Cryogenic treatment (CT) is one of the possible solutions for an environmentally friendly, sustainable, and cost-effective technology for tailoring the properties of different metallic materials.
- energy sector
- renewable energy
- fusion
- metallic materials
- cryogenic treatment
1. Introduction
2. Mechanisms of Cryogenic Treatments
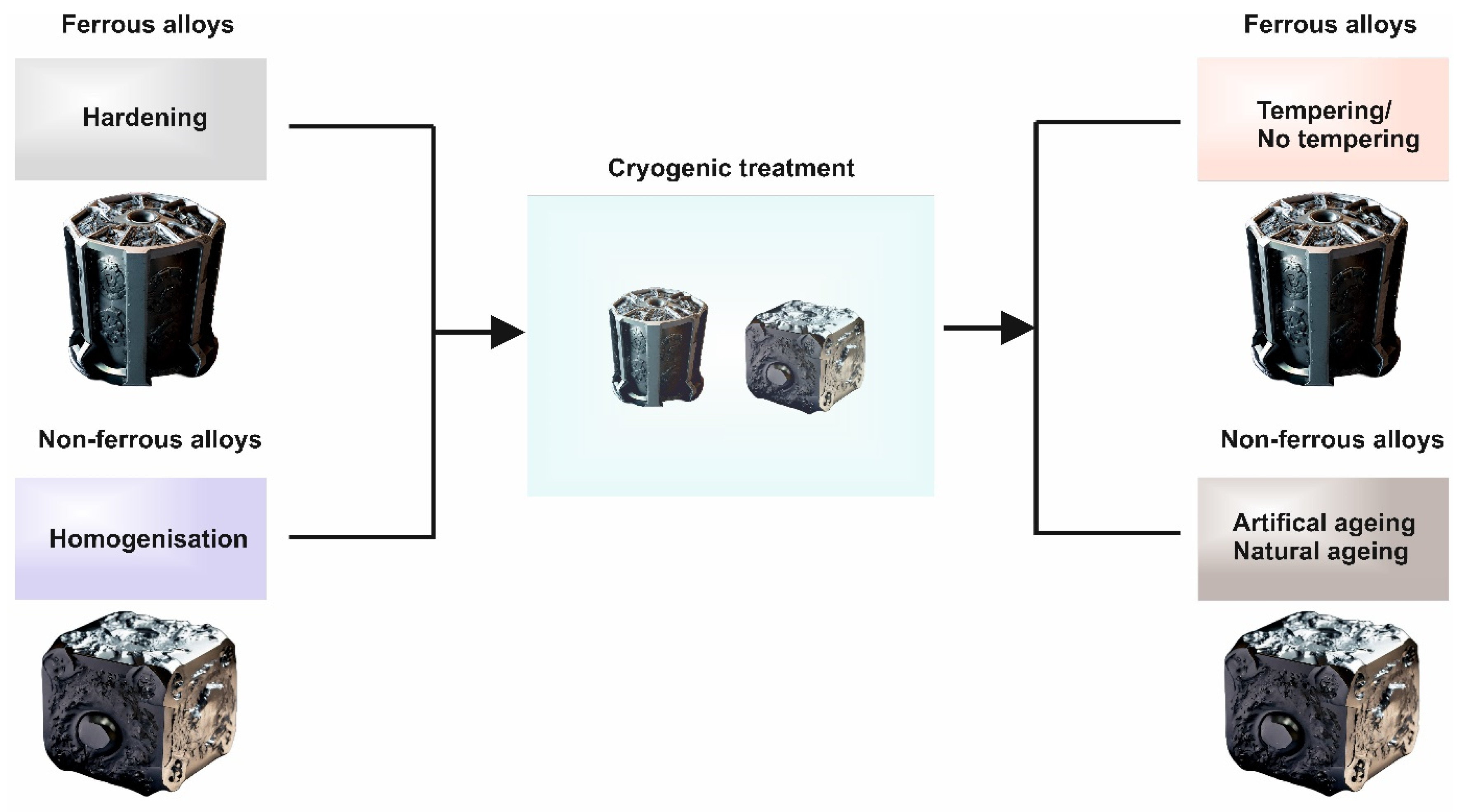
3. Energy Sector and Position of Cryogenic Treatments
4. Effect of Cryogenic Treatments on Surface, Interface, and Corrosion Properties of Metallic Mateirals Used in the Energy Sector
4.1. Metallic Materials Being Tested for the Use in the Energy Sector
|
Ferrous Alloys |
Grades of Steel |
Tested Properties |
Possibilities of Application in Selected Energy Sector |
|---|---|---|---|
|
Austenitic stainless steel |
AISI 304 [76][77][78][79][80][81][82], AISI 304L [20][31][83][84][85][86], AISI 304LN [86], AISI 316 [86][87][88][89][90][91][92], AISI 316L [53][60][93][94][95][96][97], AISI 316LN [86][98], AISI 321 [99][100], AISI 347 [101][102] |
Hardness, microhardness, wear (abrasive wear), fracture toughness, impact toughness, compressive strength, tensile strength, yield strength, elongation, friction, erosion, strain-hardening exponent, surface roughness, machining of steel, fatigue, residual stress, surface chemistry, and oxidation |
In all energy sectors |
|
Martensitic stainless steel |
AISI 410 [103], AISI 420 [61][103][104][105], AISI 420 MOD [105], AISI 430 [106][107], AISI 431 [16][21][108], AISI 440C [78], AISI P91 [109], 10Cr13Co13Mo5NiW1VE [110], 13Cr4NiMo [27], 10Cr [111]. |
Yield strength, elongation, tensile strength, wear, hardness, impact toughness, fracture toughness, magnetism tribocorrosion, electrochemistry, and corrosion resistance (also stress corrosion cracking) |
In all energy sectors. |
|
Duplex steels |
Hardness, wear, machinability, residual stress, and corrosion resistance |
Mostly in wind and solar energy |
|
|
Carbon steels |
IS 2062 [118], AISI 1045 [119][120][121][122][123][124][125], AISI 1018 [126] |
Hardness, wear, surface roughness, tensile strength, yield strength, ultimate tensile strength, elongation, and residual stress |
Steels can be used in hydroelectrical, biomass, solar, and geothermal energy |
|
Other steels |
Nitronic steels 40 [127], 50 [128] High-strength steels ASTM A36 [129] Cast steels ASTM A743 [130], SAE J431 G10 [131] ACSR [132] Bearing steel AISI 52100 [38][40][133][134][135] Low-alloyed steels SAE 1008 [136], AISI 4340 [137], AISI 4140 [137] |
Residual tress, hardness, friction, wear, fatigue, impact toughness, corrosion resistance, and machinability |
In all energy sectors |
|
Non-Ferrous Alloys |
Grades of Alloys |
Tested Properties |
Possibilities of Application in Selected Energy Sector |
|---|---|---|---|
|
Al-based alloy |
3xxx series: A356 [143][144], A390 [145] 6xxx series: 6026 [64][146][147][148], 6061 [65][149][150], 6063 [74] |
Hardness, wear (abrasion), corrosion resistance, tensile strength, machinability, fatigue, strain-hardening coefficient, residual stress, fracture toughness, and corrosion resistance |
Mostly in hydroelectrical, biomass, wind, and solar energy |
|
Ni-based alloy |
Inconel: 200 [156], 600 [157][158], 617 [159], 625 [160][161][162], 690 [163], 800 [164], 800H [165][166][167] |
Fatigue, surface roughness, machinability, durability, impact toughness, microhardness, and tensile strength |
In all energy sectors |
|
Other alloys |
HEA [172] W-based alloys [173] Cu-based alloys [174] |
Microhardness, compressive strength, and plasticity |
Mostly in advanced nuclear power (fusion), geothermal, and solar energy |
5. Effect of Cryogenic Treatments on Metallic Materials Potenitally Used in the Energy Sector
5.1. Oxide Formation
5.2. Corrosion Resistance
Corrosion Resistance of Ferrous Alloys
Corrosion Resistance of Non-Ferrous Alloys
This entry is adapted from the peer-reviewed paper 10.3390/coatings13111822
References
- Jovičević-Klug, P.; Podgornik, B. Review on the Effect of Deep Cryogenic Treatment of Metallic Materials in Automotive Applications. Metals 2020, 10, 434.
- Jovičević-Klug, P. Mechanisms and Effect of Deep Cryogenic Treatment on Steel Properties; Institute of Metals and Technology: Ljubljana, Slovenia, 2022.
- Workman, K.J.; Pitts, D.W. Method of Treating Brake Pads. U.S. Patent US5447035A, 29 September 1994.
- Leach, J.M.; Harvey, W.L. Method of Cryogenically Hardening an Insert in an Article. U.S. Patent No. 4,336,077, 3 March 1980.
- Voorhees, J.E. Process for Treating Materials to Improve Their Structural Characteristics. U.S. Patent No. 4,482,005, 13 November 1984.
- Baldissera, P.; Delprete, C. Deep Cryogenic Treatment: A Bibliographic Review. Open Mech. Eng. J. 2008, 2, 32–39.
- Vengatesh, M.; Srivignesh, R.; Pradeep, T.; Karthik, N.R. Review on Cryogenic Trearment of Steels. Int. Res. J. Eng. Technol. 2016, 3, 417–422.
- Sonar, T.; Lomte, S.; Gogte, C. Cryogenic Treatment of Metal—A Review. Mater. Today Proc. 2018, 5, 25219–25228.
- Yildiz, Y.; Nalbant, M. A Review of Cryogenic Cooling in Machining Processes. Int. J. Mach. Tools Manuf. 2008, 48, 947–964.
- Akincioğlu, S.; Gökkaya, H.; Uygur, İ. A Review of Cryogenic Treatment on Cutting Tools. Int. J. Adv. Manuf. Technol. 2015, 78, 1609–1627.
- Barron, R.F. Cryogenic Treatment of Metals to Improve Wear Resistance. Cryogenics 1982, 22, 409–413.
- Senthilkumar, D.; Rajendran, I. Influence of Shallow and Deep Cryogenic Treatment on Tribological Behavior of En 19 Steel. J. Iron Steel Res. Int. 2011, 18, 53–59.
- Senthilkumar, D.; Rajendran, I.; Pellizzari, M.; Siiriainen, J. Influence of Shallow and Deep Cryogenic Treatment on the Residual State of Stress of 4140 Steel. J. Mater. Process. Technol. 2011, 211, 396–401.
- Senthilkumar, D. Cryogenic Treatment: Shallow and Deep. In Encyclopedia of Iron, Steel, and Their Alloys; Totten, G.E., Colas, R., Eds.; Taylor and Francis: New York, NY, USA, 2016; pp. 995–1007. ISBN 9781351254496.
- Ciski, A.; Nawrocki, P.; Babul, T.; Hradil, D. Multistage Cryogenic Treatment of X153CrMoV12 Cold Work Steel. In Proceedings of the 5th International Conference Recent Trends in Structural Materials, Pilsen, Czech Republic, 14–16 November 2018; Volume 461, pp. 0120121–0120126.
- Jovičević-Klug, P.; Jovičević-Klug, M.; Thormählen, L.; McCord, J.; Rohwerder, M.; Godec, M.; Podgornik, B. Austenite Reversion Suppression with Deep Cryogenic Treatment: A Novel Pathway towards 3rd Generation Advanced High-Strength Steels. Mater. Sci. Eng. A 2023, 873, 145033.
- Jovičević-Klug, P.; Tegg, L.; Jovičević-Klug, M.; Parmar, R.; Amati, M.; Gregoratti, L.; Almasy, L.; Cairney, J.M.; Podgornik, B. Understanding Carbide Evolution and Surface Chemistry during Deep Cryogenic Treatment in High-Alloyed Ferrous Alloy. Appl. Surf. Sci. 2023, 610, 155497.
- Jovičević-Klug, P.; Puš, G.; Jovičević-Klug, M.; Žužek, B.; Podgornik, B. Influence of Heat Treatment Parameters on Effectiveness of Deep Cryogenic Treatment on Properties of High-Speed Steels. Mater. Sci. Eng. A 2022, 829, 142157.
- Jovičević-Klug, P.; Guštin, A.Z.; Jovičević-Klug, M.; Šetina Batič, B.; Lebar, A.; Podgornik, B. Coupled Role of Alloying and Manufacturing on Deep Cryogenic Treatment Performance on High-Alloyed Ferrous Alloys. J. Mater. Res. Technol. 2022, 18, 3184–3197.
- Jovičević-Klug, P.; Lipovšek, N.; Jovičević-Klug, M.; Mrak, M.; Ekar, J.; Ambrožič, B.; Dražić, G.; Kovač, J.; Podgornik, B. Assessment of Deep Cryogenic Heat-Treatment Impact on the Microstructure and Surface Chemistry of Austenitic Stainless Steel. Surf. Interfaces 2022, 35, 102456.
- Jovičević-Klug, P.; Jovičević-Klug, M.; Sever, T.; Feizpour, D.; Podgornik, B. Impact of Steel Type, Composition and Heat Treatment Parameters on Effectiveness of Deep Cryogenic Treatment. J. Mater. Res. Technol. 2021, 14, 1007–1020.
- Pellizzari, M. Influence of Deep Cryogenic Treatment on the Properties of Conventional and PM High Speed Steels. Metall. Ital. 2008, 100, 17–22.
- Senthilkumar, D. Influence of Deep Cryogenic Treatment on Hardness and Toughness of En31 Steel. Adv. Mater. Process. Technol. 2019, 5, 114–122.
- Baldissera, P. Deep Cryogenic Treatment of AISI 302 Stainless Steel: Part I—Hardness and Tensile Properties. Mater. Des. 2010, 31, 4725–4730.
- Amini, K.; Negahbani, M.; Ghayour, H. The Effect of Deep Cryogenic Treatment on Hardness and Wear Behavior of the H13 Tool Steel. Metall. Ital. 2015, 107, 53–58.
- Elango, G.; Raghunath, B.K.; Thamizhmaran, K. Effect of Cryogenic Treatment on Microstructure and Micro Hardness of Aluminium (LM25)—SiC Metal Matrix Composite. J. Eng. Res. 2014, 10, 64–68.
- Peng, J.; Zhou, B.; Li, Z.; Huo, D.; Xiong, J.; Zhang, S. Effect of Tempering Process on the Cryogenic Impact Toughness of 13Cr4NiMo Martensitic Stainless Steel. J. Mater. Res. Technol. 2023, 23, 5618–5630.
- Çakir, F.H.; Çelik, O.N. The Effects of Cryogenic Treatment on the Toughness and Tribological Behaviors of Eutectoid Steel. J. Mech. Sci. Technol. 2017, 31, 3233–3239.
- Sola, R.; Giovanardi, R.; Parigi, G.; Veronesi, P. A Novel Method for Fracture Toughness Evaluation of Tool Steels with Post-Tempering Cryogenic Treatment. Metals 2017, 7, 75.
- Nanesa, C.H.; Jahazi, M. Simultaneous Enhancement of Strength and Ductility in Cryogenically Treated AISI D2 Tool Steel. Mater. Sci. Eng. A 2014, 598, 413–419.
- Jovičević-Klug, P.; Jovičević-Klug, M.; Rohwerder, M.; Godec, M.; Podgornik, B. Complex Interdependency of Microstructure, Mechanical Properties, Fatigue Resistance and Residual Stress of Austenitic Stainless Steels AISI 304L. Materials 2023, 16, 2638.
- Weng, Z.; Gu, K.; Wang, K.; Liu, X.; Wang, J. The Reinforcement Role of Deep Cryogenic Treatment on the Strength and Toughness of Alloy Structural Steel. Mater. Sci. Eng. A 2020, 772, 138698.
- Korade, D.; Ramana, K.V.; Jagtap, K. Wear and Fatigue Behaviour of Deep Cryogenically Treated H21 Tool Steel. Trans. Indian Inst. Met. 2020, 73, 843–851.
- Bensely, A.; Shyamala, L.; Harish, S.; Mohan Lal, D.; Nagarajan, G.; Junik, K.; Rajadurai, A. Fatigue Behaviour and Fracture Mechanism of Cryogenically Treated En 353 Steel. Mater. Des. 2009, 30, 2955–2962.
- Jovicevic-Klug, M.; Jovicevic-Klug, P.; McCord, J.; Podgornik, B. Investigation of Microstructural Attributes of Steel Surfaces through Magneto-Optical Kerr Effect. J. Mater. Res. Technol. 2021, 11, 1245–1259.
- Jovičević-Klug, P.; Jovičević-Klug, M.; Podgornik, B. Unravelling the Role of Nitrogen in Surface Chemistry and Oxidation Evolution of Deep Cryogenic Treated High-Alloyed Ferrous Alloy. Coatings 2022, 12, 213.
- Jovičević-Klug, M.; Jovičević-Klug, P.; Kranjec, T.; Podgornik, B. Cross-Effect of Surface Finishing and Deep Cryogenic Treatment on Corrosion Resistance of AISI M35 Steel. J. Mater. Res. Technol. 2021, 14, 2365–2381.
- Jovičević-Klug, P.; Kranjec, T.; Jovičević-Klug, M.; Kosec, T.; Podgornik, B. Influence of the Deep Cryogenic Treatment on AISI 52100 and AISI D3 Steel’s Corrosion Resistance. Materials 2021, 14, 6357.
- Senthilkumar, D.; Bracke, J.; Slootsman, N. Corrosion and Elastic Behaviour of Cryogenically Treated En 19 Steel. Corros. Manag. 2014, 117, 16–21.
- Wang, W.; Srinivasan, V.; Siva, S.; Albert, B.; Lal, M.; Alfantazi, A. Corrosion Behavior of Deep Cryogenically Treated AISI 420 and AISI 52100 Steel. Corrosion 2014, 70, 708–720.
- Ramesh, S.; Bhuvaneswari, B.; Palani, G.S.; Lal, D.M.; Iyer, N.R. Effects on Corrosion Resistance of Rebar Subjected to Deep Cryogenic Treatment. J. Mech. Sci. Technol. 2017, 31, 123–132.
- Ma, S.; Su, R.; Li, G.; Qu, Y.; Li, R. Effect of Deep Cryogenic Treatment on Corrosion Resistance of AA7075-RRA. J. Phys. Chem. Solids 2022, 167, 110747.
- Su, R.; Ma, S.; Wang, K.; Li, G.; Qu, Y.; Li, R. Effect of Cyclic Deep Cryogenic Treatment on Corrosion Resistance of 7075 Alloy. Met. Mater. Int. 2022, 28, 862–870.
- Garrison, T.C. Process for Improving the Corrosion Resistance of Metals. CN1795278A, 24 May 2004.
- Gunes, I.; Uzun, M.; Cetin, A.; Aslantas, K.; Cicek, A. Evaluation of Wear Performance of Cryogenically Treated Vanadis 4 Extra Tool Steel. Kov. Mater. 2016, 54, 195–204.
- Koneshlou, M.; Meshinchi, A.K.; Khomamizadeh, F. Effect of Cryogenic Tratment on Microstucture, Mechanical and Wear Behaviors of AISI H13 Hot Work Tool Steel. Cryogenics 2011, 51, 55–61.
- Senthilkumar, D.; Rajendran, I. Optimization of Deep Cryogenic Treatment to Reduce Wear Loss of 4140 Steel. Mater. Manuf. Process. 2011, 27, 567–572.
- Jovičević-Klug, P.; Jenko, M.; Jovičević-Klug, M.; Šetina Batič, B.; Kovač, J.; Podgornik, B. Effect of Deep Cryogenic Treatment on Surface Chemistry and Microstructure of Selected High-Speed Steels. Appl. Surf. Sci. 2021, 548, 149257.
- Cryogenic Treatment Applications Find Potential in the Energy Sector. Available online: https://www.cryogenicsociety.org/index.php?option=com_dailyplanetblog&view=entry&year=2023&month=05&day=02&id=188:cryogenic-treatment-applications-find-potential-in-the-energy-sector (accessed on 20 July 2023).
- Industrial Heating. Deep Cryogenic Treatment for Marine and Oil-and-Gas Applications. 3 December 2018. Available online: https://www.industrialheating.com/articles/94598-deep-cryogenic-treatment-for-marine-and-oil-and-gas-applications (accessed on 20 July 2023).
- Darwin, J.D.; Mohan Lal, D.; Nagarajan, G. Optimization of Cryogenic Treatment to Maximize the Wear Resistance of 18% Cr Martensitic Stainless Steel by Taguchi Method. J. Mater. Process Technol. 2008, 195, 241–247.
- Kumar, M.C.; Vijayakumar, P.; Narayan, B. Optimization of Cryogenic Treatment Parameters to Maximise the Tool Wear of HSS Tools by Taguchi Method. Int. J. Mod. Eng. Res. 2012, 2, 3051–3055.
- Baloji, D.; Anil, K.; Satyanarayana, K.; Ul Haq, A.; Singh, S.K.; Naik, M.T. Evaluation and Optimization of Material Properties of ASS316L at Sub-Zero Temperature Using Taguchi Robust Design. Mater. Today Proc. 2019, 18, 4475–4481.
- Lance, J.W.; Jones, H.M. Material Treatment by Cryogenic Cooling. U.S. Patent No. 3,891,477, 9 September 1971.
- Jin, F.; Wu, L.; Xiong, C.; Wei, J.; Wen, X. A Kind of Cryogenic Treating Process of Cast Aluminium Alloy Piston Piece. CN103498117B, 29 September 2013.
- De Carvalho Eduardo, A.; Atem De Carvalho, R. Special Steels; Cryogenic Process for the Production Thereof; Use of Special Steels in a Saline and/or High-Pressure Environment. WO2014008564A1, 9 July 2013.
- Jin, F.; Zhou, Z.; Wei, J.; Xiong, C.; Wu, L. High-Speed Steel Cryogenic Treatment Process. CN103525997A, 29 September 2013.
- Pang, B.; Wang, B.; Zhao, X. High-Efficiency Energy-Saving Apparatus Used for Cryogenic Treatment. CN103589840A, 26 November 2013.
- Kamody, D.J. Process for the Cryogenic Treatment of Metal Containing Materials. US5875636A, 1 October 1997.
- Li, Z.; Wang, Y.; Wang, J.; Zhang, Y. Effect of Cryogenic Heat Treatment and Heat Treatment on the Influence of Mechanical, Energy, and Wear Properties of 316L Stainless Steel by Selective Laser Melting. JOM 2022, 74, 3855–3868.
- Prieto, G.; Ipiña, J.E.P.; Tuckart, W.R. Cryogenic Treatments on AISI 420 Stainless Steel: Microstructure and Mechanical Properties. Mater. Sci. Eng. A 2014, 605, 236–243.
- Zhirafar, S.; Rezaeian, A.; Pugh, M. Effect of Cryogenic Treatment on the Mechanical Properties of 4340 Steel. J. Mater. Process. Technol. 2007, 186, 298–303.
- Baldissera, P.; Delprete, C. Effects of Deep Cryogenic Treatment on Static Mechanical Properties of 18NiCrMo5 Carburized Steel. Mater. Des. 2009, 30, 1435–1440.
- Jovičević-Klug, M.; Rezar, R.; Jovičević-Klug, P.; Podgornik, B. Influence of Deep Cryogenic Treatment on Natural and Artificial Aging of Al-Mg-Si Alloy EN AW 6026. J. Alloys Compd. 2022, 899, 163323.
- Nageswara Rao, P.; Jayaganthan, R. Effects of Warm Rolling and Ageing after Cryogenic Rolling on Mechanical Properties and Microstructure of Al 6061 Alloy. Mater. Des. 2012, 39, 226–233.
- Shahsavari, A.; Karimzadeh, F.; Rezaeian, A.; Heydari, H. Significant Increase in Tensile Strength and Hardness in 2024 Aluminum Alloy by Cryogenic Rolling. Procedia Mater. Sci. 2015, 11, 84–88.
- Aamir, A.; Lei, P.; Lixiang, D.; Zengmin, Z. Effects of Deep Cryogenic Treatment, Cryogenic and Annealing Temperatures on Mechanical Properties and Corrosion Resistance of AA5083 Aluminium Alloy. Int. J. Microstruct. Mater. Prop. 2016, 11, 339–358.
- Gu, K.; Zhang, H.; Zhao, B.; Wang, J.; Zhou, Y.; Li, Z. Effect of Cryogenic Treatment and Aging Treatment on the Tensile Properties and Microstructure of Ti-6Al-4V Alloy. Mater. Sci. Eng. A 2013, 584, 170–176.
- Vinothkumar, T.S.; Kandaswamy, D.; Prabhakaran, G.; Rajadurai, A. Effect of Dry Cryogenic Treatment on Vickers Hardness and Wear Resistance of New Martensitic Shape Memory Nickel-Titanium Alloy. Eur. J. Dent. 2015, 9, 513–517.
- Anil Kumar, B.K.; Ananthaprasad, M.G.; Gopalakrishna, K. Action of Cryogenic Chill on Mechanical Properties of Nickel Alloy Metal Matrix Composites. In Proceedings of the International Conference on Advances in Materials and Manufacturing Applications, Bangalore, India, 14–16 July 2016; pp. 1–11.
- Khedekar, D.; Gogte, C.L. Development of the Cryogenic Processing Cycle for Age Hardenable AA7075 Aluminium Alloy and Optimization of the Process for Surface Quality Using Gray Relational Analysis. Mater. Today Proc. 2018, 5, 4995–5003.
- Yuanchun, H.; Li, Y.; Ren, X.; Xiao, Z. Effect of Deep Cryogenic Treatment on Aging Processes of Al–Mg–Si Alloy. Phys. Met. Metallogr. 2019, 120, 914–918.
- Adin, M.Ş. Performances of Cryo-Treated and Untreated Cutting Tools in Machining of AA7075 Aerospace Aluminium Alloy. Eur. Mech. Sci. 2023, 7, 70–81.
- Vendra, S.S.L.; Goel, S.; Kumar, N.; Jayaganthan, R. A Study on Fracture Toughness and Strain Rate Sensitivity of Severely Deformed Al 6063 Alloys Processed by Multiaxial Forging and Rolling at Cryogenic Temperature. Mater. Sci. Eng. A 2017, 686, 82–92.
- Sonia, P.; Verma, V.; Saxena, K.K.; Kishore, N.; Rana, R.S. Effect of Cryogenic Treatment on Mechanical Properties and Microstructure of Aluminium 6082 Alloy. Mater. Today Proc. 2020, 26, 2248–2253.
- Özbek, N.A.; Çİçek, A.; Gülesİn, M.; Özbek, O. Application of Deep Cryogenic Treatment to Uncoated Tungsten Carbide Inserts in the Turning of AISI 304 Stainless Steel. Met. Mater. Trans. A Phys. Met. Mater. Sci. 2016, 47, 6270–6280.
- Çiçek, A.; Kıvak, T.; Ekici, E. Optimization of Drilling Parameters Using Taguchi Technique and Response Surface Methodology (RSM) in Drilling of AISI 304 Steel with Cryogenically Treated HSS Drills. J. Intell. Manuf. 2015, 26, 295–305.
- Ramos, L.B.; Simoni, L.; Mielczarski, R.G.; Vega, M.R.O.; Schroeder, R.M.; De Fraga Malfatti, C. Tribocorrosion and Electrochemical Behavior of DIN 1.4110 Martensitic Stainless Steels After Cryogenic Heat Treatment. Mater. Res. 2017, 20, 460–468.
- Ben Fredj, N.; Sidhom, H. Effects of the Cryogenic Cooling on the Fatigue Strength of the AISI 304 Stainless Steel Ground Components. Cryogenics 2006, 46, 439–448.
- Nalbant, M.; Yildiz, Y. Effect of Cryogenic Cooling in Milling Process of AISI 304 Stainless Steel. Trans. Nonferrous Met. Soc. China 2011, 21, 72–79.
- Hong, S.Y.; Broomer, M.; Hong, S.Y.; Broomer, M. Economical and Ecological Cryogenic Machining of AISI 304 Austenitic Stainless Steel. Clean Technol. Environ. Policy 2000, 2, 157–166.
- Dhananchezian, M.; Pradeep Kumar, M.; Sornakumar, T. Cryogenic Turning of AISI 304 Stainless Steel with Modified Tungsten Carbide Tool Inserts. Mater. Manuf. Process. 2011, 26, 781–785.
- Johan Singh, P.; Guha, B.; Achar, D.R.G. Fatigue Life Improvement of AISI 304L Cruciform Welded Joints by Cryogenic Treatment. Eng. Fail. Anal. 2003, 10, 1–12.
- Singh, P.J.; Mannan, S.L.; Jayakumar, T.; Achar, D.R.G. Fatigue Life Extension of Notches in AISI 304L Weldments Using Deep Cryogenic Treatment. Eng. Fail. Anal. 2005, 12, 263–271.
- Oh, D.; Song, S.; Kim, N.; Kim, M. Effect of Cryogenic Temperature on Low-Cycle Fatigue Behavior of AISI 304L Welded Joint. Metals 2018, 8, 657.
- Read, D.T.; Reed, R.P. Fracture and Strength Properties of Selected Austenitic Stainless Steels at Cryogenic Temperatures. Cryogenics 1981, 21, 415–417.
- Manimaran, G.; Pradeep Kumar, M.; Venkatasamy, R. Influence of Cryogenic Cooling on Surface Grinding of Stainless Steel 316. Cryogenics 2014, 59, 76–83.
- Kennedy, F.E.; Ye, Y.; Baker, I.; White, R.R.; Barry, R.L.; Tang, A.Y.; Song, M. Development of a New Cryogenic Tribotester and Its Application to the Study of Cryogenic Wear of AISI 316 Stainless Steel. Wear 2022, 496–497, 204309.
- Bhaskar, L.; Raj, D.S. Evaluation of the Effect of Cryogenic Treatment of HSS Drills at Different Holding Time in Drilling AISI 316-SS. Eng. Res. Express 2020, 2, 025005.
- Norberto López de Lacalle, L.; Gandarias, A.; López de Lacalle, L.N.; Aizpitarte, X.; Lamikiz, A. High Performance Drilling of Austenitic Stainless Steels. Int. J. Mach. Mach. Mater. 2008, 3, 1–17.
- Uhlář, R.; Hlaváč, L.; Gembalová, L.; Jonšta, P.; Zuchnický, O. Abrasive Water Jet Cutting of the Steels Samples Cooled by Liquid Nitrogen. Appl. Mech. Mater. 2013, 308, 7–12.
- Çiçek, A.; Uygur, I.; Kvak, T.; Altan Zbek, N. Machinability of AISI 316 Austenitic Stainless Steel with Cryogenically Treated M35 High-Speed Steel Twist Drills. J. Manuf. Sci. Eng. 2012, 134, 061003.
- Kumar, M.; Singh Sidhu, H.; Singh Sidhu, B. Influence of Ultra-Low Temperature Treatments on the Slurry Erosion Performance of Stainless Steel-316L. In Advances in Mechanical and Materials Technology; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2022; pp. 1273–1286.
- Gao, Q.; Jiang, X.; Sun, H.; Zhang, Y.; Fang, Y.; Mo, D.; Li, X. Performance and Microstructure of TC4/Nb/Cu/316L Welded Joints Subjected to Cryogenic Treatment. Mater. Lett. 2022, 321, 132453.
- Sugavaneswaran, M.; Kulkarni, A. Effect of Cryogenic Treatment on the Wear Behavior of Additive Manufactured 316L Stainless Steel. Tribol. Ind. 2019, 41, 33–42.
- Wang, C.; Lin, X.; Wang, L.; Zhang, S.; Huang, W. Cryogenic Mechanical Properties of 316L Stainless Steel Fabricated by Selective Laser Melting. Mater. Sci. Eng. A 2021, 815, 141317.
- Kosaraju, S.; Singh, S.K.; Buddi, T.; Kalluri, A.; Ul Haq, A. Evaluation and Characterisation of ASS316L at Sub-Zero Temperature. Adv. Mater. Process. Technol. 2020, 6, 445–455.
- Kvackaj, T.; Rozsypalova, A.; Kocisko, R.; Bidulska, J.; Petrousek, P.; Vlado, M.; Pokorny, I.; Sas, J.; Weiss, K.P.; Duchek, M.; et al. Influence of Processing Conditions on Properties of AISI 316LN Steel Grade. J. Mater. Eng. Perform. 2020, 29, 1509–1514.
- Salehi, M.; Eskandari, M.; Yeganeh, M. Characterizations of the Microstructure and Texture of 321 Austenitic Stainless Steel After Cryo-Rolling and Annealing Treatments. J. Mater. Eng. Perform. 2023, 32, 816–834.
- Aletdinov, A.; Mironov, S.; Korznikova, G.; Konkova, T.; Zaripova, R.; Myshlyaev, M.; Semiatin, S.L. EBSD Investigation of Microstructure Evolution during Cryogenic Rolling of Type 321 Metastable Austenitic Steel. Mater. Sci. Eng. A 2019, 745, 460–473.
- Aurich, J.C.; Mayer, P.; Kirsch, B.; Eifler, D.; Smaga, M.; Skorupski, R. Characterization of Deformation Induced Surface Hardening during Cryogenic Turning of AISI 347. CIRP Ann. 2014, 63, 65–68.
- Mayer, P.; Skorupski, R.; Smaga, M.; Eifler, D.; Aurich, J.C. Deformation Induced Surface Hardening When Turning Metastable Austenitic Steel AISI 347 with Different Cryogenic Cooling Strategies. Procedia CIRP 2014, 14, 101–106.
- Laksanasittiphan, S.; Tuchinda, K.; Manonukul, A.; Suranuntchai, S. Use of Deep Cryogenic Treatment to Reduce Particle Contamination Induced Problem in Hard Disk Drive. Key Eng. Mater. 2017, 730, 265–271.
- Prieto, G.; Tuckart, W.R. Influence of Cryogenic Treatments on the Wear Behavior of AISI 420 Martensitic Stainless Steel. J. Mater. Eng. Perform. 2017, 26, 5262–5271.
- Ramesh, S.; Bhuvaneshwari, B.; Palani, G.S.; Mohan Lal, D.; Mondal, K.; Gupta, R.K. Enhancing the Corrosion Resistance Performance of Structural Steel via a Novel Deep Cryogenic Treatment Process. Vacuum 2019, 159, 468–475.
- Makalesi, A.; Şenel, S.; Koçar, O.; Kocaman, E.; Özdamar, O.; Bülent, Z.; Üniversitesi, E.; Fakültesi, M.; Bölümü, M.M. AISI 430 Çeliklerin Derin Kroyonejik İşlem Sonrası Mekanik ve Mikroyapısal Özelliklerinin İncelenmesi. Eur. J. Sci. Technol. 2021, 32, 1000–1005.
- ŞİRİN, Ş.; AKINCIOĞLU, S. Investigation of Friction Performance and Surface Integrity of Cryogenically Treated AISI 430 Ferritic Stainless Steel. Int. Adv. Res. Eng. J. 2021, 5, 194–201.
- Yıldız, E.; Altan Özbek, N.; Ankara Hidrolik Mak San Tic Ltd.; Şti, H. Effect of cryogenic treatment and tempering temperature on mechanical and microstructural properties of aisi 431 steel. Int. J. 3D Print. Technol. Digit. Ind. 2022, 6, 74–82.
- Tembwa, E.N. Softening Response of As-Normalized and Cryogenic-Soaked P91 Martensitic Steels p y g P91 Martensitic Steels. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2018.
- Zhao, Z.; Yu, M.; Han, C.; Yang, Z.; Teng, P.; Zhong, J.; Li, S.; Liu, J. Effects of Carbide Evolution on SCC Behaviors of 10Cr13Co13Mo5NiW1VE Martensitic Stainless Steel. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4400821 (accessed on 2 October 2023).
- Zhang, H.; Ji, X.; Ma, D.; Tong, M.; Wang, T.; Xu, B.; Sun, M.; Li, D. Effect of Aging Temperature on the Austenite Reversion and Mechanical Properties of a Fe–10Cr–10Ni Cryogenic Maraging Steel. J. Mater. Res. Technol. 2021, 11, 98–111.
- Dhananchezian, M.; Priyan, M.R.; Rajashekar, G.; Narayanan, S.S. Study the Effect of Cryogenic Cooling On Machinability Characteristics During Turning Duplex Stainless Steel 2205. Mater. Today Proc. 2018, 5, 12062–12070.
- Koppula, S.; Rajkumar, A.; Krishna, S.H.; Prudhvi, R.S.; Aparna, S.; Subbiah, R. Improving the Mechanical Properties of AISI 2205 Duplex Stainless Steel by Cryogenic Treatment Process. E3S Web Conf. 2020, 184, 01019.
- Narayanan, D.; Jagadeesha, T. Process Capability Improvement Using Internally Cooled Cutting Tool Insert in Cryogenic Machining of Super Duplex Stainless Steel 2507. In Innovative Product Design and Intelligent Manufacturing Systems; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2020; pp. 323–330.
- Narayanan, D.; Salunkhe, V.G.; Dhinakaran, V.; Jagadeesha, T. Experimental Evaluation of Cutting Process Parameters in Cryogenic Machining of Duplex Stainless Steel. In Advances in Industrial Automation and Smart Manufacturing; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2021; Volume 23, pp. 505–516.
- Kanagaraju, T.; Boopathy, S.R.; Gowthaman, B. Effect of Cryogenic and Wet Coolant Performance on Drilling of Super Duplex Stainless Steel (2507). Mater. Express 2020, 10, 81–93.
- Sastry, C.C.; Abeens, M.; Pradeep, N.; Manickam, M.A.M. Microstructural Analysis, Radiography, Tool Wear Characterization, Induced Residual Stress and Corrosion Behavior of Conventional and Cryogenic Trepanning of DSS 2507. J. Mech. Sci. Technol. 2020, 34, 2535–2547.
- Pradeep Samuel, A.; Arul, S. Effect of Cryogenic Treatment on the Mechanical Properties of Low Carbon Steel IS 2062. Mater. Today Proc. 2018, 5, 25065–25074.
- Thornton, R.; Slatter, T.; Lewis, R. Effects of Deep Cryogenic Treatment on the Wear Development of H13A Tungsten Carbide Inserts When Machining AISI 1045 Steel. Prod. Eng. 2014, 8, 355–364.
- Govindaraju, N.; Shakeel Ahmed, L.; Pradeep Kumar, M. Experimental Investigations on Cryogenic Cooling in the Drilling of AISI 1045 Steel. Mater. Manuf. Process. 2014, 29, 1417–1421.
- Dilip Jerold, B.; Pradeep Kumar, M. Experimental Investigation of Turning AISI 1045 Steel Using Cryogenic Carbon Dioxide as the Cutting Fluid. J. Manuf. Process. 2011, 13, 113–119.
- Mahendran, R.; Rajkumar, P.; Nirmal Raj, L.; Karthikeyan, S.; Rajeshkumar, L. Effect of Deep Cryogenic Treatment on Tool Life of Multilayer Coated Carbide Inserts by Shoulder Milling of EN8 Steel. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 378.
- Karnan, B.; Kuppusamy, A.; Latchoumi, T.P.; Banerjee, A.; Sinha, A.; Biswas, A.; Subramanian, A.K. Multi-Response Optimization of Turning Parameters for Cryogenically Treated and Tempered WC–Co Inserts. J. Inst. Eng. India Ser. D 2022, 103, 263–274.
- Senthilkumar, D. Deep Cryogenic Treatment of En 31 and En 8 Steel for the Development of Wear Resistance. Adv. Mater. Process. Technol. 2021, 8, 1769–1776.
- Senthilkumar, D. Effect of Deep Cryogenic Treatment on Residual Stress and Mechanical Behaviour of Induction Hardened En 8 Steel. Adv. Mater. Process. Technol. 2016, 2, 427–436.
- Arunkarthikeyan, K.; Balamurugan, K. Performance Improvement of Cryo Treated Insert on Turning Studies of AISI 1018 Steel Using Multi Objective Optimization. In Proceedings of the International Conference on Computational Intelligence for Smart Power System and Sustainable Energy, CISPSSE 2020, Keonjhar, India, 29–31 July 2020.
- Wigley, D.A. The Metallurgical Structure and Mechanical Properties at Low Temperature of Nitronic 40 with Particular Reference to Its Use in the Construction of Models for Cryogenic Wind Tunnels; National Aeronautics and Space Administration: Hampton, VA, USA, 1982.
- Gaddam, S.; Haridas, R.S.; Sanabria, C.; Tammana, D.; Berman, D.; Mishra, R.S. Friction Stir Welding of SS 316 LN and Nitronic 50 Jacket Sections for Application in Superconducting Fusion Magnet Systems. Mater. Des. 2022, 221, 110949.
- Sastry, C.C.; Hariharan, P.; Pradeep Kumar, M.; Muthu Manickam, M.A. Experimental Investigation on Boring of HSLA ASTM A36 Steel under Dry, Wet, and Cryogenic Environments. Mater. Manuf. Process. 2019, 34, 1352–1379.
- Y Chow, J.G.; Klamut, C.J. Properties of Cast C£-8 Stainless-Steel Weldments at Cryogenic Temperatures; Brookhaven National Lab.: Upton, NY, USA, 1981.
- Thornton, R.; Slatter, T.; Jones, A.H.; Lewis, R. The Effects of Cryogenic Processing on the Wear Resistance of Grey Cast Iron Brake Discs. Wear 2011, 271, 2386–2395.
- Franco Steier, V.; Kalombo Badibanga, R.; Roberto Moreira Da Silva, C.; Magalhães Nogueira, M.; Araújo, J.A. Effect of Chromium Nitride Coatings and Cryogenic Treatments on Wear and Fretting Fatigue Resistance of Aluminum. Electr. Power Syst. Res. 2014, 116, 322–329.
- Kara, F.; Çiçek, A. Multiple Regression and ANN Models for Surface Quality of Cryogenically-Treated AISI 52100 Bearing Steel Micro Machining of Ti6Al4V and Inconel 718 View Project Improvement of Machining Precesses View Project. Artic. J. Balk. Tribol. Assoc. 2013, 19, 570–584.
- Gunes, I.; Cicek, A.; Aslantas, K.; Kara, F. Effect of Deep Cryogenic Treatment on Wear Resistance of AISI 52100 Bearing Steel. Trans. Indian Inst. Met. 2014, 67, 909–917.
- Villa, M.; Pantleon, K.; Somers, M.A.J. Enhanced Carbide Precipitation during Tempering of Sub-Zero Celsius Treated AISI 52100 Bearing Steel. In Proceedings of the Heat Treat & Surface Engineering Conference & Expo, Chennai, India, May 16–18 2013; pp. 1–7.
- Hong, S.Y.; Ding, Y. Micro-Temperature Manipulation in Cryogenic Machining of Low Carbon Steel. J. Mater. Process Technol. 2001, 116, 22–30.
- Jamali, A.R.; Khan, W.; Chandio, A.D.; Anwer, Z.; Jokhio, M.H.; Karachi, P. Effect of Cryogenic Treatment on Mechanical Properties of AISI 4340 and AISI 4140 Steel. J. Eng. Technol. 2019, 38, 2413–7219.
- Lisiecki, A.; Ślizak, D.; Kukofka, A. Laser Cladding of Co-Based Metallic at Cryogenic Conditions. J. Achiev. Mater. Manuf. Eng. 2019, 95, 20–31.
- Keseler, H.; Westermann, I.; Kandukuri, S.Y.; Nøkleby, J.O.; Holmedal, B. Permanent Effect of a Cryogenic Spill on Fracture Properties of Structural Steels. IOP Conf. Ser. Mater. Sci. Eng. 2015, 102, 012004.
- Abdin, A.; Feyzabi, K.; Hellman, O.; Nordström, H.; Rasa, D.; Thaung Tolförs, G.; Öqvist, P.-O. Methods to Create Compressive Stress in High Strength Steel Components; Ångströmlaboratoriet: Uppsala, Sweden, 2018.
- Walters, C.L.; Alvaro, A.; Maljaars, J. The Effect of Low Temperatures on the Fatigue Crack Growth of S460 Structural Steel. Int. J. Fatigue 2016, 82, 110–118.
- Wang, C.; Yi, Y.; Huang, S.; Dong, F.; He, H.; Huang, K.; Jia, Y. Experimental and Theoretical Investigation on the Forming Limit of 2024-O Aluminum Alloy Sheet at Cryogenic Temperatures. Met. Mater. Int. 2021, 27, 5199–5211.
- Fiedler, T.; Al-Sahlani, K.; Linul, P.A.; Linul, E. Mechanical Properties of A356 and ZA27 Metallic Syntactic Foams at Cryogenic Temperature. J. Alloys Compd. 2020, 813, 152181.
- Sagar, S.R.; Srikanth, K.M.; Jayasimha, R. Effect of Cryogenic Treatment and Heat Treatment on Mechanical and Tribological Properties of A356 Reinforced with SiC. Mater. Today Proc. 2021, 45, 184–190.
- Zhao, Z.; Hong, S.Y. Cooling Strategies for Cryogenic Machining from a Materials Viewpoint. J. Mater. Eng. Perform. 1992, 1, 669–678.
- Jovičević-Klug, M.; Jovičević-Klug, P.; Sever, T.; Feizpour, D.; Podgornik, B. Extraordinary Nanocrystalline Pb Whisker Growth from Bi-Mg-Pb Pools in Aluminum Alloy 6026 Moderated through Oriented Attachment. Nanomaterials 2021, 11, 1842.
- Jovičević-Klug, M.; Tegg, L.; Jovičević-Klug, P.; Dražić, G.; Almásy, L.; Lim, B.; Cairney, J.M.; Podgornik, B. Multiscale Modification of Aluminum Alloys with Deep Cryogenic Treatment for Advanced Properties. J. Mater. Res. Technol. 2022, 21, 3062–3073.
- Jovičević-Klug, M.; Verbovšek, T.; Jovičević-Klug, P.; Batič, B.Š.; Ambrožič, B.; Dražić, G.; Podgornik, B. Revealing the Pb Whisker Growth Mechanism from Al-Alloy Surface and Morphological Dependency on Material Stress and Growth Environment. Materials 2022, 15, 2574.
- Wang, X.; Fan, X.; Chen, X.; Yuan, S. Forming Limit of 6061 Aluminum Alloy Tube at Cryogenic Temperatures. J. Mater. Process. Technol. 2022, 306, 117649.
- Wang, X.; Fan, X.; Chen, X.; Yuan, S. Cryogenic Deformation Behavior of 6061 Aluminum Alloy Tube under Biaxial Tension Condition. J. Mater. Process. Technol. 2022, 303, 117532.
- Bouzada, F.; Cabeza, M.; Merino, P.; Trillo, S. Effect of Deep Cryogenic Treatment on the Microstructure of an Aerospace Aluminum Alloy. Adv. Mat. Res. 2012, 445, 965–970.
- Mohan, K.; Suresh, J.A.; Ramu, P.; Jayaganthan, R. Microstructure and Mechanical Behavior of Al 7075-T6 Subjected to Shallow Cryogenic Treatment. J. Mater. Eng. Perform. 2016, 25, 2185–2194.
- Siyi, M.; Su, R.; Wang, K.; Yang, Y.; Qu, Y.; Li, R. Effect of Deep Cryogenic Treatment on Wear and Corrosion Resistance of an Al–Zn–Mg–Cu Alloy. Russ. J. Non-Ferr. Met. 2021, 62, 89–96.
- Wei, L.; Wang, D.; Li, H.; Xie, D.; Ye, F.; Song, R.; Zheng, G.; Wu, S. Effects of Cryogenic Treatment on the Microstructure and Residual Stress of 7075 Aluminum Alloy. Metals 2018, 8, 273.
- Zhang, P.; Liu, Z.; Liu, J.; Yu, J.; Mai, Q.; Yue, X. Effect of Aging plus Cryogenic Treatment on the Machinability of 7075 Aluminum Alloy. Vacuum 2023, 208, 111692.
- Deshpande, Y.V.; Andhare, A.B.; Padole, P.M. How Cryogenic Techniques Help in Machining of Nickel Alloys? A Review. Mach. Sci. Technol. 2018, 22, 543–584.
- Baig, A.; Jaffery, S.H.I.; Khan, M.A.; Alruqi, M. Statistical Analysis of Surface Roughness, Burr Formation and Tool Wear in High Speed Micro Milling of Inconel 600 Alloy under Cryogenic, Wet and Dry Conditions. Micromachines 2022, 14, 13.
- Satyanarayana, K.; Krishna, B.R.; Bhargavi, M.; Vasuki, R.E.; Kiran, K.R. Taguchi Optimization in Machining Inconel 600 with WEDM Process Using Cryogenically Treated Brass Wire. E3S Web Conf. 2021, 309, 01110.
- Mandal, P.K.; Michael Saji, A.; Kurian Lalu, A.; Krishnan, A.; Nair, A.S.; Jacob, M.M. Microstructural Study and Mechanical Properties of TIG Welded Inconel 617 Superalloy. Mater. Today Proc. 2022, 62, 3561–3568.
- Akgün, M.; Demir, H. Optimization of Cutting Parameters Affecting Surface Roughness in Turning of Inconel 625 Superalloy by Cryogenically Treated Tungsten Carbide Inserts. SN Appl. Sci. 2021, 3, 277.
- Yıldırım, Ç.V. Experimental Comparison of the Performance of Nanofluids, Cryogenic and Hybrid Cooling in Turning of Inconel 625. Tribol. Int. 2019, 137, 366–378.
- Anburaj, R.; Pradeep Kumar, M. Experimental Studies on Cryogenic CO2 Face Milling of Inconel 625 Superalloy. Mater. Manuf. Process. 2020, 36, 814–826.
- Makhesana, M.A.; Patel, K.M.; Khanna, N. Analysis of Vegetable Oil-Based Nano-Lubricant Technique for Improving Machinability of Inconel 690. J. Manuf. Process. 2022, 77, 708–721.
- Jovičevič Klug, P.; Jovičević Klug, M.; Podgornik, B. Potential in Deep Cryogenic Treatment of Non-Ferrous Alloys. In Proceedings of the European Cryogenics Days 2021, Virtual Conference, 4 November 2021; Cryogenics Society of Europe: Darmstadt, Germany, 2021.
- Palanisamy, A.; Jeyaprakash, N.; Sivabharathi, V.; Sivasankaran, S. Effects of Dry Turning Parameters of Incoloy 800H Superalloy Using Taguchi-Based Grey Relational Analysis and Modeling by Response Surface Methodology. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2022, 236, 607–623.
- Palanisamy, A.; Selvaraj, T.; Sivasankaran, S. Optimization of Turning Parameters of Machining Incoloy 800H Superalloy Using Cryogenically Treated Multilayer CVD-Coated Tool. Arab. J. Sci. Eng. 2018, 43, 4977–4990.
- Palanisamy, A.; Selvaraj, T. Optimization of Turning Parameters for Surface Integrity Properties on Incoloy 800 h Superalloy Using Cryogenically Treated Multi-Layer Cvd Coated Tool. Surf. Rev. Lett. 2019, 26, 1850139.
- Dhananchezian, M. Study the Machinability Characteristics of Nicked Based Hastelloy C-276 under Cryogenic Cooling. Measurement 2019, 136, 694–702.
- Nas, E.; Kara, F. Optimization of EDM Machinability of Hastelloy C22 Super Alloys. Machines 2022, 10, 1131.
- Akincioğlu, S.; Gökkaya, H.; Akincioğlu, G.; Karataş, M.A. Taguchi Optimization of Surface Roughness in the Turning of Hastelloy C22 Super Alloy Using Cryogenically Treated Ceramic Inserts. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2020, 234, 3826–3836.
- Ekambaram, P. Study of Mechanical and Metallurgical Properties of Hastelloy X at Cryogenic Condition. J. Mater. Res. Technol. 2019, 8, 6413–6419.
- Wu, Y.; Yuan, X.; Wen, X.; Jiao, M. Body-Centered Cubic High-Entropy Alloys. In Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2022; pp. 3–34.
- Hu, W.; Dong, Z.; Wang, H.; Ahamad, T.; Ma, Z. Microstructure Refinement and Mechanical Properties Improvement in the W-Y2O3 Alloys via Optimized Freeze-Drying. Int. J. Refract. Met. Hard Mater. 2021, 95, 105453.
- Yildiz, Y.; Sundaram, M.M.; Rajurkar, K.P.; Nalbant, M. The Effects of Cold and Cryogenic Treatments on the Machinability of Beryllium-Copper Alloy in Electro Discharge Machinability. In Proceedings of the 44th CIRP Conference on Manufacturing Systems, Madison, WI, USA, 1–3 June 2011.
- Bagherzadeh, A.; Kuram, E.; Budak, E. Experimental Evaluation of Eco-Friendly Hybrid Cooling Methods in Slot Milling of Titanium Alloy. J. Clean. Prod. 2021, 289, 125817.
- Uygur, I.; Gerengi, H.; Arslan, Y.; Kurtay, M. The Effects of Cryogenic Treatment on the Corrosion of AISI D3 Steel. Mater. Res. 2015, 18, 569–574.
- Shinde, T.; Pruncu, C.; Dhokey, N.B.; Parau, A.C.; Vladescu, A. Effect of Deep Cryogenic Treatment on Corrosion Behavior of AISI H13 Die Steel. Materials 2021, 14, 7863.
- Wang, Y.M.; Liang, Y.; Zhai, Y.D.; Zhang, Y.S.; Sun, H.; Liu, Z.G.; Su, G.Q. Study on the Role of Cryogenic Treatment on Corrosion and Wear Behaviors of High Manganese Austenitic Steel. J. Mater. Res. Technol. 2023, 24, 5271–5285.
- Akhbarizadeh, A.; Amini, K.; Javadpour, S. Effects of Applying an External Magnetic Field during the Deep Cryogenic Heat Treatment on the Corrosion Resistance and Wear Behavior of 1.2080 Tool Steel. Mater. Des. 2012, 41, 114–123.
- Wang, L.; Dong, C.; Cao, Y.; Liang, J.; Xiao, K.; Li, X. Co-Enhancing the Mechanical Property and Corrosion Resistance of Selective Laser Melted High-Strength Stainless Steel via Cryogenic Treatment. J. Mater. Eng. Perform. 2020, 29, 7052–7062.
- Baldissera, P.; Delprete, C. Deep Cryogenic Treatment of AISI 302 Stainless Steel: Part II—Fatigue and Corrosion. Mater. Des. 2010, 31, 4731–4737.
- Cai, Y.; Luo, Z.; Zeng, Y. Influence of Deep Cryogenic Treatment on the Microstructure and Properties of AISI304 Austenitic Stainless Steel A-TIG Weld. Sci. Technol. Weld. Join. 2016, 22, 236–243.
- He, X.; Lü, X.-Y.; Wu, Z.-W.; Li, S.-H.; Yong, Q.-L.; Liang, J.-X.; Su, J.; Zhou, L.-X.; Li, J. M23C6 Precipitation and Si Segregation Promoted by Deep Cryogenic Treatment Aggravating Pitting Corrosion of Supermartensitic Stainless Steel. J. Iron Steel Res. Int. 2021, 28, 629–640.
- Cabeza, M.; Feijoo, I.; Merino, P.; Trillo, S. Effect of the Deep Cryogenic Treatment on the Stress Corrosion Cracking Behaviour of AA 2017-T4 Aluminium Alloy. Mater. Corros. 2016, 67, 504–512.
