Imaging within the realm of biomedical applications can be categorized into two domains based on object size: macroscopic and microscopic imaging. The substantive importance of macroscopic imaging has been demonstrated prominently in medical practices, encompassing X-ray imaging, positron emission tomography (PET), magnetic resonance imaging (MRI), and ultrasonic echo imaging. Although these modalities offer undeniable utility, they are not devoid of limitations. Even with recent progress in X-ray detection, the ionizing radiation inherent to X-ray imaging engenders challenges related to repeated exposure. Similarly, the utilization of PET and MRI is impeded by the considerable scale of the necessary apparatus, thereby hindering seamless bedside deployment. The domain of ultrasound imaging presents difficulty involving a tradeoff between spatial resolution and penetration depth in animal bodies. An additional contender for noninvasive macroscopic structural imaging of animal bodies has emerged: optical imaging.
- biomedical imaging
- diffusion
- functional imaging
- medical imaging
- near-infrared light
- near-axis scattered light
- noninvasive measurement
- scattering
1. Two-Dimensional Transillumination Imaging
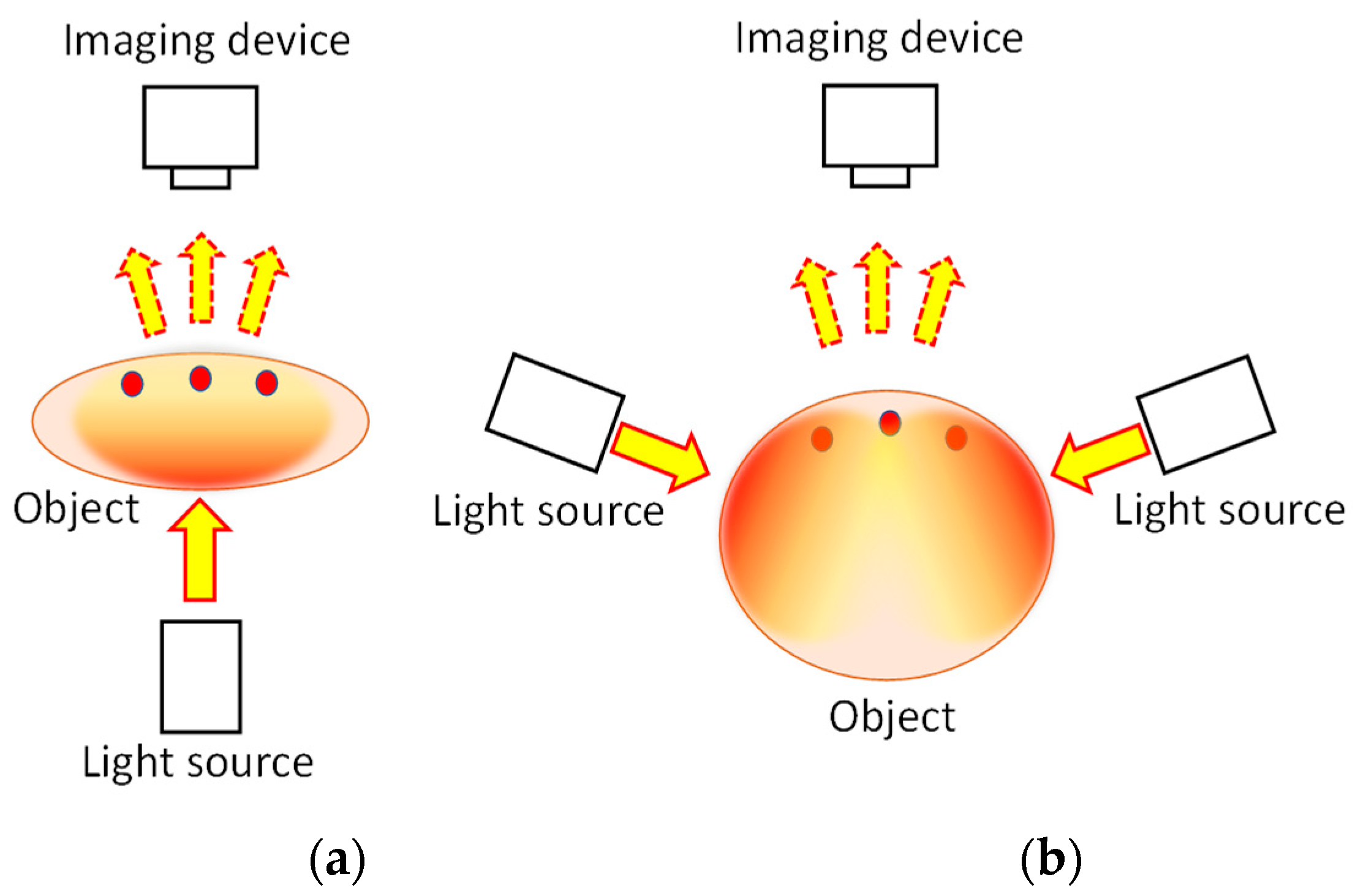
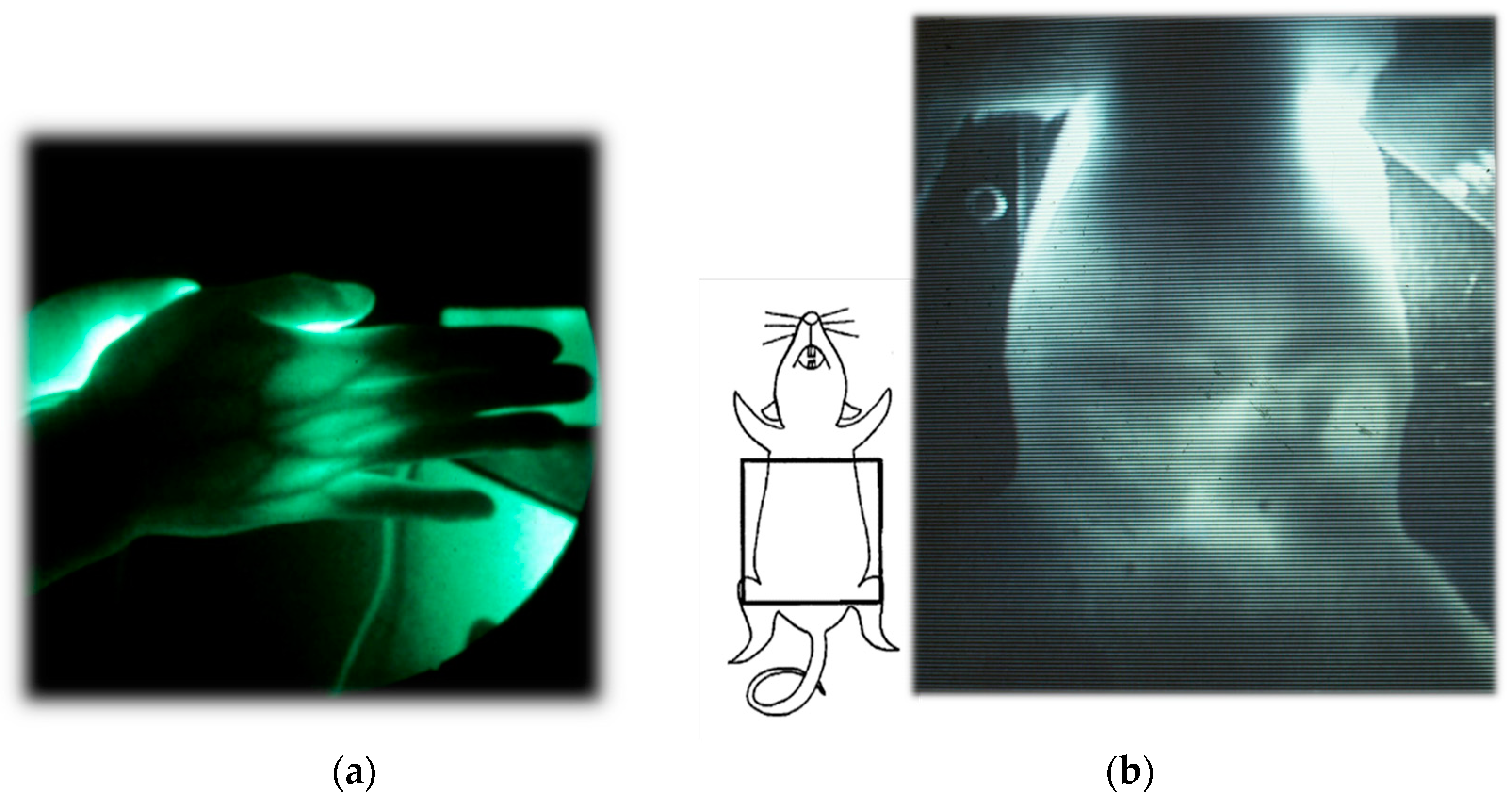
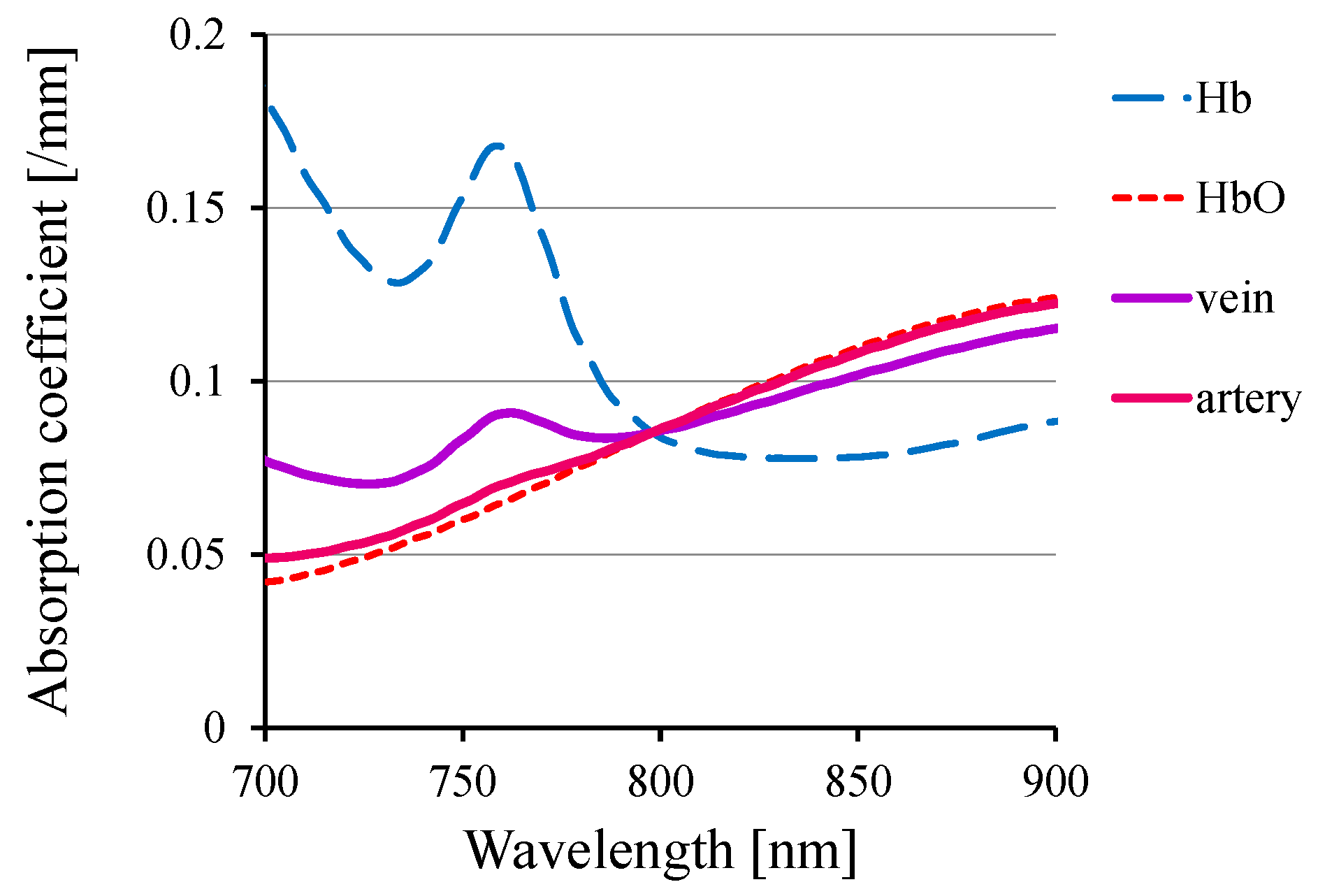
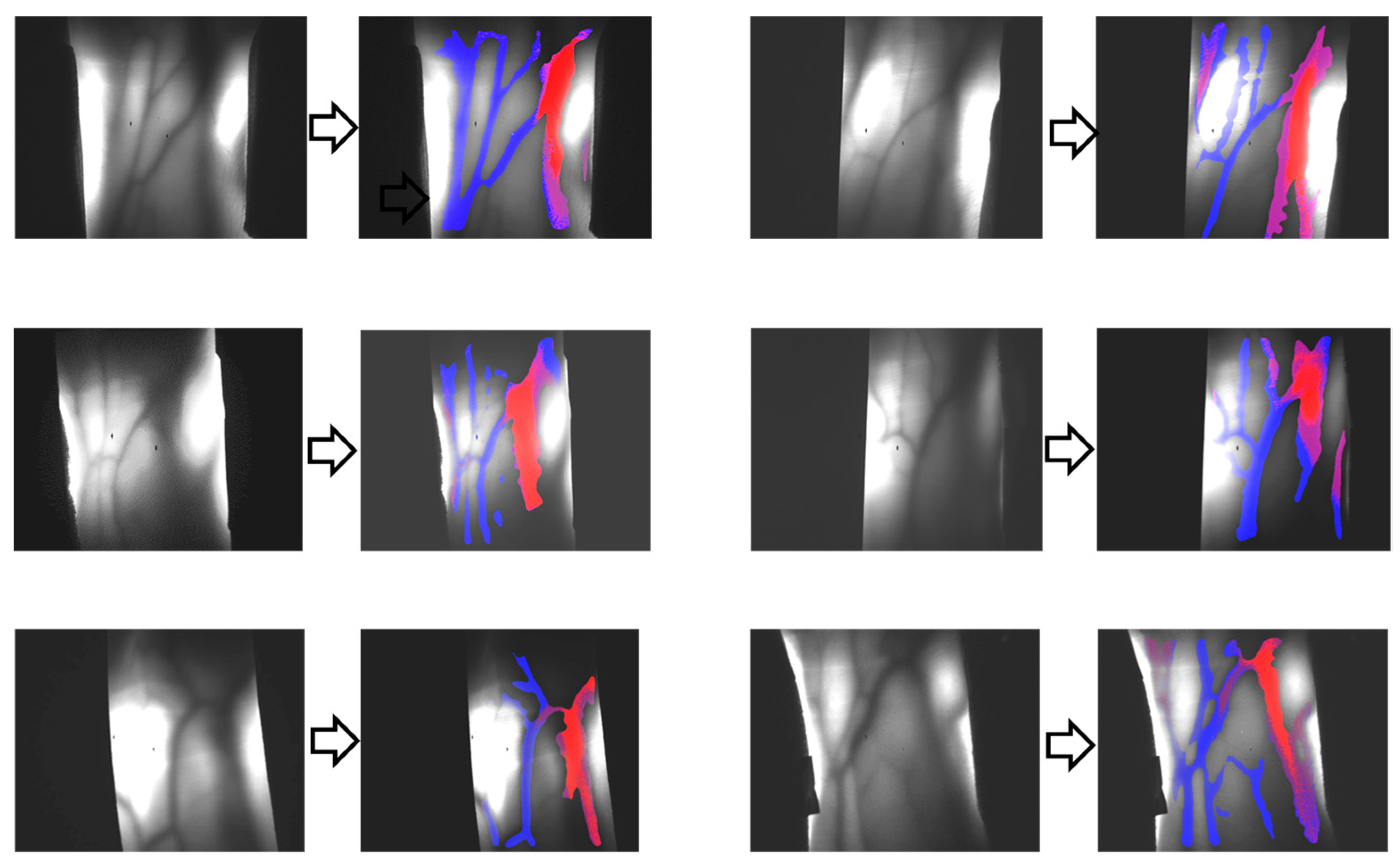
2. Functional Imaging
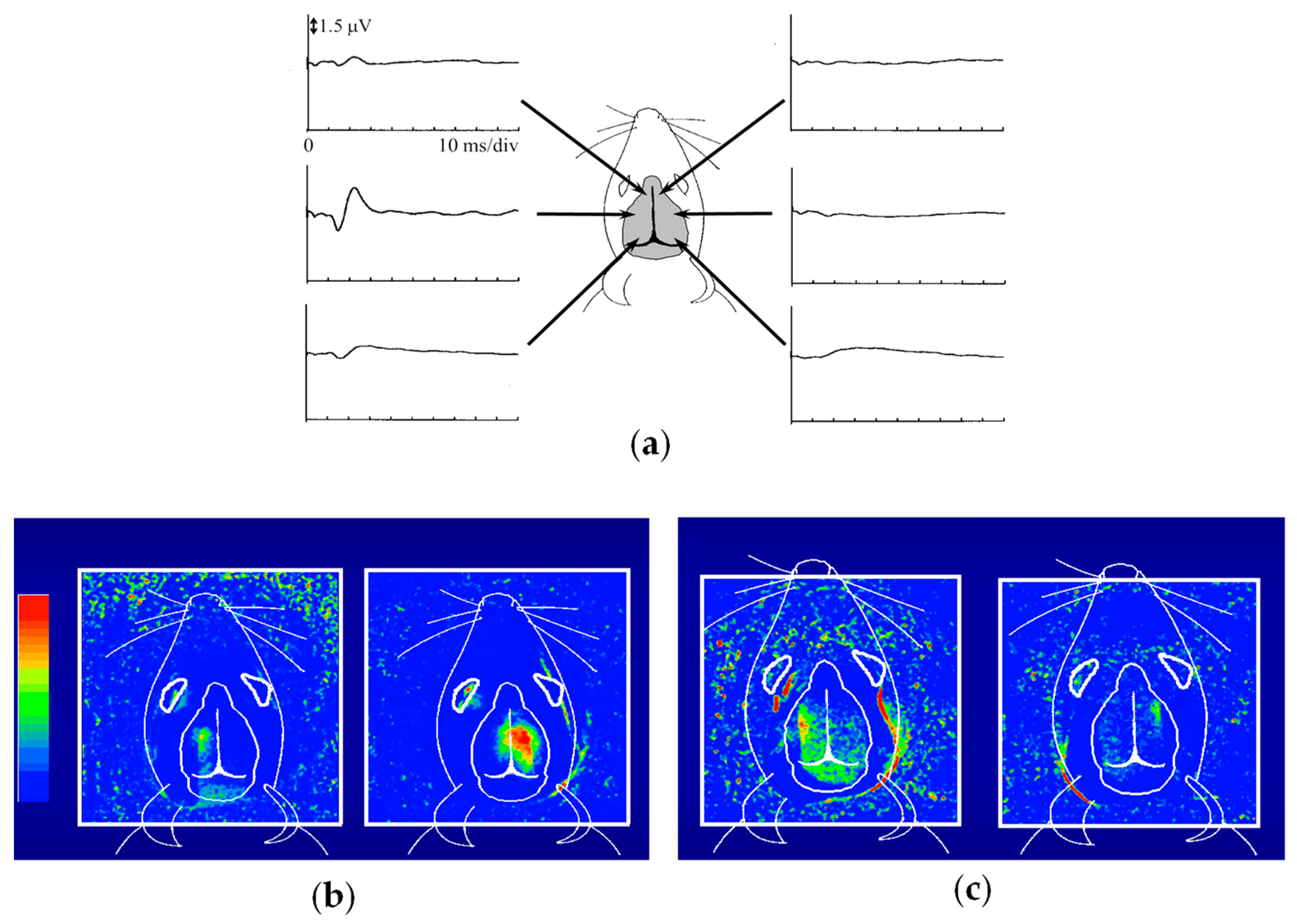
This entry is adapted from the peer-reviewed paper 10.3390/biology12111362
References
- Key, H.; Jackson, P.C.; Wells, P.N.T. New approaches to transillumination imaging. J. Biomed. Eng. 1988, 10, 113–118.
- Gandjbakhche, A.H.; Bonner, R.F.; Nossal, R.; Weiss, G.H. Absorptivity contrast in transillumination imaging of tissue abnormalities. Appl. Opt. 1996, 35, 1767–1774.
- Gurcan, M.N.; Roysam, B.; Dhawan, A.P.; Wang, L.V. Special Letters Issue on Emerging Technologies in Multiparameter Biomedical Optical Imaging and Image Analysis. IEEE Trans. Biomed. Eng. 2010, 57, 2551–2630.
- D’Alessandro, B.; Dhawan, A.P. Depth-dependent hemoglobin analysis from multispectral transillumination images. IEEE Trans. Biomed. Eng. 2010, 57, 2568–2571.
- D’Alessandro, B.; Dhawan, A.P. Transillumination imaging for blood oxygen saturation estimation of skin lesions. IEEE Trans. Biomed. Eng. 2012, 59, 2660–2667.
- Cutler, M. Transillumination of the breast. Ann. Surg. 1931, 93, 223–234.
- Shimizu, K.; Xian, S.; Guo, J. Reconstructing a deblurred 3D structure in a turbid medium from a single blurred 2D image—For near-infrared transillumination imaging of a human body. Sensors 2022, 22, 5747.
- Tuchin, V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, 3rd ed.; The Society of Photo-Optical Instrumentation Engineers (SPIE): Bellingham, WA, USA, 2015.
- Boas, D.A.; Pitris, C.; Ramanujam, N. Handbook of Biomedical Optics; CRC Press: Boca Raton, FL, USA, 2011.
- Wang, L.V.; Wu, H. Biomedical Optics: Principles and Imaging; Wiley: Hoboken, NJ, USA, 2007.
- Splinter, R.; Hooper, B.A. An Introduction to Biomedical Optics; Taylor and Francis: New York, NY, USA, 2007.
- Vo-Dinh, T. Biomedical Photonics Handbook; CRC Press: Boca Raton, FL, USA, 2003.
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320.
- Shimizu, K.; Kitama, M. Fundamental study on near-axis scattered light and its application to optical computed tomography. Opt. Rev. 2000, 7, 383–388.
- Wang, L.; Ho, P.P.; Liu, C.; Zhang, G.; Alfano, R.R. Ballistic 2-D imaging through scattering walls using an ultrafast optical Kerr gate. Science 1991, 253, 769–771.
- Das, B.B.; Yoo, K.M.; Alfano, R.R. Ultrafast time-gated imaging in thick tissues: A step toward optical mammography. Opt. Lett. 1993, 18, 1092–1094.
- Das, B.B.; Yoo, K.M.; Alfano, R.R. Snake Photon Imaging in Thick Tissues in Visible and Near Infrared Radiation Spectral Region. Adv. Opt. Imaging Photon Migr. 1994, 21, 166–169.
- Freund, I.; Kaveh, M.; Rosenbluh, M. Dynamic Multiple Scattering: Ballistic Photons and the Breakdown of the Photon-Diffusion Approximation. Phys. Rev. Lett. 1988, 60, 1130–1133.
- Chen, N.G.; Bai, J. Estimation of quasi-straightforward propagating light in tissues. Phys. Med. Biol. 1999, 44, 1669–1676.
- Vasefi, F.; Najiminaini, M.; Ng, E.; Kaminska, B.; Chapman, G.H.; Carson, J.J.L. Angular domain transillumination imaging optimization with an ultrafast gated camera. J. Biomed. Opt. 2010, 15, 061710.
- Gopal, V.; Mujumdar, S.; Ramachandran, H.; Sood, A.K. Imaging in turbid media using quasi-ballistic photons. Opt. Commun. 1999, 170, 331–345.
- Shimizu, K.; Mouri, M.; Yamamoto, K. Trans-body imaging of physiological functions with light. In Optical Methods in Biomedical and Environmental Sciences; Ohzu, H., Komatsu, S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1994; pp. 63–66.
- Uhl, A.; Busch, C.; Marcel, S.; Veldhuis, R. (Eds.) Handbook of Vascular Biometrics; Springer Nature: Cham, Switzerland, 2020.
- Yang, L.; Yang, G.; Yin, Y.; Zhou, L. A survey of finger vein recognition. In Biometric Recognition; Sun, Z., Shan, S., Sang, H., Zhou, J., Wang, Y., Yuan, W., Eds.; Springer: Cham, Switzerland, 2014.
- Kamiyama, H.; Kitama, M.; Shimizu, H.O.; Yamashita, M.; Kojima, Y.; Shimizu, K. Fundamental study for optical transillumination imaging of arteriovenous fistula. Adv. Biomed. Eng. 2021, 10, 1–10.
- Zhao, M.; Hao, Y.; Zhang, C.; Zhai, R.; Liu, B.; Liu, W.; Wang, C.; Jafri, S.H.M.; Razak, A.; Papadakis, R.; et al. Advances in two-dimensional materials for optoelectronics applications. Crystals 2022, 12, 1087.
- Xu, K. Silicon electro-optic micro-modulator fabricated in standard CMOS technology as components for all silicon monolithic integrated optoelectronic systems. J. Micromech. Microeng. 2021, 31, 054001.
- Matsuda, Y.; Namita, T.; Kato, Y.; Shimizu, K. Arteriovenous discrimination by spectroscopic analysis of transillumination images. IEICE Tech. Rep. 2012, 111, 151–156.
- Taka, Y.; Sakatani, K.; Kato, Y.; Shimizu, K. Non—Invasive lmaging of absorption changes in rat brain by NIR transillumination. Med. Imaging Tech. 1999, 7, 545–555.
- Tandon, S.; Kambi, N.; Jain, N. Overlapping representations of the neck and whiskers in the rat motor cortex revealed by mapping at different anaesthetic depths. Eur. J. Neurosci. 2008, 27, 228–237.
- Zhang, M.; Watanabe, H.; Sarkisyan, D.; Andersen, M.S.; Nosova, O.; Galatenko, V.; Carvalho, L.; Lukoyanov, N.; Thelin, J.; Schouenborg, J.; et al. Hindlimb motor responses to unilateral brain injury: Spinal cord encoding and left-right asymmetry. Brain Commun. 2020, 2, fcaa055.
