Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Rotational thromboelastometry (ROTEM) is a viscoelastic method, which provides a graphical and numerical representation of induced hemostasis in whole blood samples. Its ability to quickly assess the state of hemostasis is used in the management of bleeding from a variety of causes.
- rotational thromboelastometry
- management
- fibrinogen disorders
1. Introduction
Rotational thromboelastometry (ROTEM) is a point-of-care testing devices, which means that tests can be performed at the bedside. Test results are delivered quickly, enabling rapid treatment modification [1]. Increasingly, ROTEM analysis is being incorporated into the diagnostic algorithm and the treatment of bleeding in high-risk patients, such as those undergoing cardiac surgery or suffering from extensive trauma. This system also plays an important role in the management of peri- and postpartum bleeding, in the diagnosis of inherited and acquired bleeding disorders, or in complex procedures, such as liver transplantation. According to the latest findings, ROTEM, as a fast and reliable diagnostic tool, is an essential component of blood management since it can significantly reduce the number of allogeneic transfusions and increase patient safety [2].
2. The Measuring Principle of ROTEM
The ROTEM device measures changes in viscoelastic properties in blood during clot formation in a small sample of citrated or heparinized blood (300–340 μL) after the addition of clotting factors. A blood sample is heated to 37 °C in a stationary disposable cup, which is subjected to the constant rotational force of an oscillating pin. The oscillating axis has an attached mirror to which a light beam is directed. As the clot forms around the pin, pin oscillation is increasingly restricted, and changes in light reflectance are captured by a photodetector [2][3][4].
Thus, based on the emerging thromboelastometry curve (Figure 1), it can visually evaluate coagulation from the formation of the clot through its promotion and stabilization of its dissolution in the process of fibrinolysis.
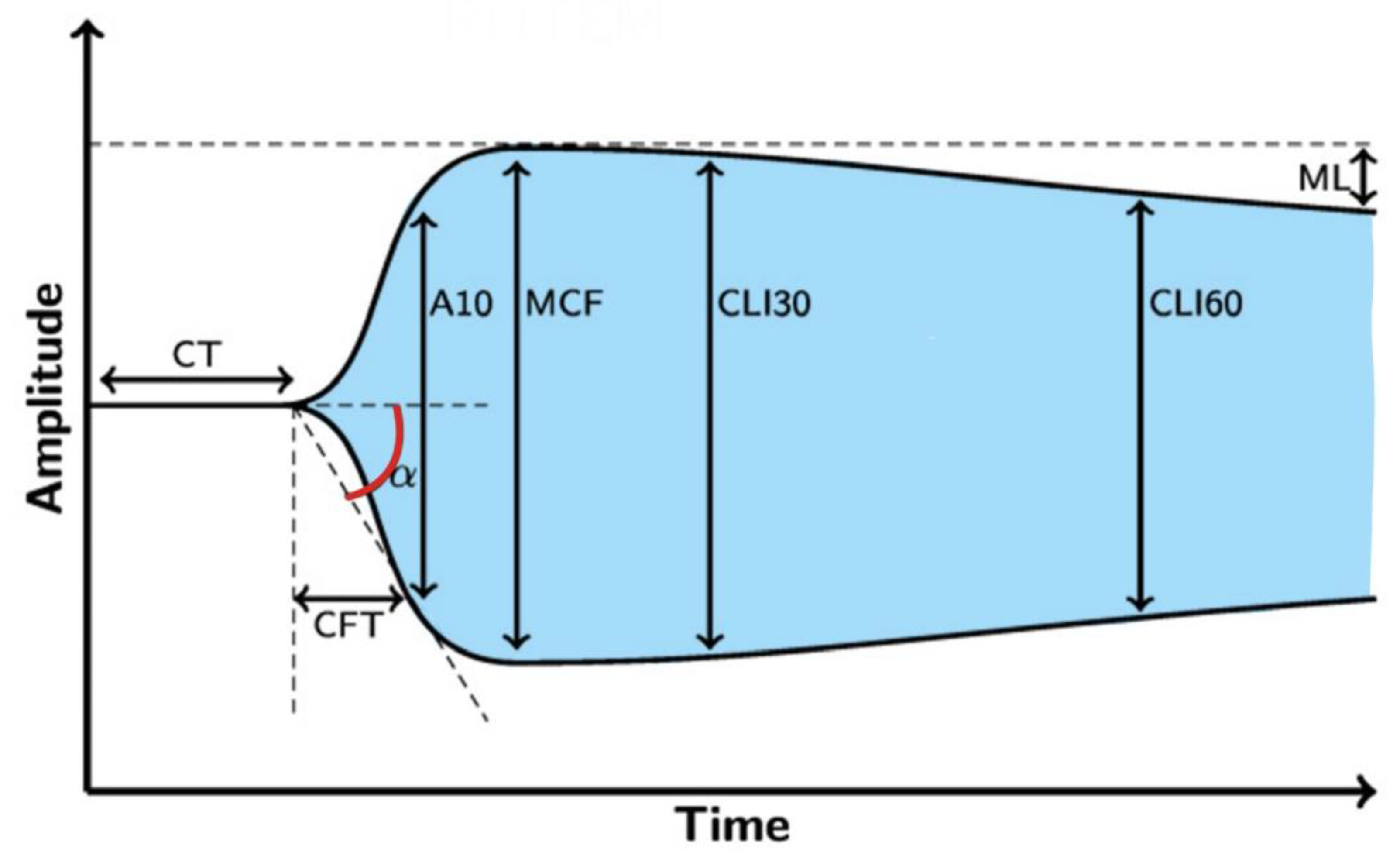
Figure 1. The resulting curve of rotational thromboelastometry. Legend: CT—clotting time (s), CFT—clot formation time (s), α—angle of clot polymerization rate (°), A10—clot strength value in time of 10 min from CT (mm), MCF—maximum clot firmness (mm), CLI30—lysis index 30 min after clotting time (%), CLI60—lysis index 60 min after clotting time (%), ML—maximum clot lysis (%).
The advantage of this POC test is the possibility of separating the activation of the intrinsic coagulation cascade (by kaolin and ellagic acid) and the extrinsic coagulation cascade (by tissue factor), which helps evaluate the functionality of the particular components of coagulation and thus allows for targeted therapeutic interventions in terms of the missing factor substitution. At the same time, after the addition of a platelet inhibitor, independent of platelet influence, it is possible to monitor the influence of fibrinogen on the final clot strength [5].
The ROTEM system allows for four independent measurements that can be performed independently of each other in separate chambers, giving a comprehensive view of patient’s coagulation.
2.1. INTEM Assay
Negatively charged surfaces (kaolin or ellagic acid) activate the intrinsic pathway of the coagulation cascade in the INTEM tests, thus providing information similar to that provided by the activated partial thromboplastin time (aPTT) examination, especially the clotting time (CT) parameter.
2.2. EXTEM Assay
By using tissue factor as a reagent, it is possible to assess the extrinsic pathway of the coagulation cascade, with clotting occurring faster than in the intrinsic coagulation pathway assay, similar to the living organism. The resulting parameters of the EXTEM assay correspond to the prothrombin time (PT) in basic coagulation. Similar to the INTEM and APTEM assays, clot strength is mainly influenced by fibrinogen and platelets [6]. Pathological values of particular parameters reflect a factor deficiency of the extrinsic coagulation pathway, as well as the possible influence of vitamin K antagonists or direct-acting oral anticoagulants (DOACs).
2.3. FIBTEM Assay
The addition of cytochalasin, which has an inhibitory effect on myctofilaments in platelets, prevents platelet-mediated clot retraction. Thus, the FIBTEM test best reflects fibrinogen activity. FIBTEM also correlates very well with fibrinogen activity determined by the Clauss assay [7]. The Clauss fibrinogen assay is the most often used laboratory method to measure plasma fibrinogen levels. The main advantage of the FIBTEM assay is that it provides information on fibrinogen activity within minutes, whereas the Clauss test requires 30–60 min before the result is available [7]. There is an evidence that early FIBTEM assay parameters predict the final strength of the blood clot. The FIBTEM assay detects fibrinolysis sooner than assays for the intrinsic and extrinsic pathways of blood coagulation. Therefore, this type of fibrinogen determination should be used in the management of active bleeding in trauma patients to provide early antifibrinolytic therapy [8].
2.4. APTEM Assay
The addition of the plasmin inhibitor aprotinin as a reagent is used to assess and confirm whether the decrease in clot amplitude is caused by fibrinolysis. The results are compared with those of the EXTEM assay, with shortened clotting time (CT) and higher maximum clot strength (MCF) in the APETM test indicating fibrinolysis.
2.5. HEPTEM Assay
HAPTEM test is an optional used in heparinized patients. Due to the fact that the INTEM assay is particularly sensitive to heparin, leading to a distortion of the results, in the HEPTEM assay, the effect of heparin is inhibited by adding heparinase, and the results are compared to the parameters in the INTEM assay. Thus, the inhibitory effect of heparin on coagulation is proven.
Five possible examinations (INTEM, EXTEM, FIBTEM, APTEM, HEPTEM) together with the necessary reagents are summarized in Table 1.
Table 1. Summary of possible examinations and necessary reagents on the ROTEM® delta device [9].
| Assay | Investigated Area | Used Reagent |
|---|---|---|
| INTEM | Intrinsic coagulation pathway FXII, FXI, FIX, FVIII, FX, FV, FII, FI, fibrin, platelets, fibrinolysis |
partial thromboplastin, ellagic acid |
| EXTEM | Extrinsic coagulation pathway FVII, FX, FV, FII, FI, fibrin, platelets, fibrinolysis |
recombinant tissue factor, phospholipids |
| FIBTEM | Contribution of fibrinogen to the clot formation after platelet inactivation | recalcification and platelet inhibitor cytochalasin D |
| APTEM | Inhibition of fibrinolysis, comparison to EXTEM can indicate/detect hyperfibrinolysis | recalcification and fibrinolysis inhibitor aprotinin/tranexamic acid |
| HEPTEM | Heparin inactivation in heparinized patients | recalcification, heparinase I * |
Legend: F—factor. * heparinase I (neutralase I) is an enzyme that specifically degrades heparin by catalyzing the cleavage of the saccharide bonds found in the heparin molecule.
3. The Application of ROTEM in the Management of Bleeding in Patients with Congenital Fibrinogen Disorders
ROTEM-guided substitution therapy also plays an important role in the management of bleeding in patients with inherited fibrinogen disorders (IFD). IFD are rare diseases affecting either the amount of circulating fibrinogen (afibrinogenemia and hypofibrinogenemia), its quality (dysfibrinogenemia), or both (hypodysfibrinogenemia). They are also associated with different clinical phenotypes, including bleeding, and thrombotic or pregnancy complications. Some patients with IFD are also without any clinical manifestation. Most cases of IFD are caused by a causal pathogenic variant in one of the three genes coding for fibrinogen (FGA, FGB, or FGG) [10].
Fibrinogen concentrates were originally approved for the treatment of bleeding episodes in patients with these fibrinogen disorders with an elimination half-life of approximately 80 h. Currently, there are many reasons to consider fibrinogen concentrates as a supplementary therapy for the treatment of coagulopathy [11][12].
Rotational thromboelastometry is also a useful tool in the diagnosis of IFD, as the Clauss fibrinogen assay is able to determine the fibrinogen activity, although it does not distinguish between qualitative and quantitative defects of fibrinogen [13]. Currently, the FIBTEM assay is sensitive to the detection of a clot polymerization disorder. Thus, the clot firmness MCF parameter is used to predict fibrinogen deficiency and the need for fibrinogen concentrate [14][15]. An example of a ROTEM test in a patient with congenital dysfibrinogenemia with a significant decrease in the FIBTEM A10 and FIBTEM MCF parameters is presented in Figure 2.
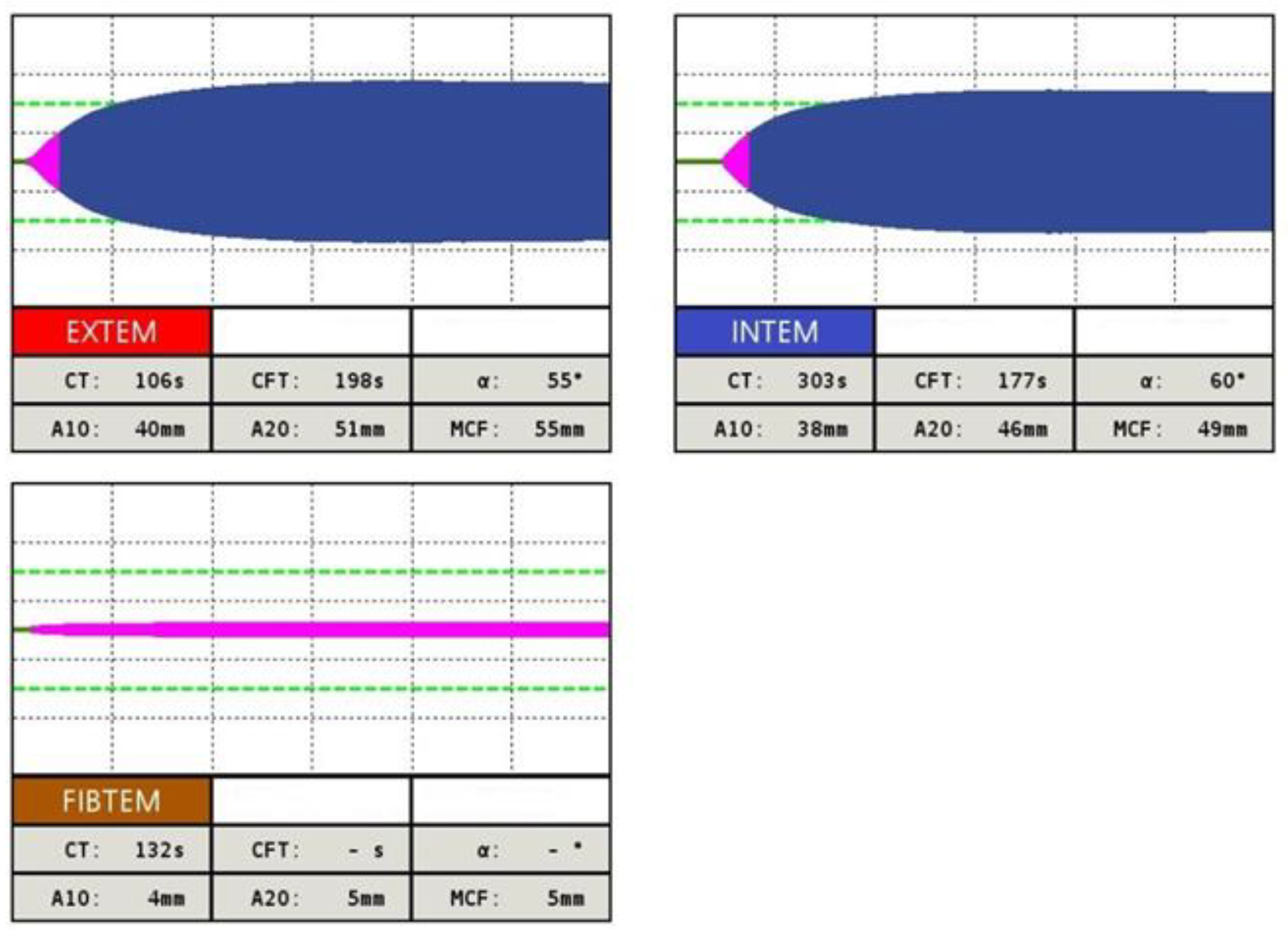
Figure 2. Example of INTEM, EXTEM and FIBTEM assay in patient with inherited dysfibrinogenemia. Thromboelastometric curve in EXTEM test shows prolonged CT and CFT, parameter A10 is shortened. In INTEM test parallel CT and CFT parameters are prolonged, A10, A20 and MCF parameters are shortened. In FIBTEM test a significant decrease in A10, A20 and FIBTEM MCF parameters is observed, what testifies for a clot polymerization disorder due to fibrinogen deficiency.
The fibrinogen dose can be calculated according to the following formula: Fibrinogen concentrate dose (g) = (target FIBTEM MCF (mm) − actual FIBTEM MCF (mm)) × (body weight (kg)/70) × 0.5 g/mm [7]. The increase in the MCF also serves as a parameter of efficacy after fibrinogen infusion.
In the diagnosis of hereditary hypofibrinogenemia, an abnormal median CT and MCF by FIBTEM assay is present compared to those of a dysfibrinogenemia diagnosis, of which the parameters of FIBTEM assay are ambiguous (median values of MCF are often higher in comparison with patients with hypofibrinogenemia). ROTEM, especially MCF and FIBTEM tests, are useful, e.g., in the perioperative management of fibrinogen replacement therapy in patients with a-, hypo-, or dysfibrinogenemia [16][17]. However, their effectiveness in predicting the clinical phenotypes of these disorders are unambiguous and must be confirmed by larger prospective studies [15][18]. A series of thromboelastometric curves after administration of 23.5 mg/kg fibrinogen concentrate in a patient with congenital afibrinogenemia is presented in Figure 3.
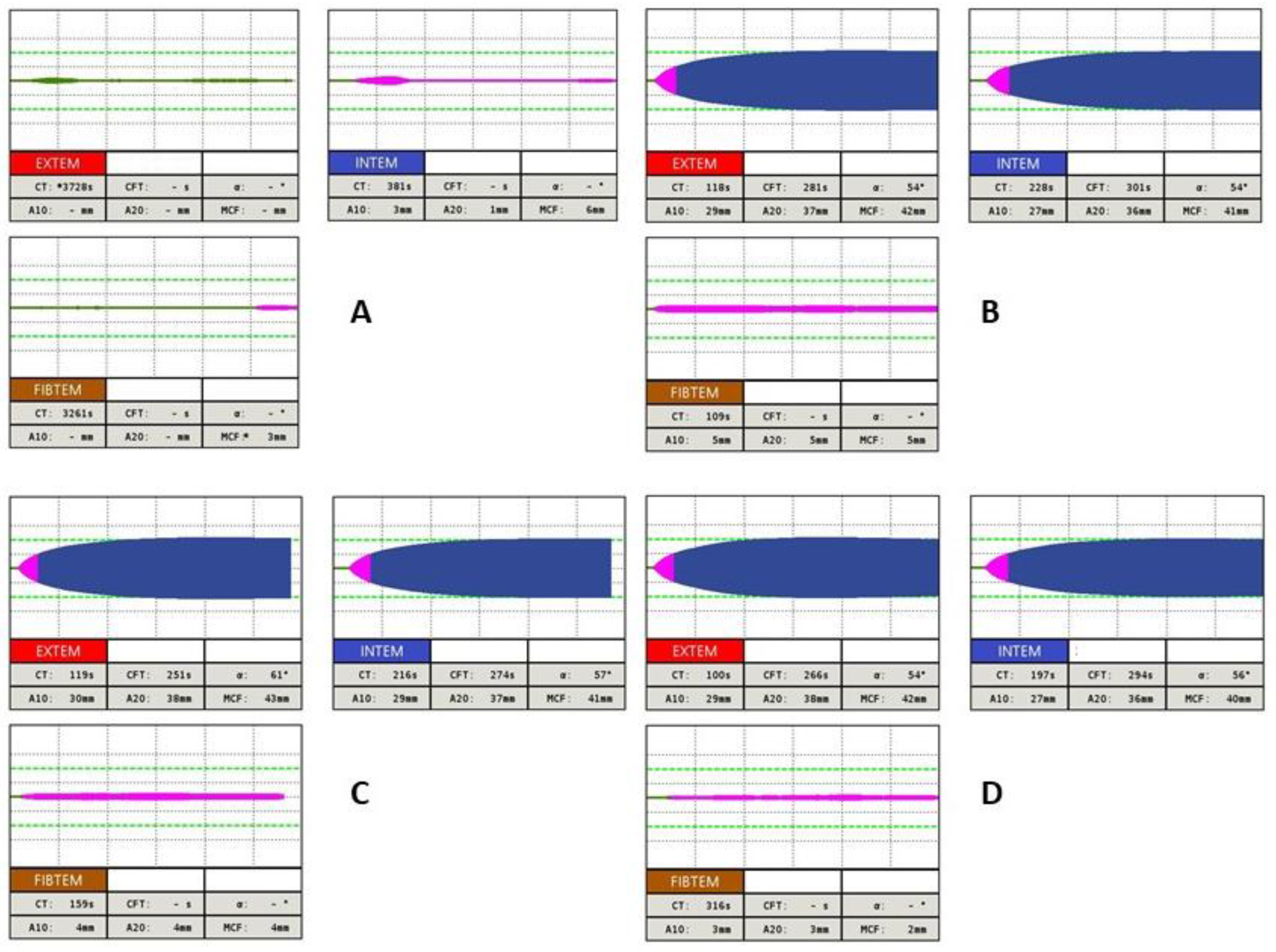
Figure 3. Example of using ROTEM in patient with congenital afibrinogenemia after administration of 23.5 mg/kg fibrinogen concentrate: (A) basal values (Fbg 0.54 g/L [ref. 1.80–4.20], PT 74% [ref. 75.0–120.0], INR 1,25 [ref. 0.80–1.20], APTT 35.9 s [ref. 25.0–36.0], TT 20 s [ref. 12.0–18.0]) (B) one hour after fibrinogen substitution (Fbg 0.57 g/L [ref. 1.80–4.20], PT 76% [ref. 75.0–120.0], INR 1.24 [ref. 0.80–1.20], APTT 35.0 s [ref. 25.0–36.0], TT 21 s [ref. 12.0–18.0]) (C) four hours after fibrinogen replacement therapy (Fbg 0.45 g/L [ref. 1.80–4.20], PT 72% [ref. 75.0–120.0], INR 1.31 [ref. 0.80–1.20], APTT 36.2 s [ref. 25.0–36.0], TT 25 s [ref. 12.0–18.0]) (D) twelve hours after fibrinogen replacement therapy (Fbg 0.34 g/L [ref. 1.80–4.20], PT 62% [ref. 75.0–120.0], INR 1,48 [ref. 0.80–1.20], APTT 35.1 s [ref. 25.0–36.0], TT 28.3 s [ref. 12.0–18.0]).
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13203219
References
- Nichols, J.H. Point-of-Care Testing. In Contemporary Practice in Clinical Chemistry, 4th ed.; AACC Press: Washington, DC, USA, 2020; pp. 323–336. ISBN 9780128154991.
- Görlinger, K.; Dirkmann, D.; Hanke, A. Rotational Thromboelastometry (ROTEM®). In Trauma Induced Coagulopathy; Gonzalez, E., Moore, H., Moore, E., Eds.; Springer: Cham, Switzerland, 2016; pp. 267–298.
- Carll, T.; Wool, G.D. Basic principles of viscoelastic testing. Transfusion 2020, 60, S1–S9.
- Volod, O.; Bunch, C.M.; Zackariya, N.; Moore, E.E.; Moore, H.B.; Kwaan, H.C.; Neal, M.D.; Al-Fadhl, M.D.; Patel, S.S.; Wiarda, G.; et al. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. J. Clin. Med. 2022, 11, 860.
- Whiting, D.; DiNardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232.
- Theusinger, O.M.; Schröder, C.M.; Eismon, J.; Emmert, M.Y.; Seifert, B.; Spahn, D.R.; Baulig, W. The influence of laboratory coagulation tests and clotting factor levels on Rotation Thromboelastometry (ROTEM(R)) during major surgery with hemorrhage. Anesth. Analg. 2013, 117, 314–321.
- De Vries, J.J.; Veen, C.S.B.; Snoek, C.J.M.; Kruip, M.J.H.A.; de Maat, M.P.M. FIBTEM clot firmness parameters correlate well with the fibrinogen concentration measured by the Clauss assay in patients and healthy subjects. Scand. J. Clin. Lab. Investig. 2020, 80, 600–605.
- Harr, J.N.; Moore, E.E.; Chin, T.L.; Chapman, M.P.; Ghasabyan, A.; Stringham, J.R.; Banerjee, A.; Silliman, C.C. Viscoelastic hemostatic fibrinogen assays detect fibrinolysis early. Eur. J. Trauma. Emerg. Surg. 2015, 41, 49–56.
- Skornova, I.; Slavik, L.; Stasko, J.; Kubisz, P.; Krcova, V.; Bartova, L.; Bradacova, P.; Macichova, M.; Ulehlova, J.; Vazanova, A.; et al. Hemostáza-Laboratórne Metódy, ich Využitie a Interpretácia vo Vybraných Klinických Situáciách. ; Martin Slovakia: Bratislava, Slovakia, 2020; pp. 148–154. ISBN 978-80-89694-78-5.
- Simurda, T.; Zolkova, J.; Snahnicanova, Z.; Loderer, D.; Skornova, I.; Sokol, J.; Hudecek, J.; Stasko, J.; Lasabova, Z.; Kubisz, P. Identification of Two Novel Fibrinogen Bβ Chain Mutations in Two Slovak Families with Quantitative Fibrinogen Disorders. Int. J. Mol. Sci. 2017, 19, 100.
- Koller, T.; Parera Ruiz, A.; Diaz-Ricart, M.; Gómez Caro, A.M. Role of fibrinogen concentrates for treatment of critical perioperative hemorrhage. Drugs Today 2021, 57, 219–239.
- Manco-Johnson, M.J.; Dimichele, D.; Castaman, G.; Fremann, S.; Knaub, S.; Kalina, U.; Peyvandi, F.; Piseddu, G.; Mannucci, P.; FIBRINOGEN CONCENTRATE STUDY GROUP. Pharmacokinetics and safety of fibrinogen concentrate. J. Thromb. Haemost. 2009, 7, 2064–2069.
- Peng, H.T.; Nascimento, B.; Beckett, A. Thromboelastography and Thromboelastometry in Assessment of Fibrinogen Deficiency and Prediction for Transfusion Requirement: A Descriptive Review. BioMed Res. Int. 2018, 2018, 7020539.
- Simurda, T.; Asselta, R.; Zolkova, J.; Brunclikova, M.; Dobrotova, M.; Kolkova, Z.; Loderer, D.; Skornova, I.; Hudecek, J.; Lasabova, Z.; et al. Congenital Afibrinogenemia and Hypofibrinogenemia: Laboratory and Genetic Testing in Rare Bleeding Disorders with Life-Threatening Clinical Manifestations and Challenging Management. Diagnostics 2021, 11, 2140.
- Szanto, T.; Lassila, R.; Lemponen, M.; Lehtinen, E.; Neerman-Arbez, M.; Casini, A. Whole Blood Thromboelastometry by ROTEM and Thrombin Generation by Genesia According to the Genotype and Clinical Phenotype in Congenital Fibrinogen Disorders. Int. J. Mol. Sci. 2021, 22, 2286.
- Zhou, J.; Xin, Y.; Ding, Q.; Chen, Y.; Dai, J.; Lu, Y.; Wu, X.; Liang, Q.; Wang, H.; Wang, X. Thromboelastography predicts risks of obstetric complication occurrence in (hypo)dysfibrinogenemia patients under non-pregnant state. Clin. Exp. Pharmacol. Physiol. 2016, 43, 149–156.
- Khunakanan, S.; Akaraborworn, O.; Sangthong, B.; Thongkhao, K. Correlation between Maximum Clot Firmness in FIBTEM and Fibrinogen Level in Critical Trauma Patients. Crit. Care Res. Pr. 2019, 2019, 2756461.
- Treliński, J.; Pachniewska, K.; Matczak, J.; Robak, M.; Chojnowski, K. Assessment of Selected ROTEM Parameters, Kinetics of Fibrinogen Polymerization and Plasmin Amidolytic Activity in Patients with Congenital Fibrinogen Defects. Adv. Clin. Exp. Med. 2016, 25, 1255–1263.
This entry is offline, you can click here to edit this entry!
