigen 8 (SPAG8), a novel regulator of activator of CREM in testis during spermatogenesis. . FEBS Lett 2010, 584(13), 2807-2815, .
- He, X.J.; Song, B.; Du, W.D.; et al; CREM variants rs4934540 and rs2295415 conferred susceptibility to nonobstructive azoospermia risk in the Chinese population. Biol. Reprod 2014, 91(2), 52, .
- Abofoul-Azab, M.; Lunenfeld, E.; Kleiman, S.; et al; Determining the expression levels of CSF-1 and OCT4, CREM-1, and protamine in testicular biopsies of adult Klinefelter patients: Their possible correlation with spermatogenesis. Andrologia 2022, 54(10), e14558, .
- Song, B.; Wang, C.; Chen, Y.; et al; Sperm DNA integrity status is associated with DNA methylation signatures of imprinted genes and non-imprinted genes. J. Assist. Reprod. Genet 2021, 38(8), 2041-2048, .
- Grozdanov, P.N.; Amatullah, A.; Graber, J.H.; et al; TauCstF-64 Mediates Correct mRNA Polyadenylation and Splicing of Activator and Repressor Isoforms of the Cyclic AMP-Responsive Element Modulator (CREM) in Mouse Testis. Biol. Reprod 2016, 94(2), 34, .
- Dvorakova-Hortova, K.; Sidlova, A.; Ded, L.; et al; Toxoplasma gondii decreases the reproductive fitness in mice. PLoS ONE 2014, 9(6), e96770, .
- Sang, Y.; Liu, J.; Li, X.; et al; The effect of SiNPs on DNA methylation of genome in mouse spermatocytes. Environ. Sci. Pollut. Res. Int 2021, 28(32), 43684-43697, .
- Boekelheide, K.; Mechanisms of toxic damage to spermatogenesis. J. Natl. Cancer. Inst. Monogr 2005, 34, 6-8, .
- Chen, H.Y.; Yu, Y.H.; Yen, P.H.; DAZAP1 regulates the splicing of Crem, Crisp2 and Pot1a transcripts. Nucleic Acids Res 2013, 41(21), 9858-9869, .
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; et al; Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23(6), 646-659, .
- Centola, G.M.; Blanchard, A.; Demick, J.; et al; Decline in sperm count and motility in young adult men from 2003 to 2013: Observations from a U.S. Sperm Bank. Andrology 2017, 4(2), 270-276, .
- Zhang, Y.; Li, G.; Zhong, Y.; et al; Dichloroethane Induces Reproductive Toxicity Mediated by the CREM/CREB Signaling Pathway in Male NIH Swiss Mice. Toxicol. Sci. 2017, 160(29), 299-314, .
- Wang, C.; Chen, Y.; Manthar, R.K.; et al; Abnormal spermatogenesis following sodium fluoride exposure is associated with the downregulation of CREM and ACT in the mouse testis. Toxicol. Sci 2017, 160(2), 299-314, .
- Liu, J.; Zhang, P.; Zhao, Y.; et al; Low dose carbendazim disrupts mouse spermatogenesis might Be through estrogen receptor related histone and DNA methylation. Ecotoxicol. Environ. Saf 2019, 176, 242-249, .
- Kim, S.W.; Yoo, S.H.; Lee, H.J.; et al; Cistanches herba induces testis cytotoxicity in male mice. Bull. Environ. Contam. Toxicol 2012, 88(1), 112-117, .
- Nagahori, K.; Qu, N.; Kuramasu, M.; et al; Changes in Expression of Specific mRNA Transcripts after Single- or Re-Irradiation in Mouse Testes. Genes 2022, 13(19), 151, .
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G.; Roles of reactive oxygen species in the spermatogenesis regulation.. Front. Endocrinol 2014, 5, 56, .
- Kim, E.J.; Lee, Y.J.; Shin, H.K.; Park, J.H.; Induction of apoptosis by the aqueous extract of Rubus coreanum in HT-29 human colon cancer cells.. Nutrition 2005, 21(11-12), 1141-1148, .
- Bensky, D.; Barolet, R. . Chinese Herbal Medicine Formulas and Strategies; Eastland Press: Seattle, WA, USA, 1990; pp. 263-264.
- Pang, G.C.; Kim, M.S.; Lee, M.W.; Hydrolyzable tannins from the fruits of Rubus coreanum. Korean J. Pharmacogn 1996, 27(4), 366-370, .
- Kim, S.W.; Shin, M.H.; Jung, J.H. et al; A triterpene glucosyl ester from the roots of Rubus crataegifolius. . Arch. Pharmacal Res 2001, 24(5), 412-415, .
- Patel, A.V.; Rojas-Vera, J.; Dacke, C.G.; Therapeutic Constituents and Actions of Rubus Species. Curr. Med. Chem. 2004, 11(11), 1501-1512, .
- Oh, M.S.; Yang, W.M.; Chang, M.S. et al; Effects of Rubus coreanus on sperm parameters and cAMP-responsive element modulator (CREM) expression in rat testes. J. Ethnopharmacol 2007, 114(3), 463-467, .
- Oh, M.S.; Chang, M.S.; Park, W. et al; Yukmijihwang-tang protects against cyclophosphamide-induced reproductive toxicity. Reprod. Toxicol 2007, 24(3-4), 365-370, .
- Arafa, M.; Agarwal, A.; Majzoub, A. et al; Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility.. Fertil. Steril. 2019, 112(39), e362, .
- Al-Maghrebi, M.; Renno, W.M.; Al-Somali, H.F. et al; Lutein modulates transcription dysregulation of adhesion molecules and spermatogenesis transcription factors induced by testicular ischemia reperfusion injury: It could be SAFE. Naunyn-Schmiedeberg’s Arch. Pharmacol 2016, 389(5), 539-551, .
- Ye, N.; Lv, Z.; Huang, Z. et al; Dietary folic acid supplementation improves semen quality and spermatogenesis through altering autophagy and histone methylation in the testis of aged broiler breeder roosters.. Theriogenology 2022, 181, 8-15, .
- Agarwal, A.; Panner Selvam, M.K.; Samanta, L. et al; Effect of Antioxidant Supplementation on the Sperm Proteome of Idiopathic Infertile Men.. Antioxidants 2019, 8(10), 488, .
- Mioduszewska, B.; Jaworski, J.; Szklarczyk, A.W. et al; Inducible cAMP early repressor (ICER)-evoked delayed neuronal death in the organotypic hippocampal culture. J. Neurosci. Res 2008, 86(1), 61-70, .
- Kojima, N.; Borlikova, G.; Sakamoto, T. et al; . Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory. J. Neurosci 2008, 28(25), 6459-6472, .
- Porter, B.E.; Lund, I.V.; Varodayan, F.P. et al; The role of transcription factors cyclic-AMP responsive element modulator (CREM) and inducible cyclic-AMP early repressor (ICER) in epileptogenesis. Neuroscience 2008, 152(3), 829-836, .
- Hu, Y.; Lund, I.V.; Gravielle, M.C. et al; Surface expression of GABAA receptors is transcriptionally controlled by the interplay of cAMP-response element-binding protein and its binding partner inducible cAMP early repressor. J. Biol. Chem 2008, 283(14), 9328-9340, .
- Lund, I.V.; Hu, Y.; Raol, Y.H. et al; BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway.. Sci. Signal 2008, 1(41), ra9, .
- Favre, D.; Le Gouill, E.; Fahmi, D. et al; Impaired expression of the inducible cAMP early repressor accounts for sustained adipose CREB activity in obesity. Diabetes 2011, 60(12), 3169-3174, .
- Brajkovic, S.; Marenzoni, R.; Favre, D. et al; Evidence for tuning adipocytes ICER levels for obesity care. . Adipocyte 2012, 1(3), 157-160, .
- Abderrahmani, A.; Cheviet, S.; Ferdaoussi, M. et al; ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis.. EMBO J 2006, 25(5), 977-986, .
- Favre, D.; Niederhauser, G.; Fahmi, D. et al; Role for inducible cAMP early repressor in promoting pancreatic beta cell dysfunction evoked by oxidative stress in human and rat islets. Diabetologia 2011, 54(9), 2337-2346, .
- Zmrzljak, U.P.; Korenčič, A.; Košir, R. et al; Inducible cAMP early repressor regulates the Period 1 gene of the hepatic and adrenal clocks.. J. Biol. Chem 2013, 288(15), 10318–10327, .
- Ohtsubo, H.; Ichiki, T.; Miyazaki, R. et al; Inducible cAMP early repressor inhibits growth of vascular smooth muscle cell. Arter. Thromb. Vasc. Biol 2007, 27(7), 1549-1555, .
- Ahlmann, M.; Varga, G.; Sturm, K. et al; The cyclic AMP response element modulator {alpha} suppresses CD86 expression and APC function. J. Immunol. 2009, 182(7), 4167-4174, .
- Hedrich, C.M.; Crispin, J.C.; Rauen, T. et al; cAMP response element modulator α controls IL2 and IL17A expression during CD4 lineage commitment and subset distribution in lupus. . Proc. Natl. Acad. Sci. USA 2012, 109(41), 16606-16611, .
- Juang, Y.T.; Rauen, T.; Wang, Y.; Ichinose, K.; Benedyk, K.; Tenbrock, K.; Tsokos, G.C. Transcriptional activation of the cAMP-responsive modulator promoter in human T cells is regulated by protein phosphatase 2A-mediated dephosphorylation of SP-1 and reflects disease activity in patients with systemic lupus erythematosus. J. Biol. Chem. 2011, 286, 1795–1801.
- Zhang, Q.; Ding, S.; Zhang, H.; Long, H.; Wu, H.; Zhao, M.; Chan, V.; Lau, C.S.; Lu, Q. Increased Set1 binding at the promoter induces aberrant epigenetic alterations and up-regulates cyclic adenosine 5′-monophosphate response element modulator alpha in systemic lupus erythematosus. Clin. Epigenet. 2016, 8, 126.
- Hedrich, C.M.; Rauenl, T.; Kis-Tothl, K.; Kyttaris, V.C.; Tsokos, G.C. cAMP-responsive element modulator α (CREMα) suppresses IL-17F protein expression in T lymphocytes from patients with systemic lupus erythematosus (SLE). J. Biol. Chem. 2012, 287, 4715–4725.
- Lippe, R.; Ohl, K.; Varga, G.; Rauen, T.; Crispin, J.C.; Juang, Y.T.; Kuerten, S.; Tacke, F.; Wolf, M.; Roebrock, K.; et al. CREMα overexpression decreases IL-2 production, induces a T(H)17 phenotype and accelerates autoimmunity. J. Mol. Cell. Biol. 2012, 4, 121–123.
