Suprachoroidal (SC) injections offer a potential therapeutic approach for diseases involving photoreceptor loss. These injections involve delivering therapeutic agents or cells into the space between the choroid and the sclera, known as the suprachoroidal space (SCS). This targeted delivery allows for direct access to the choroid, retinal pigment epithelium (RPE), and photoreceptor cells. Many retinal diseases such as age-related macular degeneration and retinitis pigmentosa can have shown positive responses to suprachoroidal treatments in multiple studies. Notably, suprachoroidal injections offer a novel route of administration for treatments such as gene therapy and stem cell treatments.
- Suprachoroidal delivery
- Gene therapy
- Retinal diseases
- Visual improvement
- Mesenchymal stem cells
- Choroidal thickening
- Ophthalmology
1. Suprachoroidal Injection of AAV Vectors for the Treatment of Inherited and Acquired Retinal Disorders
Suprachoroidal (SC) delivery of adeno-associated vectors (AAV) holds promise for treating inherited and acquired retinal diseases [1][2].For example, Peden et al. (2011) demonstrated successful SC delivery of AAV5 using a microcatheter in rabbits, resulting in effective gene transfer and tolerability [1]. Martorana et al. (2012) compared AAV2 and AAV5 for SC and subretinal transduction in rabbits, with AAV2 showing the strongest GFP expression [3]. Woodard et al. (2016) evaluated various routes of AAV2 administration in mice and found that SC injections resulted in transduction across multiple retinal layers without inducing temporary retinal detachment [2].
These studies highlight the feasibility and potential advantages of SC delivery for AAV vectors, offering a less invasive alternative to conventional approaches in retinal gene therapy.
Recent studies have also investigated the efficacy of SC AAV delivery using a hypodermic needle and free-hand method [4][5]. Ding et al. (2020) injected a GFP-reporter gene with RGX-314, an AAV8 vector, into the SCS in animal models. SC injection resulted in robust fluorescence and GFP expression in the RPE, outer retina, and remote quadrants, while subretinal injection showed limited staining. AAV8 and AAV9 displayed strong GFP expression, while AAV2 exhibited limited fluorescence [4]. In another study by Ding et al. (2020), AAV2tYF-CBA and AAV2tYF-GRK1 demonstrated efficient and widespread transduction of retinal cells, particularly photoreceptors, via the SC route [6]. Yiu et al. (2020) compared SC, subretinal, and IV gene delivery in non-human primates, with SC injection resulting in widespread transgene expression in the RPE [5].
These studies suggest that SC delivery of AAV vectors offers advantages such as widespread transduction and reduced retinal complications, although transduction efficiency may vary among serotypes [5][7].
AAV-associated inflammation has emerged as a concern in gene therapy [8][9][10]. Yiu et al. (year) observed transient expression following SC AAV8 delivery, attributed to cellular damage and local inflammatory cells. Subretinal and IV delivery showed lower chorioretinitis, but IV administration elicited a stronger systemic immune response [8]. Ching et al. (2021) found that SC delivery induced a lower systemic immune response but higher intraocular pressure compared to subretinal delivery [9]. Wiley et al. (2023) reported varying levels of inflammation with different AAV vectors, with AAV2 and AAV6 consistently inducing higher inflammation levels [10].
While SC delivery holds promise for transducing multiple retinal layers, challenges remain in targeting specific regions and managing immune responses [11]. Optimization strategies such as catheter-based delivery or pushing formulations may improve SC targeting. Local inflammation associated with SC gene administration requires further investigation, as does the exploration of advanced AAV technologies for SC delivery [11].
2. Suprachoroidal Injection of DNPs for the Treatment of Inherited and Acquired Retinal Disorders
SC injection of DNA nanoparticles (DNPs) is an emerging method for ocular gene therapy [6]. Kansara et al. (2019) performed SC injection of ellipsoid-shaped and rod-shaped DNPs in non-human primates and rabbits, observing luciferase activity in the retina, choroid, and RPE. Ellipsoid-shaped DNPs exhibited persistent activity, while rod-shaped DNPs declined over time [5].
In a follow-up study, Kansara et al. (2020) compared SC and subretinal injections of DNPs in rabbits. SC administration using microneedles resulted in effective transfection and wider coverage of the peripheral retina, with minimal toxicity. Subretinal injections, however, showed ocular toxicity [10].
These studies demonstrate the successful transfection of chorioretinal cells using SC-delivered DNPs, highlighting the potential of nonviral-based gene delivery in the chorioretina [5][10].
Poly (β-amino ester)s nanoparticles (PBAE NPs) have been investigated for SC gene delivery [11]. Shen et al. (year) performed SC injections of PBAE NPs in rats, resulting in widespread GFP expression throughout the retina, although less intense in the RPE and photoreceptors compared to AAV8 injections. Lateral and radial NP penetration was attributed to transient pressure increase in the SCS space. SC injection of PBAE NPs with a VEGF expression plasmid led to subretinal neovascularization, while PBAE NPs with a VEGF-binding protein suppressed vascular leakage and neovascularization, demonstrating therapeutic potential [11].
SC injection of nanoparticles shows promise for treating retinal diseases, providing a nonviral-based and repeatable gene therapy option. Nanoparticles can transfer large genes and offer potential for addressing inherited retinal disorders [7]. However, they may exhibit variable gene expression intensity and neovascularization risks. AAVs, on the other hand, may induce immune responses due to pre-existing antibodies. Continued research may establish SC injection of nanoparticles as a valuable therapeutic approach for genetic retinal diseases [11].
3. Suprachoroidal Injection for the Treatment Dry-Aged Macular Degeneration and Stargardt’s Macular Dystrophy
Ongoing research investigates the use of non-retinal-derived mesenchymal stem cells (MSCs) for degenerative retinal diseases, providing trophic support to damaged retinal cells [12]. Recent studies have explored SC delivery of MSCs for dry AMD and SMD. Kahraman et al. (2021) conducted a prospective study with eight patients, showing improvements in visual acuity, visual field, and mfERG after SC implantation of adipose tissue-derived MSCs. No serious complications were reported, and improvements persisted throughout the 1-year follow-up period, accompanied by choroidal thickening [13]. Further research should focus on larger patient cohorts at earlier stages to refine treatment timing, graft replacement, and delivery methods. Nevertheless, these findings offer promising evidence for effective treatment of degenerative retinal diseases.
4. Suprachoroidal Injection for the Treatment of Retinitis Pigmentosa
Retinitis pigmentosa (RP), a group of inherited retinal disorders, leads to progressive photoreceptor loss and visual impairment. Developing effective treatments for RP is challenging due to a wide range of genetic mutations. Figure 1 illustrates potential therapeutic strategies in RP management. SC injection has been proposed as a delivery method for gene therapy in RP, and this section extends the discussion to cell therapy using umbilical cord-derived mesenchymal stem cells (UCMSCs) [14].
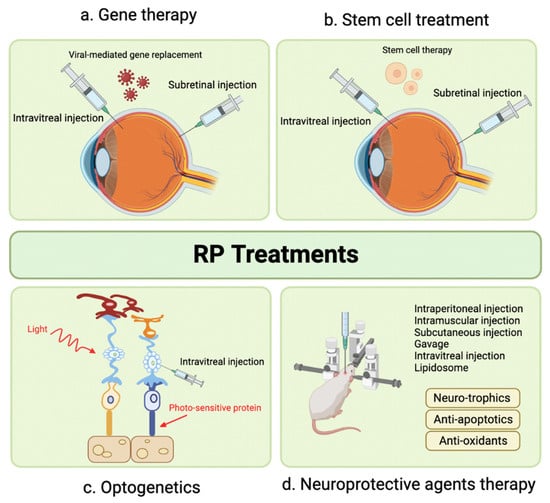
Figure 1. Emerging Therapeutic Modalities for Retinitis Pigmentosa.
Oner et al. conducted two studies evaluating UCMSC implantation in RP patients. In the first study, significant improvements were observed in mean BCVA, visual field scores, disease score, and grade over 12 months [14]. The second study focused on pediatric RP patients and demonstrated significant enhancements in BCVA, visual field examination, and mfERG measurements in all 46 eyes, with no reported complications [15]. Evaluation of SC mesenchymal spheroidal stem cell implantation is still ongoing, and results are pending [16]. Further research is necessary to fully understand the potential benefits and limitations of this treatment approach for RP.
5. Suprachoroidal Injection for Solar Retinopathy
A case report by Marashi et al. (2021) described a 17-year-old female with solar retinopathy and a sudden scotoma. The patient underwent a single SCTA injection (4.0 mg/0.1 mL) using a custom-made needle. Within 1 week, her BCVA improved from 0.1 to 1.0, and the scotoma disappeared. Mild elevation in IOP to 28 mmHg at 7 weeks resolved with topical anti-glaucoma agents. After 4 weeks, BCVA fully recovered, and OCT showed anatomical improvement in the ellipsoid zone layer. No serious adverse events (AEs) occurred [17]. This case suggests SCTA as a promising therapeutic option for solar retinopathy, leading to significant visual acuity improvements and anatomical changes without serious AEs. However, further research in larger patient cohorts is necessary to establish efficacy, safety, and long-term outcomes.
References
- Peden, M.C.; Min, J.; Meyers, C.; Lukowski, Z.; Li, Q.; Boye, S.L.; Levine, M.; Hauswirth, W.W.; Ratnakaram, R.; Dawson, W.; et al. Ab-Externo AAV-Mediated Gene Delivery to the Suprachoroidal Space Using a 250 Micron Flexible Microcatheter. PLoS ONE 2011, 6, e17140.
- Woodard, K.T.; Vance, M.; Gilger, B.; Samulski, R.J.; Hirsch, M. 544. Comparison of AAV Serotype2 Transduction by Various Delivery Routes to the Mouse Eye. Mol. Ther. 2016, 24, S217–S218.
- Martorana, G.; Levine, M.; Peden, M.; Boye, S.; Lukowski, Z.; Min, J.; Meyers, C.; Boye, S.; Sherwood, M. Comparison of Suprachoroidal Delivery via an Ab-Externo Approach with the ITrack Microcatheter versus Vitrectomy and Subretinal Delivery for 3 Different AAV Serotypes for Gene Transfer to the Retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1931.
- Ding, K.; Shen, J.; Hafiz, Z.; Hackett, S.F.; Silva, R.L.E.; Khan, M.; Lorenc, V.E.; Chen, D.; Chadha, R.; Zhang, M.; et al. AAV8-Vectored Suprachoroidal Gene Transfer Produces Widespread Ocular Transgene Expression. J. Clin. Investig. 2019, 129, 4901–4911.
- Kansara, V.; Yoo, J.; Cooper, M.J.; Laird, O.S.; Taraborelli, D.; Moen, R.; Noronha, G. Suprachoroidally Delivered Non-Viral DNA Nanoparticles Transfect Chorioretinal Cells in Non-Human Primates and Rabbits. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2909.
- Ding, K.; Shen, J.; Hackett, S.; Campochiaro, P.A. Transgene Expression in RPE and Retina after Suprachoroidal Delivery of AAV Vectors. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4490.
- Kansara, V.; Muya, L.; Wan, C.-R.; Ciulla, T.A. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392.
- Yiu, G.; Chung, S.H.; Mollhoff, I.N.; Nguyen, U.T.; Thomasy, S.M.; Yoo, J.; Taraborelli, D.; Noronha, G. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol. Ther. Methods Clin. Dev. 2020, 16, 179–191.
- Chung, S.H.; Mollhoff, I.N.; Mishra, A.; Sin, T.-N.; Ngo, T.; Ciulla, T.; Sieving, P.; Thomasy, S.M.; Yiu, G. Host Immune Responses after Suprachoroidal Delivery of AAV8 in Nonhuman Primate Eyes. Hum. Gene Ther. 2021, 32, 682–693.
- Kansara, V.S.; Cooper, M.; Sesenoglu-Laird, O.; Muya, L.; Moen, R.; Ciulla, T.A. Suprachoroidally Delivered DNA Nanoparticles Transfect Retina and Retinal Pigment Epithelium/Choroid in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 21.
- Shen, J.; Kim, J.; Tzeng, S.Y.; Ding, K.; Hafiz, Z.; Long, D.; Wang, J.; Green, J.J.; Campochiaro, P.A. Suprachoroidal Gene Transfer with Nonviral Nanoparticles. Sci. Adv. 2020, 6, eaba1606.
- Lin, Y.; Ren, X.; Chen, Y.; Chen, D. Interaction Between Mesenchymal Stem Cells and Retinal Degenerative Microenvironment. Front. Neurosci. 2021, 14, 617377.
- Habot-Wilner, Z.; Noronha, G.; Wykoff, C.C. Suprachoroidally Injected Pharmacological Agents for the Treatment of Chorio-Retinal Diseases: A Targeted Approach. Acta Ophthalmol. 2019, 97, 460–472.
- Öner, A.; Kahraman, N.S. Does Stem Cell Implantation Have an Effect on Severity of Retinitis Pigmentosa: Evaluation with a Classification System? Open J. Ophthalmol. 2021, 11, 36–48.
- Oner, A.; Kahraman, N.S. Suprachoroidal Umbilical Cord Derived Mesenchymal Stem Cell Implantation for the Treatment of Retinitis Pigmentosa in Pediatric Patients. Am. J. Stem Cell Res. 2023, 5, 1–7.
- Özkan, B. Suprachoroidal Spheroidal Mesenchymal Stem Cell Implantation in Retinitis Pigmentosa: Clinical Results of 6 Months Follow-Up. 2023. Available online: clinicaltrials.gov (accessed on 6 July 2023).
- Marashi, A.; Baba, M.; Zazo, A. Managing Solar Retinopathy with Suprachoroidal Triamcinolone Acetonide Injection in a Young Girl: A Case Report. J. Med. Case Rep. 2021, 15, 577.
