Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Breast cancer (BC) is the most diagnosed cancer worldwide, mainly affecting the epithelial cells from the mammary glands. When it expresses the estrogen receptor (ER), the tumor is called luminal BC, which is eligible for endocrine therapy with hormone signaling blockade. Natural products are being continuously screened for treating cancer due to their chemical diversity, reduced toxicity, lower side effects, and low price.
- breast cancer
- hormone therapy
- natural products
1. Introduction
Epidemiological data on cancer are alarming. According to the World Health Organization (WHO), in 2020, more than 19 million new cases of the disease and approximately 10 million associated deaths were recorded worldwide. In 2023, 1,958,310 new cancer cases and 609,820 cancer deaths are expected in the United States. Breast cancer (BC) has the highest incidence rate of all cancers, accounting for 2.3 million diagnoses in 2020. Most of the new cases and disease-related mortality from BC occur in low- and middle-income countries. In high-income countries, the chance of survival exceeds 80%, in contrast to developing countries where the diagnosis still occurs late [1,2]. In addition, prognostic factors such as tumor size, grade, lymph node involvement, and estrogen receptor (ER) expression are essential in choosing the therapeutic strategy. In fact, BC is classified molecularly according to the expression of human epidermal growth factor receptor 2 (HER2), progesterone receptor, and ER [3].
ER-positive tumors are defined as luminal, account for about two-thirds of cases, and show an intrinsic heterogeneity from the histological, transcriptional, and mutational points of view, with different clinical courses and therapeutic strategies [4]. Although patients with luminal BC have a better prognosis, in 30% of these cases there is late recurrence of the disease (after 5 to 10 years), mainly at a distance, with a predominance of bone metastases. This scenario directly impacts the data on the overall survival, and the risk of recurrence for patients with luminal BC is real. The time and characteristics of this progression are affected not only by the prognosis, but also by the adjuvant therapeutic strategies adopted [5].
For ER-positive tumors, surgery, radiotherapy, chemotherapy, targeted therapy, and hormone therapy are recommended as established methods. However, different questions are still raised in clinical practice: Which patients really benefit from chemotherapy? How to reverse endocrine resistance? What are the challenges in the development of new drugs for the treatment of luminal BC? In this context, natural compounds have shown to be potentially promising.
Plants produce secondary metabolites in responses to stress, damage, and infections caused by pathogens. Interestingly, these compounds are responsible for around 25% of drugs currently marketed, with examples included in cancer treatment [6,7,8,9]. Recently, the ability of some secondary plant metabolites to modulate estrogen signaling and hallmarks of cancer, such as proliferation and apoptosis, was discovered, which makes them applicable to the treatment of luminal BC, especially when resistant to endocrine therapy [10,11].
2. Natural Compounds and Their Effects on Luminal Tumors
Plants are considered to be important sources of substances for the treatment of cancer, being effective, safe, and with structures subject to modification. Natural products have antioxidant, growth-inhibiting, apoptosis-inducing, and invasion- and metastasis-control activities [204,205,206]. Despite advances in scientific studies focused on this area, it is estimated that only 15% of existing plant species have already been investigated for their pharmacological potential [207]. Therefore, the need to develop effective natural therapeutic agents is recognized, especially in the face of therapeutic resistance.
Natural products have been used in adjuvant therapy and proven to be versatile and capable of modulating hormonal signaling, interfering with the cell cycle, proliferation, invasion, metastasis, and angiogenesis [208]. Phytoestrogens, for example, are natural compounds derived from plants and are analogous to estrogens in structure and function [11,209]. In addition, other natural compounds have been shown to be active in luminal tumors, capable of reversing cases of resistance to hormone therapy [210,211,212]. The richness of the therapeutic potential of plants is due to the presence of active phytochemicals.
2.1. Flavonoids
Flavonoids are polyphenolic secondary metabolites that have been evaluated for the treatment of BC due to their antitumor potential through epigenetic changes, expression of tumor-suppressor genes, and activation of pro-apoptotic pathways. Interestingly, flavonoids can modulate ERs, especially hesperidin, hesperetin, luteolin, and apigenin [251,252,253] (Figure 1).
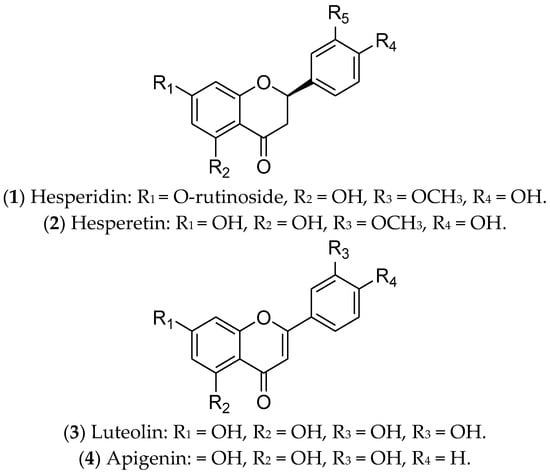
Figure 1. Flavonoids with effects on luminal breast cancer.
Hesperidin (1) is a glycosylated flavanone compound composed of hesperetin (2) (main part) and a disaccharide called rutinose (a type of glucose-linked rhamnose) [213]. Hesperidin and its derivatives are found in citrus fruits from the Rutaceae family, such as oranges, tangerines, limes, lemons, and grapefruit [254]. They have antimitotic, pro-apoptotic, antimetabolic, and antimetastatic potential, due to their anti-inflammatory and antioxidant properties [213,253]. These compounds, separately or in combination, have been used in the treatment of luminal BC cells (MCF 7 and T-47D) and have been responsible for downregulation of ER1 expression. Therefore, hesperidin and its derivatives can modulate estrogenic signaling [213,255,256]. In vivo assays using an MCF 7 xenograft model demonstrated that hesperidin inhibits tumor growth and metastasis, mainly through overexpressing estrogen synthase-aromatase [257]. To date, there are no reports of clinical trials with these compounds. Despite their relevant biological functions, both hesperidin and hesperetin have poor water solubility and limited bioavailability. For this reason, several studies have been focused on creating nanoformulations to increase their bioavailability [257].
Luteolin (3) is a natural flavonoid that regulates cancer-related signaling pathways. It is commonly found in carrots, broccoli, sweet peppers, celery, parsley, onion leaves, and chrysanthemum flowers [216]. Recent evidence has shown that luteolin promotes cell death by apoptosis, acting as an antioxidant and anticancer agent in different tumors, including BC [215,216]. In luminal BC cells, luteolin upregulates caspases 3, 8, and 9, as well as BAX (Bcl-2-associated X-protein—pro-apoptotic regulator) and miR-16. Also, this compound downregulates the expression of BCL-2 (an anti-apoptotic protein) and inhibits IGF-1 (insulin growth factor-1) activation by modulating the P13K-Akt signal transduction pathway [258]. Furthermore, the combination of luteolin with Taxol is synergistic and can increase the sensitivity of BC cells to the treatments adopted [259]. An interesting study conducted by Markaverich and collaborators demonstrated that the treatment of MCF 7 cells with luteolin modulated different genes of the estrogen pathway, such as GTF2H2 (general transcription factor IIH, polypeptide 2) (-), NCOR1 (nuclear receptor co-repressor 1) (-), TAF9 (+), NRAS (neuroblastoma viral RAS (v-ras) oncogene homolog) (-), NRIP1 (nuclear receptor interacting protein 1) (-), POLR2A (polymerase (RNA) II (DNA-directed) polypeptide A) (-), DDX5 (DEAD (Asp-Glu-Ala-Asp) box polypeptide 5) (-), and NCOA3 (nuclear receptor co-activator 3) (-) [260]. The joint action of luteolin and indole-3-carbinol effectively inhibited ER-positive BC. This treatment targeted two key therapeutic elements—ERα and the CDK 4/6/retinoblastoma (Rb) pathway—in cell lines and xenograft tumors [261]. Currently, there are no ongoing or completed clinical trials using luteolin in the treatment of BC patients.
Finally, apigenin (4) is a flavonoid that is found in species belonging to the Asteraceae family [262]. This compound inhibits cell growth and induces apoptosis of luminal BC cells through the regulation of caspases, cytochrome c release, the NF-κB, PI3K, and Akt/mTOR pathways [263,264], and poly-ADP ribose polymerase (PARP) cleavage [217,218]. In vivo, Yao and colleagues demonstrated that apigenin partially antagonizes ERs [265]. However, this compound is poorly soluble in water, and nanotechnology has contributed to advances related to its therapeutic applications [266]. Although promising for the treatment of luminal BC, there are no reports of completed or ongoing clinical trials.
2.2. Isoflavonoids
Isoflavonoids, or isoflavones, are phytoestrogens that have demonstrated greater affinity for ERβ [267,268]. The ingestion of isoflavones either in childhood or in puberty seems to contribute to the prevention of BC [268]. Regarding treatment of luminal BC, the isoflavones daidzein, genistein, glycitein, biochanin A, formononetin, glabridin, glabrene, puerarin, calycosin, and equol have already shown promising effects (Figure 2).
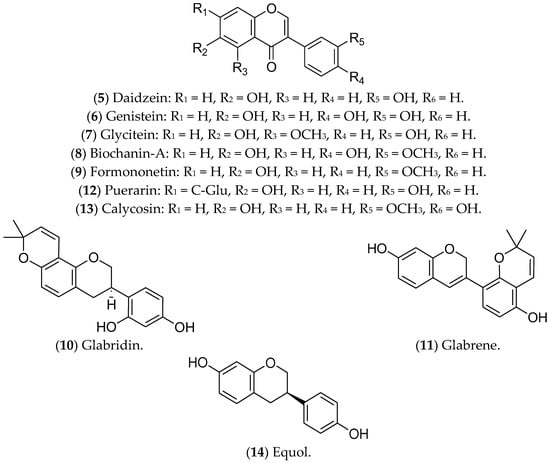
Figure 2. Isoflavonoids with effects on luminal breast cancer.
Daidzein (5) is an isoflavone present in soybeans that shares similarities with human estrogens, acting in a dual way: replacing or blocking the action of the hormones in ERs [269,270,271]. In luminal BC cells, such as MCF 7 cells, daidzein inhibits the NF-kB pathway, CYP1, and topoisomerase, leading to cell-cycle arrest and apoptosis [272]. A phase I multiple-dose clinical investigation indicated that this compound was safe in healthy postmenopausal women (ClinicalTrials.gov Identifier: NCT00491595) [273].
The isoflavone genistein (6) is a phytoestrogen found in soy and its derivatives [274] that inhibits ER-positive BC tumors, suppressing MAPK and DNA polymerase II, reducing cell proliferation, and triggering apoptosis [270,275]. This compound also increases ERβ and decreases ERα expression at the transcriptional and protein levels [276,277]. Genistein has been recognized as one of the most biologically active and potent isoflavones for cancer prevention [270,275]. However, it is noteworthy that studies in animal models found that genistein (6) and daidzein (5), even at lower concentrations, promote the development of BC, highlighting the need for further experiments focused on the characterization of its metabolites and their effects on breast tumors. Moreover, in in vitro tests, these substances counteracted the antitumor effects of tamoxifen [278,279].
The compounds glycitein (7), biochanin A (8), formononetin (9), glabridin (10), and glabrene (11) are phytoestrogens that are capable of positively regulating BCL-2 and modulating important oncogenic pathways such as IGF-1/IGF-1R, MAPK, and P13K/Akt [227]. Glycitein (7), an O-methylated isoflavone that is present in soybean foods, can decrease the glucose uptake of MCF 7 cells and modulate the metabolic status of ERα-positive cells [225]. The results available in the literature about glycitein are limited to in vitro assays. Biochanin A (BCA) (8) is an isoflavonoid that is present in large quantities in chickpeas, soybeans, red clover, and other herbs [280]. BCA can reduce migration/invasion and activate pro-inflammatory pathways of MCF 7 cells through ROS production and inhibition of the ERK-1/2 pathway [281,282]. In addition, BCA has a preventive effect against BC, whether administered alone or combined with other flavonoids [283]. In vivo assays demonstrated that BCA has a synergistic effect with 5-fluorouracil, reducing tumor size, mainly associated with the ER-α/Akt axis [284].
Formononetin (9), in turn, is an active component extracted from the traditional Chinese medicinal herb Astragalus membranaceus that can reverse resistance to chemotherapy. This compound regulates the expression of CXCL12, ESR1, and IGF1, modulates the Akt and mTOR pathways, and increases the sensitivity of Taxol-resistant BC cells [285,286]. In nude xenograft mice, the treatment with formononetin controlled the growth of BC [287]. Preclinical assays have also demonstrated the ability of this compound to inhibit angiogenesis through modulating the Akt pathway and downregulating the effect of basic fibroblast growth factor 2 (FGF2). There are no ongoing clinical trials [288,289].
Glabridin (10), a flavonoid from the root of Glycyrrhiza glabra, traditionally called licorice, has antiproliferative activity in MCF 7 cells [290], associated with oxidative stress, mitochondrial dysfunction [291], and modulation of EGFR expression [292]. Similar to glycitein (7), this flavonoid modulates ERs, with a proliferative effect at lower concentrations and an antiproliferative effect at higher concentrations [293]. In an animal model of TNBC, glabridin combined with a low concentration of paclitaxel significantly reduced the tumor burden and the formation of lung metastases [294]. Glabrene (11), also present in licorice, has a higher affinity for ERs and a dual effect on BC cells; at lower concentrations (10 nM–10 µM), it promotes ER-dependent growth, while at higher concentrations (>15 µM) it shows ER-independent antiproliferative activity [293]. In vivo studies have demonstrated that glabridin is similar to ETD, but both glabridin and glabrene have limitations for the treatment of luminal BC, especially given the lack of validation of their therapeutic effects [295].
Puerarin (12), a natural isoflavone from Pueraria lobata (a plant from China and Japan known as the “kudzu vine”), has therapeutic potential for luminal BC cells, inhibiting cell migration, adhesion, and invasion, and triggering apoptosis through modulation of non-coding RNAs (ncRNAs) [296]. Furthermore, treatment with puerarin reduces multidrug resistance in Adriamycin-resistant MCF 7 cells [297]. In particular, the action of puerarin in the estrogenic pathway in luminal BC has not yet been described, but in vivo studies indicate that this compound increases the expression of ER-α in cardiac tissues in ovariectomized animals [298]. A previous study investigating the anti-osteoporotic action of puerarin showed that this compound weakly binds to ERs [299]. However, as the results with puerarin in BC are limited to a few in vitro experiments, its clinical application is still incipient.
Calycosin (13), derived from Astragali root, exhibits antiproliferative and antimetastatic activities in the luminal BC cell lines T-47D and MCF 7. The mechanisms of action include suppression of basic leucine zipper transcription factor ATF-like (BATF) and modulation of the expression of E-cadherin, N-cadherin, vimentin, CD147, matrix metallopeptidase (MMP)-2, MMP-9, and the long non-coding RNA (lncRNA) WDR7-7 [300,301]. Furthermore, in MCF 7 cells, puerarin (12) and calycosin (13) activate caspase-3 [228,229]. Regarding the estrogenic pathway, a significant increase in the expression of ERβ was observed in MCF 7 cells after treatment with calycosin. This effect was associated with a reduction in IGF-1R, the activation of PARP-1, and the downregulation of miR-375. In that same study, the researchers noted that calycosin is more promising than formononetin [302]. Again, there are no ongoing or completed clinical trials with calycosin.
Finally, equol (14), also found in soybeans, has been associated with increased effectiveness of tamoxifen, which suggests a possible combined treatment for luminal BC. The ability of equol to bind with high affinity to ERβ has also been reported, resulting in the inhibition of cell proliferation and induction of apoptosis [248]. However, this activity is under investigation due to the possible controversial activity of equol [303,304,305]. In vitro studies have shown that the translation factor eIF4G is upregulated in cells that are treated with equol, resulting in increased translation of pro-oncogenic mRNAs, for example, the transcription factor c-Myc, which can consequently increase the viability of metastatic cells [306].
2.3. Alkaloids and Catechins
Alkaloids are a class of natural substances that have received considerable interest due to their therapeutic potential, including anti-inflammatory, antiviral, and antimicrobial activities. In cancer cells, they can trigger apoptosis and autophagy, reduce tumor size, inhibit cell proliferation, and can be used in combined therapeutic approaches [307,308]. Among the alkaloids, piperine is commonly cited for its antitumor activities in luminal BC.
Piperine (15) (Figure 3), an alkaloid obtained from black pepper (Piper longum), is an active compound in luminal BC cells [309]. It inhibits the Wnt/β-catenin, Hedgehog, and Notch pathways, which are involved in cancer stem cells’ self-renewal. In addition, piperine induces apoptosis and regulates the expression of proteins such as EGFR, VEGF, CDK, and NF-kβ [310]. Nanoparticles and liposomes have been used for piperine formulations with enhanced effectiveness, including reversion of multidrug resistance and sensitivity to paclitaxel and tamoxifen [310,311]. The mechanism of action by which piperine regulates the estrogen pathway remains unclear, given the lack of in vitro and in vivo assays focused on hormonal signaling.
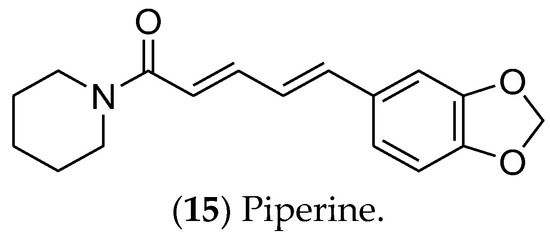
Figure 3. The alkaloid piperine, which has effects on luminal breast cancer.
In turn, catechins are polyphenolic phytonutrients that are found in green tea (Camellia sinensis). Among the catechins, epigallocatechin (16) [312,313] (Figure 4) is cytotoxic and selective for MCF 7 cells, regulating the EGFR, STAT3, ERK, ERK1/2, NF-κB, and Akt pathways [314,315,316,317]. Moreover, this compound is currently in phase I clinical trials for the prevention and treatment of radiodermatitis in patients with BC (ClinicalTrials.gov identifier: NCT01481818) [318].
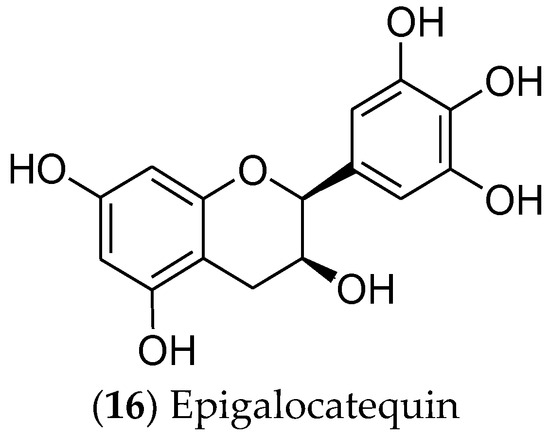
Figure 4. The catechin epigalocatequin, which has effects on luminal breast cancer.
2.4. Lignans
Lignans are phytoestrogens that are absorbed from plant sources and are associated with lower risks of postmenopausal BC, with promised effects against luminal BC [319]. Their main mechanism of action includes inhibition of NF-κB [320,321], with special attention devoted to pinoresinol, arctigenin, enterolactone, enterodiol, matairesinol, sesamin, and secoisolariciresinol (Figure 5).
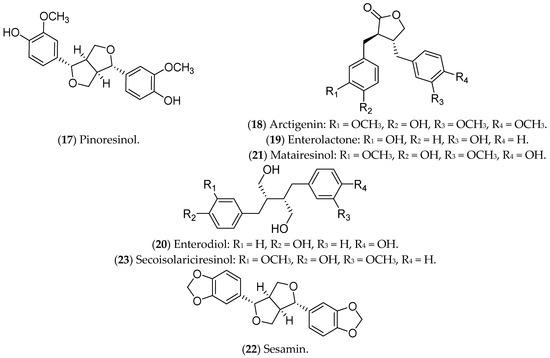
Figure 5. Lignans with effects on luminal breast cancer.
Pinoresinol (17), a lignan commonly found in olives, binds to ERs, being selective and cytotoxic to luminal BC cells, with a pro-oxidant action [232]. Furthermore, pinoresinol selectively inhibits cell proliferation, induces apoptosis, blocks HER2 receptors, and increases ROS production [232,322]. Regarding the estrogenic pathway, it was previously demonstrated that pinoresinol apparently increases the viability of MCF 7 cells, with an ERα agonist action [323]. Another study, also conducted in vitro, demonstrated that the antiproliferative and cytotoxic action of pinoresinol is independent of the estrogen pathway when low concentrations of the compound are used. Therefore, pinoresinol’s activity in luminal BC is poorly understood and limited to in vitro assays [232].
Arctigenin (18), a bioactive compound from Arctium lappa L., also binds to ERs and reduces pro-tumor signals, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), MMP-3, MMP-9, and thymic stromal lymphopoietin (TSLP). In addition, it inhibits the proliferation and invasion of luminal BC cells [233,324,325,326].
Some lignans are converted in the intestine into estrogenic enterolignans such as enterolactone (19) and enterodiol (20). Although both act through ERs, the mechanisms are different. Enterodiol binds ERα via the N-terminal activation domains 1 (AF-1) and 2 (AF-2) of the receptor, like ETD. Enterolactone, in turn, acts mainly via AF-2. Both compounds, however, affect the proliferation of MCF 7 cells [327]. Furthermore, enterolactone, in the presence of ETD, reduces the proliferation of MCF 7 cells, possibly modulating the hormonal effects [328]. Mali et al. (2012) showed that enterolactone downregulates the expression of MMP2, MMP9, and MMP14 and inhibits the adhesion, invasion, and migration of MCF 7 cells [329]. Along with enterolactone, enterodiol has also been evaluated for its antitumor activity against the proliferation of MCF 7 cells [330]. In addition, in vitro (MCF 7 lineage) and in vivo treatments with enterodiol and enterolactone inhibited hormone-induced tumor growth, even controlling the production of VEGF and angiogenesis [331]. This information highlights the potential of these compounds in the prevention and treatment of luminal BC, including some clinical trials that have already been carried out [332].
Matairesinol (21), found in seeds, vegetables, and fruits, has antiangiogenic, antitumor, and antifungal activities [333]. Abarzua and collaborators (2012) demonstrated the antitumor potential of matairesinol in MCF 7 cells, with a significant reduction in cell viability. However, the effects of matairesinol did not surpass those of enterolactone (19) and enterodiol (20) [236].
Sesamin (22), a phytochemical identified in Sesamum indicum, is metabolized by the liver and induces G1 cell-cycle arrest in MCF 7 cells [334], regulates ER and programmed death-ligand 1 (PD-L1) expression, and inhibits growth factors and tyrosine kinase pathways [238,335,336]. In murine models, sesamin reduced the expression of HER2 and VEGF, and it inhibited the MAPK signaling pathway [238,336]. The above results were limited to in vitro and in vivo tests in animal models.
Also, secoisolariciresinol (23), a compound extracted from seeds of Linum usitatissimum, modulates inflammation through the NF-kB pathway in MCF 7 cells [239], and it also alters the expression of ER1, ER2, EGF, BCL2, and IGF1R [240]. Moreover, secoisolariciresinol and its derivatives can also induce apoptosis in MCF 7 cells and potentiate the action of chemotherapeutic agents such as doxorubicin, being considered safe and tolerable in phase IIB studies [337]. These data demonstrate the potential of this compound for the treatment of luminal BC.
2.5. Coumestans and Stilbenes
Coumestans are polycyclic aromatic compounds that have a heterocyclic structure with four oxygenated rings, including coumarin and benzofuran moieties, connected by a carbon–carbon double bond. They exhibit biological effects similar to those of phytoestrogens and polyphenols, showing in vitro anticancer potential. However, in vivo studies using these compounds are still limited [338,339,340,341]. Among the coumestans with antitumor activity, it highlight coumestrol and 4′-methoxycumestrol, which have previously shown activity against luminal BC (Figure 6). Coumestans are mainly produced during the germination of beans, clovers, Brussels sprouts, and soybeans. The amounts of coumestans in plants may vary depending on the variety, growth stage, presence of diseases, location, and use of fungicides and insecticides [342].
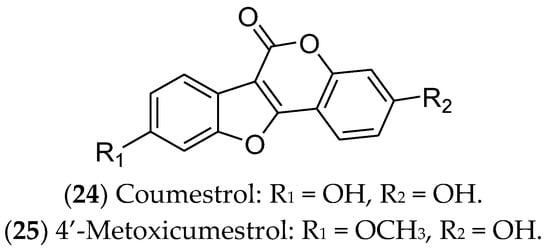
Figure 6. Coumestans with effects on luminal breast cancer.
Coumestrol (24) has already been identified in soybeans, clover, and spinach. It inhibits 17β-HSD enzymes and aromatase and binds to ERα and ERβ. In this sense, coumestrol regulates the hormone receptor pathways and expression, with anti-estrogenic activity 30–100 times greater than that of isoflavones. Therefore, this compound can be used as a complementary strategy in hormone therapy and chemotherapy for luminal BC [343,344,345,346]. In ER-positive cells, in addition to reducing cell viability, coumestrol significantly reduces the expression of genes that drive epithelial-to-mesenchymal transition (Snail), bone fixation (CXCR4 and integrin αV), and osteolysis (PTHrP and TNF-α) [344] Likewise, 4′-metoxicumestrol (25) is also cited as a phytoestrogen with an antiproliferative effect on luminal BC cells, downregulating Akt phosphorylation [242].
Stilbenes are natural substances isolated from vines, sorghum, pine, fir, and mulberry. These compounds have a core structure of 1,2-diphenylethylene and are used by plants as a defense against external threats, including pests, microorganisms, and the harmful effects of ultraviolet radiation [347]. Among the stilbenes, resveratrol (26) and pterostilbene (27) have been reported as active agents for luminal BC cells (Figure 7).

Figure 7. Stilbenes with effects on luminal breast cancer.
Resveratrol (26), also known as 3,5,4′-trihydroxy-trans-stilbene, is one of the most famous polyphenols and phytoestrogens, found mainly in grape skins, especially those of red grapes. Additionally, it can be found in blueberries, raspberries, cranberries, blackberries, peanuts, and cocoa powder [348]. This compound inhibits cell proliferation and reduces the migration and viability of BC cells [243,244]. Moreover, it exhibits synergistic effects when combined with chemotherapy agents such as doxorubicin, cisplatin, docetaxel, and paclitaxel [349]. Resveratrol triggers apoptosis in MCF 7 cells [350], arrests the cell cycle in the S phase [351], and causes DNA damage [352] and epigenetic alterations, such as in genomic methylation and miRNA expression [353]. Regarding the estrogen pathway, the compound is characterized as a weak agonist/antagonist of both ERs, being structurally similar to ETD [348,354]. Clinical trials indicated that resveratrol was safe and well tolerated, in addition to its action as a chemopreventive agent for BC patients [355].
Pterostilbene (27) is an analogue of resveratrol, found mainly in blueberries, and it also demonstrates antitumor activity against MCF 7 cells. Previous studies demonstrated that pterostilbene functions as an ERα inhibitor, while also inducing apoptosis [243,245,356]. Pterostilbene can induce apoptosis in mammary tumor cells by antagonizing ETD and specifically inhibiting ERα36 [357]. In addition, this stilbene can lead to an accumulation of neutral lipids in the intracellular environment, activating autophagy, reducing mitosis and metastasis [358], blocking the cell cycle, inducing morphological alterations and DNA degradation, increasing caspase-9 expression, and modulating the Akt/mTOR pathway [359,360].
2.6. Other Compounds
Other compounds such as thymoquinone, sulforaphane, and ginsenosides have been studied as potentially active in luminal BC (Figure 8). Thymoquinone (28), a monoterpene found in Nigella sativa, can induce apoptosis via p53 in MCF 7 cells [361]. In addition, this compound can modulate NF-κB levels, arrest the cell cycle in the S phase [361], and alter the expression of genes related to the estrogen pathway [362]. Isothiocyanate sulforaphane (29) is found in broccoli, especially broccoli sprouts, and in cruciferous vegetables such as cabbage, cauliflower, and kale [363]; it decreases ER1 expression [364]. This compound is found in cruciferous vegetables and regulates gene expression through epigenetics, inhibiting histone deacetylase (HDAC) [365]. Furthermore, sulforaphane inhibits cell proliferation, induces apoptosis, and arrests the cell cycle at the G2/M phase in MCF 7 cells [366]. Recently, a phase II clinical study found that the effect of a broccoli sprout preparation, in which sulforaphane is a key component, could increase the levels of protective enzymes in BC tissues (ClinicalTrials.gov Identifier: NCT00982319) [367].
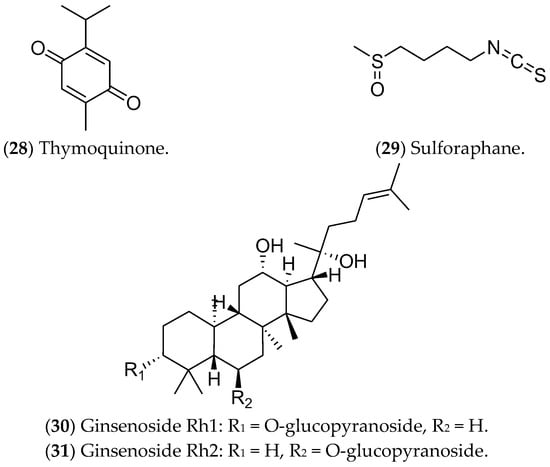
Figure 8. Other compounds with effects on luminal breast cancer.
Finally, ginsenosides (30, 31), present in ginseng root (Panax), have been considered as possible antitumor agents for triggering apoptosis [368]. Huynh et al. (2021) showed high cytotoxicity of these compounds in MCF 7 cells, as well as increases in apoptosis, autophagy, and cell-cycle arrest. Furthermore, the ROS production after the treatments inhibited the PI3K/Akt pathway [249,250,369]. Despite showing antitumor action during in vitro tests, the use of these compounds in clinical trials is limited by the scarcity of data related to their metabolic regulation and modulated pathways [370].
This entry is adapted from the peer-reviewed paper 10.3390/ph16101466
This entry is offline, you can click here to edit this entry!
