Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Macrophages serve as vital defenders, protecting the body by exhibiting remarkable cellular adaptability in response to invading pathogens and various stimuli. These cells express nicotinic acetylcholine receptors, with the α7-nAChR being extensively studied due to its involvement in activating the cholinergic anti-inflammatory pathway. Activation of this pathway plays a crucial role in suppressing macrophages’ production of proinflammatory cytokines, thus mitigating excessive inflammation and maintaining host homeostasis.
- macrophage
- inflammation
- acetylcholine receptor
- cholinergic receptor
- HIV
- SARS CoV-2
1. Introduction
Macrophages are a vital component of the immune system responsible for defending the body against pathogens and foreign substances. Found throughout the body, particularly in tissues and organs, macrophages play a crucial role in innate immunity. Their primary function is to engulf and eliminate harmful microorganisms, debris, and dead cells through phagocytic processes involving cytokines [1,2,3,4,5].
Macrophages exhibit remarkable plasticity, capable of adopting different phenotypes and functions in response to signals from their microenvironment [6]. In the presence of pathogens, insults, or tissue alterations, macrophages fulfill their critical role by polarizing and differentiating into distinct subtypes with specific roles and functions. Currently, four main subgroups (M1–M4) have been identified, working in conjunction with other immune cells to defend the body and maintain organ and systemic homeostasis [7]. Importantly, macrophages switch between different functional phenotypes in response to the local cytokine milieu, a process of particular significance during infectious processes [8]. Of note, macrophages are equipped with cholinergic constituents that enable them to respond in an anti-inflammatory manner through a neuroimmune circuit known as the cholinergic anti-inflammatory response (CAR) [9,10]. In macrophages, this route is dependent on the expression of the alpha7 nicotinic acetylcholine receptor (α7-nAChR) [9], an ion channel with anti-inflammatory properties upon stimulation.
2. Activation and Polarization of Human Macrophages
Human macrophages are white immune cells responsible for destroying pathogens. They also play crucial roles in tissue remodeling, wound healing, angiogenesis, the removal of dead immune cells, metabolism, and the secretion of cytokines and chemokines to stimulate other immune system cells, contributing to the immune response, among other functions. Macrophages become activated and polarized as part of the natural response process that maximizes their antimicrobial properties and maintains host homeostasis. Their cellular plasticity allows them to polarize into two distinct populations: classically activated inflammatory (M1) and alternatively activated anti-inflammatory (M2) macrophages (Figure 1). The latter can be divided into four subsets (M2a, b, c, and d) based on the type of stimulus they receive (Figure 2). The specialization and tropism of macrophages toward organs is an example of cellular plasticity in both health and disease. This is the case with the relatively recent discovery of five types of macrophages (M4, Mhem, M(Hb), HA-mac, Mox, and M17) associated with the development of atherosclerosis and atheroinflammation [11,12,13,14,15,16] (Figure 3). There are also M3 macrophages known as commuting macrophages. The first investigation to look into the origins of this previously unidentified lineage discovered that it was dependent on the switching response between M1 and M2 macrophage pathways [7,17]. It has recently been demonstrated that these macrophages have antitumor activity and limiting tumor proliferation [18], and that they may be a pharmacological target for inducing antitumor immunity [19]. In the context of cancer, there are tumor-associated macrophages (TAMs), and their presence has been linked to metastasis [20]. The polarization state of TAMs is influenced by the tumor microenvironment, especially by cytokines [21]. These TAM-type cells can exhibit phenotypes akin to M1 macrophages [22] and M2 macrophages [23]. Macrophages exhibiting the M17 phenotype can emerge through activation with corticosteroids, granulocyte-macrophage colony-stimulating factor, or IL-10 [7,24]. On the other hand, stimulation with IL-17 imparts an anti-apoptotic signature [7,24]. Finally, M4 macrophages are a subset of macrophages that are polarized by platelet factor 4. This population is found in human lesions and is distinguished by high levels of matrix metalloprotease 7 and S100A8 expression. M4 macrophages are considered atherogenic because they produce proinflammatory cytokines (IL-6 and TNF-α) and have poor phagocytic properties [25,26]. It has been reported that these cells are reproducibly found in coronary artery plaques [27]. In HIV infection, these inflammatory M4 macrophages form a major tissue reservoir of replication-competent HIV-1, which reactivates viral production upon autocrine/paracrine S100A8-mediated glycolytic stimulation [28]. Furthermore, it has been discovered that the level of cholesterol oxidation influences the profile of these M4 macrophages, with LDL being the molecule that causes the most significant changes. This results in the polarization of M2 macrophages and Kupffer cells towards the M4 phenotype. These cells also demonstrate increased neutrophil recruitment and more effective induction of neutrophil extracellular traps [29].
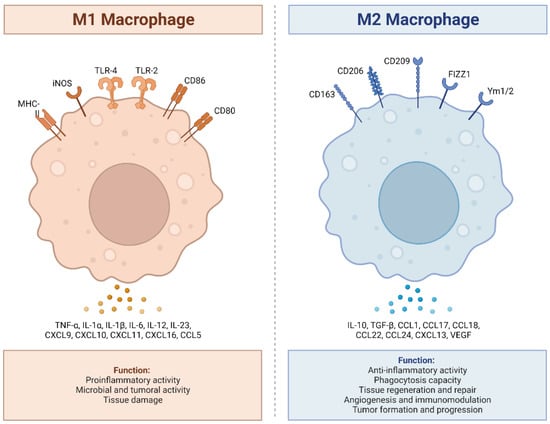
Figure 1. M1 and M2 macrophages exhibit distinct inflammatory phenotypes by expressing and secreting pro-inflammatory and anti-inflammatory cytokines, respectively. M1 macrophages, classically activated, play a vital role in pro-inflammatory responses, expressing receptors like MHC-II, iNOS, TLR-2, TLR-4, CD86, and CD80. They secrete pro-inflammatory cytokines (TNF-α, IL-1α, IL-1β, IL-6, IL-12, IL-23, CXCL9 (MIG), CXCL10, (IP-10), CXCL11 (I-TAC), CXCL16, and CCL5 (RANTES), produce ROS and NO, and promote Th1 responses. In contrast, M2 macrophages, alternatively activated, exhibit anti-inflammatory and tissue repair functions, expressing receptors such as CD163, CD206, CD209, FIZZ1, and Ym1/2. They secrete anti-inflammatory cytokines (IL-10, TGF-β, CCL1 (I-309), CCL17 (TARC), CCL18 (MIP-4), CCL22 (MDC), CCL24 (Eotaxin-2), CXCL13 (BCA-1), and VEGF), enhance phagocytosis, and support Th2 responses. The balance between M1 and M2 states is crucial for immune homeostasis and effective immune responses. Adapted from “Macrophage Polarization: M1 and M2 Subtypes”, by BioRender.com (2019). Retrieved from https://app.biorender.com/biorender-templates (accessed on 23 September 2023).
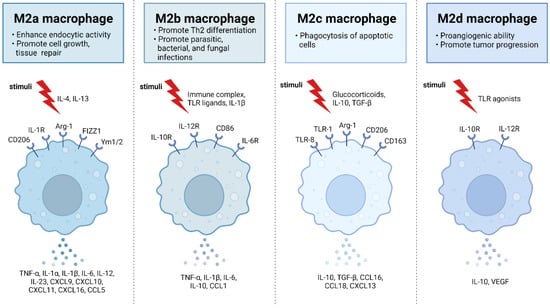
Figure 2. M2 macrophages are divided into M2a, M2b, M2c, and M2d subtypes. These macrophages differ in their cell surface mark ers, secreted cytokines, and biological functions. Once stimulated, each subtype exhibits unique functions and expresses characteristic receptors that influence their distinct roles in immune regulation and tissue homeostasis. M2a macrophages, alternatively activated by IL-4 and IL-13, are involved in tissue repair, high endocytic activity, and anti-inflammatory responses. They express CD206, IL-1R, Arg-1, FIZZ1, and Ym1/2 which contribute to their polarization. M2b macrophages, activated by immune complexes, IL-1β, and Toll-like receptor (TLR) signaling, exhibit regulatory functions in immune responses and Th2 differentiation. Their characteristic receptors include IL-10R, IL-12R, CD86, and IL-6R. M2c macrophages, activated by IL-10, TGF-β, and glucocorticoids, are known for their anti-inflammatory and immune-regulatory functions such as phagocytosis of dying cells. They express TLR-8, TLR-1, Arg-1, CD163, and CD206. M2d macrophages, activated by TLR ligands, are involved in immune modulation and express IL-10R and IL-12R. Adapted from “M2 Macrophage Subtypes”, by BioRender.com (2019). Retrieved from https://app.biorender.com/biorender-templates (accessed on 23 September 2023).
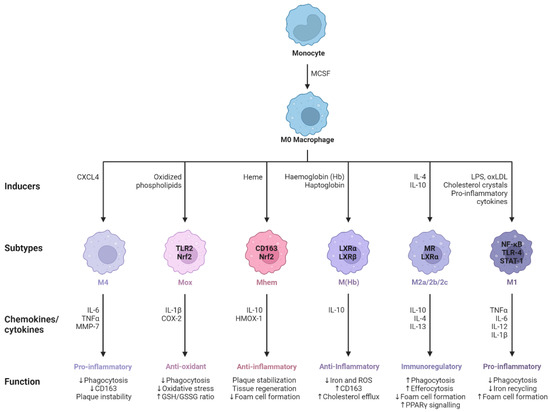
Figure 3. M0 macrophages are the precursors that give rise to specialized subtypes found in atherosclerotic lesions. Monocytes are differentiated to M0 macrophages via monocyte colony stimulating factor (MCSF). Depending on the tissue microenvironment, the M0 macrophage is polarized to a particular subtype. The primary subtypes are pro-inflammatory M1 and anti-inflammatory M2 macrophages with immunoregulatory properties. In addition, multiple other subtypes have been identified including M(Hb), Mhem, Mox, and M4. All of these subtypes produce various chemokines and cytokines and have different functions within the atherosclerotic plaque. Arrows pointing upwards indicate an increase, whereas arrows pointing downwards indicate a decrease. Adapted from “Macrophage Subtypes in Atherosclerosis”, by BioRender.com (2014). Retrieved from https://app.biorender.com/biorender-templates (accessed on 23 September 2023).
Polarization of Macrophages during Viral Infection
One of the hallmark events of macrophages during viral infections is their polarization switch during the immune response. This polarization could be triggered by TLR4 or IL-1R ligand activation, IFN-γ binding to its receptor (IFN-γR), interaction of Notch proteins with Delta-like and Jagged ligands, and IL-4 or IL-13 binding to its corresponding receptor [30]. Depending on the virus, the stage of infection, and even the infected person’s gender, macrophages adopt different inflammatory phenotypes: either M1, M2, or a biphasic identity beginning with M1 during the acute phase of infection and then changing to M2 during the chronic phase. Depending on the virus, macrophage polarization plays a variety of roles during viral infection. For example, HIV-1-induced polarization has been shown to influence macrophage susceptibility to infection and replication [31]. The polarization involves the conversion of macrophages into M1 and M2a types through a cytokine-dependent mechanism. Also, studies on the Epstein–Barr virus have shown that M1 macrophage polarization persists even in asymptomatic patients, despite the presence of anti-inflammatory cytokines like IL-10 and TGF-β [32]. Moreover, Japanese encephalitis virus and dengue virus can cause microglia (macrophages from CNS) and infiltrated macrophages to undergo M1 polarization and related proinflammatory activation in mice, and it has been proposed that targeting the occurrence of type 1 immunity may alleviate the pathologically lethal effect of viral encephalitis [33]. Furthermore, during COVID-19, both classically polarized macrophages (M1) and alternatively polarized macrophages (M2) inhibit SARS-CoV-2 infection. However, upon viral infection, M1 and non-activated (M0) macrophages, but not M2 macrophages, significantly up-regulate inflammatory factors [34]. This up-regulation of inflammatory factors is undoubtedly a significant contributor to the inflammation observed in COVID-19 [35]. Additionally, recent studies on the street rabies virus (RABV) have demonstrated its capability to affect macrophage polarization, shifting the macrophages toward an M2-c phenotype. Remarkably, the authors also discovered that a RABV glycoprotein can activate the α7-nAChR in monocyte-derived macrophages (MDMs), thus triggering the cholinergic anti-inflammatory response (CAR) [36]. These collective findings suggest that the RABV can induce an anti-inflammatory phenotype in human macrophages, potentially impacting the functioning of T cells [36].
It is not always the virus itself that polarizes macrophages, but rather viral proteins or cytokines released during infection. This is the case with HIV, where, in addition to the polarizing activity of granulocyte-macrophage colony-stimulating factor (M1) and macrophage colony-stimulating factor (M2) [41,42], the viral protein, Nef, polarizes macrophages to an M1-like phenotype [43]. Similarly, hepatitis C virus core protein engagement with Toll-like receptor 2 of macrophages inhibits M2a, M2b, and M2c macrophage polarization [44]. Furthermore, in the case of a respiratory syncytial virus (RSV) infection, the polarization of alveolar macrophages occurs as a result of cytokines (IFN and GM-CSF), intercellular communication via the Notch–Jagged pathway, and RSV’s direct activation signal [45]. Interestingly, the soluble spike protein of SARS-CoV-2 has recently gained prominence as a potential culprit in the deregulation of macrophage polarization via the α7-nAChR in COVID-19 [46]. The latter is crucial because the activation of this cholinergic ion channel in macrophages plays a significant role in reducing the production of proinflammatory cytokines in these cells [9]. The well-known CAR is responsible for achieving this effect, emphasizing the significance of this nAChR in suppressing inflammation during disease states.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242115732
This entry is offline, you can click here to edit this entry!
