Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
To achieve optimal growth and productivity of poultry, ensuring their health and well-being is essential. Studies have illuminated the functional roles of certain amino acids, highlighting their unique contributions to various physiological processes and the synthesis of metabolically important molecules. Methionine and arginine are two notable examples of such amino acids.
- arginine
- methionine
- intestinal health
- functional amino acids
- poultry
1. Introduction
The global poultry industry holds immense significance in meeting the continuously increasing demand for high-quality protein-rich food, driven by the continuous rise in the global population [1]. To achieve optimal growth and productivity of the birds, ensuring their health and well-being is essential.
The intestines are responsible for the digestion and absorption of essential nutrients from the feed consumed by poultry [2]. Furthermore, the intestinal tract is immensely important in the immune system of the animals. It serves as the physical barrier and also hosts abundant organized lymphoid tissue and immune effector cells, which collectively provide protection against pathogens and toxins [3][4][5]. Nevertheless, the intestines possess the most extensive exposed surface in the body [6]. The constant exposure to a wide range of potentially harmful substances makes them susceptible to many diseases, such as coccidiosis and necrotic enteritis, which have a significant impact on the poultry industry [7][8]. Maintaining a healthy intestinal tract has been a focus of research for decades, and this area is now gaining more attention due to the growing awareness of animal welfare, food safety, and the increasing public scrutiny towards the use of antibiotic growth promoters [9].
2. Methionine and Arginine in Intestinal Health
2.1. Methionine and Arginine in Intestinal Development and Repair
The intestinal epithelium, which comprises a single layer of epithelial cells and a protein-rich mucus layer, has one of the highest turnover rates in the body [10]. The renewal of epithelial cells in the intestinal epithelium involves a highly coordinated process of cellular proliferation, differentiation, migration, and apoptosis. This constant cell turnover relies on ongoing protein synthesis, which is largely mediated through the activation of mTOR pathway [11]. In addition to serving as essential building blocks of proteins, Met and Arg have been shown to activate mTORC1 [12][13]. Met activates the mTOR signaling pathway by promoting the methylation of phosphatase 2A through the action of SAM. Furthermore, previous studies showed that SAM could bind to the S-adenosylmethionine sensor upstream of mTORC1 (SAMTOR), counteracting its inhibitory effects on mTORC1 activity [13][14]. On the other hand, Arg activates the mTOR pathway through inhibiting the activity of tuberous sclerosis complex 2, a protein that normally suppresses the mTOR pathway [15]. The mTOR pathway is essential for maintaining intestinal epithelial cell proliferation during both homeostasis and regeneration, as research showed that a disruption in this signaling pathway would lead to intestinal epithelial cell defects and hinder the intestinal regeneration [16][17].
The Wnt/β-catenin signaling pathway is also well established for its role in maintaining intestinal structure and homeostasis as it regulates the self-renewal and differentiation of the intestinal stem cells [18][19][20]. Interestingly, studies have also shown that both Met and Arg can activate the Wnt/β-catenin pathway to enhance the intestinal epithelial development [21][22]. Met is required for the sequestration of glycogen synthase kinase 3, which is an essential step in activating the Wnt signaling pathway [23]. The modulating effects of Arg on the Wnt/β-catenin signaling pathway can be attributed to the synthesis of NO, which is a known activator of this pathway [24]. Intriguingly, activation of the Wnt/β-catenin signaling pathway is closely regulated by the methylation of the Arg residues in Ras GTPase-activating protein-binding protein 1 (G3BP1) with SAM as the methyl donor. This linkage underscores the collaborative regulatory roles of Met and Arg in this pathway [23][25]. The activation roles of Met and Arg in both pathways contribute to the regenerative capacity and development of the intestine.
Beyond their roles in pathway activation, Met and Arg are indispensable for polyamine synthesis. Arg serves as the precursor for polyamine synthesis, while SAM, a product of Met metabolism, functions as the methyl donor in this process [26]. Polyamines are essential for intestinal epithelial renewal and repair, ensuring its important roles in cell proliferation, development, and migration [27][28]. Studies have demonstrated that providing dividing cells in the crypts with polyamines can stimulate mucosal growth and facilitate the repair of damaged mucosa [29][30][31]. The proposed mechanism underlying the stimulation effect of polyamines in mucosal growth involves their ability to regulate expression of various genes encoding growth promoting and inhibiting factors [26][32]. Polyamines are also shown to be vital for the expression of tight junction and adhesion junction proteins which maintain the intestinal barrier function [33][34].
Overall, Met and Arg are essential for the development and repair of the intestinal epithelium. They contribute to this process by activating pathways essential for intestinal regeneration and by participating in polyamine synthesis.
2.2. Antioxidant Effects of Methionine and Arginine on Intestinal Health
2.2.1. Oxidative Stress
The integrity of the intestinal barrier can be disrupted by various factors, among which oxidative stress is a significant contributor. Reactive oxygen species (ROS) are byproducts generated during normal metabolic processes [35][36]. Under normal conditions, the production of ROS is balanced by the antioxidant system [37]. However, an excessive production of ROS or a decline in antioxidant defenses can disrupt this equilibrium, leading to the accumulation of ROS. The highly reactive ROS will react with the cellular components causing cellular damage, dysfunction, and apoptosis, which ultimately leads to impaired organ functions and the development of oxidative stress [35]. Several factors during poultry production can cause oxidative stress to the birds, such as nutritional factors like nutrient imbalances and feed toxins, environmental factors like heat stress and stocking density stress, as well as pathological factors [38][39][40].
Oxidative stress can damage the structure and function of tight junctions, resulting in compromised intestinal barrier functions and increased permeability [41]. Oxidative stress is also known to damage the intestinal epithelial cells directly, leading to further disruption of the barrier function and inflammation. The recruited macrophage and heterophils intensify the production of ROS, stimulating a positive feedback loop that exacerbates oxidative stress and inflammation [42][43]. Oxidative stress is also reported to cause morphometric changes in the intestinal tract by reducing the villi height and lowering the villus: crypt ratio, interfering with nutrient absorption [44][45].
2.2.2. Antioxidant Effects of Methionine and Arginine
Methionine is reported to exhibit potent antioxidant capacity through two major mechanisms [46][47]. Firstly, it produces antioxidant metabolites by undergoing metabolic processes. SAM, as one of the metabolites in the pathway, is not only an important methyl donor, but also a key metabolite modifying antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT) [46][47]. Such modification effects were confirmed by different research groups observing increased activities of antioxidant enzymes in birds fed Met supplemented diets [48][49][50][51][52]. Cysteine, another sulfur-containing amino acid, also exhibits antioxidant ability and is further metabolized to GSH, a well-known intrinsic antioxidant. Previous studies have also demonstrated increased GSH or improved GSH: glutathione disulfide (GSSG) ratio in broilers fed Met supplemented diets [39][53][54]. Secondly, Met residues in proteins can directly scavenge ROS. As a sulfur-containing amino acid, Met residues on the surface of proteins are readily oxidized. By scavenging ROS and being oxidized into methionine sulfoxide, Met can protect other critical components from oxidation, thus maintaining their integrity and function [25][55]. Furthermore, methionine sulfoxide can be reduced by methionine sulfoxide reductases (MSR) back to Met to restore its antioxidant capacity [56][57][58]. The antioxidant capacity of methionine and its metabolites is presented in Figure 1.
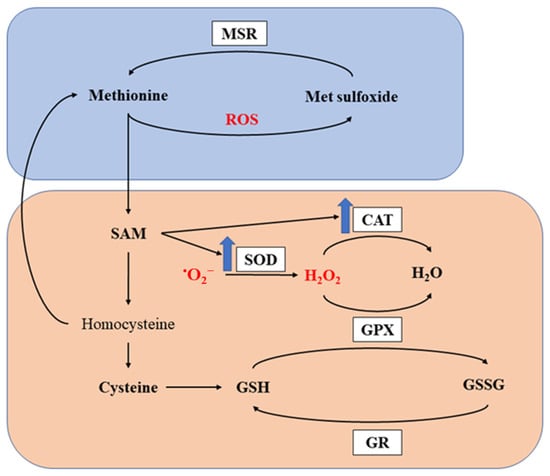
Figure 1. Antioxidant capacity of methionine. MSR, methionine sulfoxide reductase; SOD, superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase; GR, glutathione reductase; ROS, reactive oxygen species; SAM, S-adenosylmethionine; GSH, glutathione; GSSG, glutathione disulfide. The orange and boxed represents the first and second mechanism for methionine to exert its antioxidant capacity. The blue arrows indicate upregulation of the enzymes.
Previous research has provided evidence on the beneficial effects of Met supplementation in intestinal health through its antioxidant capacity. Dietary Met supplementation in broilers improved the GSH:GSSG ratio and glutathione peroxidase activity as well as increased the villus height (VH) and ratio of villus height to crypt depth (VH:CD) with or without stocking density challenge [59]. Met supplementation also improved the tight junction protein expression, whereas it decreased the expression of proinflammatory cytokines in broilers under heat stress [60]. Another study also demonstrated that Met supplementation increased the concentration of GSH, while it reduced the malondialdehyde (MDA) content in the duodenum mucosa. The VH and VH:CD were again improved by Met supplementation [61]. Despite not being widely recognized for its antioxidant capacity, several studies have shown that Arg supplementation can improve the oxidative status and benefit intestinal health. In an in vitro study conducted in ovine intestinal epithelial cells [62], the researchers found that Arg supplementation significantly reduced the hydrogen peroxide-induced ROS production. Furthermore, Arg also increased levels of glutathione peroxidase and tight junction protein 1, whereas it decreased the level of proinflammatory cytokines. Moreover, supplementing Arg in broiler breeders improved the oxidative status of the breeder birds as well as the one-day-old offspring [63]. Another study conducted in rats challenged with sodium nitrite showed that Arg supplementation reduced the serum MDA content and increased GSH production [64]. Additionally, both Met and Arg have been shown to activate the nuclear factor erythroid 2-related factor 2–antioxidant responsive element (Nrf2-ARE) pathway, leading to the upregulation of antioxidant enzymes and the generation of antioxidant effects [65][66].
Considering the potent antioxidant capacity and effects of Met and Arg, incorporating both Met and Arg as functional dietary supplements for poultry holds significant promise in mitigating the detrimental effects of oxidative stress on intestinal health and the overall well-being of the birds.
2.3. Methionine and Arginine in the Immune System
The intestinal tract is a major compartment of the immune system. The gut-associated lymphoid tissues (GALT) are estimated to comprise more immune cells than any other tissues [67]. The definable structures of GALT include lymphoid aggregates located within the lamina propria, Meckel’s diverticulum, Peyer’s patches, cecal tonsils, and bursa of Fabricius. In contrast to mammals, birds lack traditional lymph nodes; however, they possess numerous lymphoid aggregates that represent most of the secondary lymphoid tissues. Overall, the gut harbors diverse immune cell types, including heterophils, macrophages, dendritic cells, natural killer cells, and B and T cells, with the cellular compositions differing among different lymphoid tissues [68].
Methionine plays a critical role in both the humoral and cell-mediated immune responses in animals [69]. The impact of methionine on the humoral immune function of animals is primarily reflected in its effects on immunoglobulin levels in the body [70]. As for the cell-mediated immune responses, Met exerts regulatory effects on T cell activation and development [71]. Research showed that the activation of T cells is typically associated with an upregulation of Met transporters and SAM metabolism enzymes [72]. It was proposed that Met as well as its metabolites are taken up by the T helper cells for the synthesis of new protein and methylation of RNA and DNA, which drives activation, proliferation and differentiation of T cells [71][73]. Sufficient levels of methionine can significantly enhance antibody production as well as improve T cell proliferation in broilers [70][74][75][76]. Conversely, a deficiency of methionine significantly reduces the levels of immunoglobins in the bloodstream and inhibits the proliferation and differentiation of lymphocytes in broilers [77][78].
Numerous studies have extensively explored the immunoregulatory roles of Arg. Arg plays a significant modulatory role in immune function, primarily through the synthesis of NO by inducible NOS (iNOS) in macrophages [79][80]. NO is a versatile molecule that serves as a pivotal mediator in various immunological processes, including antimicrobial defense, immune cell regulation, and cytotoxicity [81][82]. Research has also shown its protective effects against protozoan infections [83][84]. Over the years, researchers have also shown that arginine availability is crucial for maintaining normal T cell proliferation and function. Rodriguez et al. [85] demonstrated that T cell function was significantly impaired under conditions of limited arginine supply, while this effect was completely reversed when arginine was replenished. It was proposed that arginine is indispensable for the expression of the T cell antigen receptor CD3ζ, which subsequently influences T cell function [85][86]. A recent study has also reported that Arg supplementation ameliorated the negative effect in Eimeria-infected broiler birds by enhancing the T cell function and elevating NO production [87].
Given the important roles of Met and Arg in immunity, the supplementation of Met and Arg could be a promising approach to enhance the immune response of the poultry intestine that constantly faces various challenges.
2.4. Methionine and Arginine in the Intestinal Microbiome
Numerous bacterial species inhabit the gastrointestinal tract (GIT) of chickens, with Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria being the dominant ones at the phylum level [88]. Studies have revealed that maintaining a well-balanced microbiota profile is crucial for preserving intestinal health and normal functions [89]. For instance, these microbial communities break down polysaccharides to provide amino acids and short-chain fatty acids, serving as crucial energy sources for the epithelial cells [90]. Moreover, the healthy intestinal microorganism plays a protective role against pathogens by inhibiting the adherence and colonization of the pathogens as well as modulating the immune responses of the host [88][91].
The intestinal microbiota profile is known to be influenced by dietary factors [92]. Recent research has shed light on the impact of Arg supplementation on the intestinal microbiota of chickens. Multiple studies have consistently shown that Arg supplementation can lead to an increase in the relative abundance of Firmicutes and a decrease in Proteobacteria in the ileum [93][94][95]. At the genus level, Ruan et al. [94] demonstrated that Arg supplementation resulted in a higher relative abundance of Romboutsia and a lower abundance of Clostridium sensu stricto 1. Brugaletta et al. [96] found that in the ceca, Arg supplementation decreased alpha diversity and the relative abundance of Proteobacteria, while increasing the relative abundances of Bacteroidetes and Lactobacillus salivarius. There is limited evidence regarding the effects of Met supplementation on the chicken intestinal microbiota profiles. However, Kumar et al. [97] reported that Met supplementation could enhance glycolysis and energy generation by the cecal microbiota. Another study performed in germ-free pigs revealed that in the absence of intestinal microbiota, Met levels in the ileum and hindgut increased significantly, indicating that the intestinal microbiota were actively involved in Met metabolism [98].
In conclusion, the findings so far suggest that Arg supplementation has notable effects on the intestinal microbiota composition in chickens. However, further investigation is needed to explore the potential impacts of Met supplementation on the chicken intestinal microbiota.
This entry is adapted from the peer-reviewed paper 10.3390/ani13182949
References
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53.
- Ravindran, V.; Abdollahi, M.R. Nutrition and Digestive Physiology of the Broiler Chick: State of the Art and Outlook. Animals 2021, 11, 2795.
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208.
- Adedokun, S.A.; Olojede, O.C. Optimizing Gastrointestinal Integrity in Poultry: The Role of Nutrients and Feed Additives. Front. Vet. Sci. 2018, 5, 348.
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013, 4, 27.
- Yegani, M.; Korver, D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008, 87, 2052–2063.
- Alizadeh, M.; Shojadoost, B.; Boodhoo, N.; Astill, J.; Taha-Abdelaziz, K.; Hodgins, D.C.; Kulkarni, R.R.; Sharif, S. Necrotic enteritis in chickens: A review of pathogenesis, immune responses and prevention, focusing on probiotics and vaccination. Anim. Health Res. Rev. 2021, 22, 147–162.
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115.
- Cervantes, H.M. Antibiotic-free poultry production: Is it sustainable? J. Appl. Poult. Res. 2015, 24, 91–97.
- Arike, L.; Seiman, A.; van der Post, S.; Rodriguez Piñeiro, A.M.; Ermund, A.; Schütte, A.; Bäckhed, F.; Johansson, M.E.V.; Hansson, G.C. Protein Turnover in Epithelial Cells and Mucus along the Gastrointestinal Tract Is Coordinated by the Spatial Location and Microbiota. Cell Rep. 2020, 30, 1077–1087.e73.
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976.
- Kong, X.; Tan, B.; Yin, Y.; Gao, H.; Li, X.; Jaeger, L.A.; Bazer, F.W.; Wu, G. l-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J. Nutr. Biochem. 2012, 23, 1178–1183.
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Mechanism of Activation of Mechanistic Target of Rapamycin Complex 1 by Methionine. Front. Cell Dev. Biol. 2020, 8, 715.
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: A variety of regulatory modes. J. Biomed. Sci. 2020, 27, 87.
- Carroll, B.; Maetzel, D.; Maddocks, O.D.; Otten, G.; Ratcliff, M.; Smith, G.R.; Dunlop, E.A.; Passos, J.F.; Davies, O.R.; Jaenisch, R.; et al. Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity. eLife 2016, 5, e11058.
- Sampson, L.L.; Davis, A.K.; Grogg, M.W.; Zheng, Y. mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice. FASEB J. 2016, 30, 1263–1275.
- Guan, Y.; Zhang, L.; Li, X.; Zhang, X.; Liu, S.; Gao, N.; Li, L.; Gao, G.; Wei, G.; Chen, Z. Repression of mammalian target of rapamycin complex 1 inhibits intestinal regeneration in acute inflammatory bowel disease models. J. Immunol. 2015, 195, 339–346.
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205.
- Ootani, A.; Li, X.; Sangiorgi, E.; Ho, Q.T.; Ueno, H.; Toda, S.; Sugihara, H.; Fujimoto, K.; Weissman, I.L.; Capecchi, M.R. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009, 15, 701–706.
- Schuijers, J.; Junker, J.P.; Mokry, M.; Hatzis, P.; Koo, B.-K.; Sasselli, V.; Van Der Flier, L.G.; Cuppen, E.; Van Oudenaarden, A.; Clevers, H. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 2015, 16, 158–170.
- Zhong, C.; Tong, D.Q.; Zhang, Y.R.; Wang, X.Q.; Yan, H.C.; Tan, H.Z.; Gao, C.Q. (DL)-methionine and (DL)-methionyl-(DL)-methionine increase intestinal development and activate Wnt/β-catenin signaling activity in domestic pigeons (Columba livia). Poult. Sci. 2022, 101, 101644.
- Hou, Q.; Dong, Y.; Huang, J.; Liao, C.; Lei, J.; Wang, Y.; Lai, Y.; Bian, Y.; He, Y.; Sun, J.; et al. Exogenous L-arginine increases intestinal stem cell function through CD90+ stromal cells producing mTORC1-induced Wnt2b. Commun. Biol. 2020, 3, 611.
- Albrecht, L.V.; Bui, M.H.; De Robertis, E.M. Canonical Wnt is inhibited by targeting one-carbon metabolism through methotrexate or methionine deprivation. Proc. Natl. Acad. Sci. USA 2019, 116, 2987–2995.
- Du, Q.; Zhang, X.; Liu, Q.; Zhang, X.; Bartels, C.E.; Geller, D.A. Nitric oxide production upregulates Wnt/β-catenin signaling by inhibiting Dickkopf-1. Cancer Res. 2013, 73, 6526–6537.
- Bikkavilli, R.K.; Malbon, C.C. Arginine methylation of G3BP1 in response to Wnt3a regulates β-catenin mRNA. J. Cell Sci. 2011, 124, 2310–2320.
- Larqué, E.; Sabater-Molina, M.; Zamora, S. Biological significance of dietary polyamines. Nutrition 2007, 23, 87–95.
- Sánchez-Jiménez, F.; Medina, M.Á.; Villalobos-Rueda, L.; Urdiales, J.L. Polyamines in mammalian pathophysiology. Cell. Mol. Life Sci. 2019, 76, 3987–4008.
- McCORMACK, S.A.; Viar, M.J.; Johnson, L.R. Polyamines are necessary for cell migration by a small intestinal crypt cell line. Am. J. Physiol.-Gastrointest. Liver Physiol. 1993, 264, G367–G374.
- Lan, A.; Blachier, F.; Benamouzig, R.; Beaumont, M.; Barrat, C.; Coelho, D.; Lancha, A., Jr.; Kong, X.; Yin, Y.; Marie, J.-C. Mucosal healing in inflammatory bowel diseases: Is there a place for nutritional supplementation? Inflamm. Bowel Dis. 2015, 21, 198–207.
- Wang, J.-Y.; Johnson, L.R. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology 1991, 100, 333–343.
- Wang, J.-Y.; McCormack, S.; Viar, M.; Johnson, L. Stimulation of proximal small intestinal mucosal growth by luminal polyamines. Am. J. Physiol.-Gastrointest. Liver Physiol. 1991, 261, G504–G511.
- Wang, J.-Y. Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 2007, 33, 241–252.
- Guo, X.; Rao, J.N.; Liu, L.; Zou, T.-T.; Turner, D.J.; Bass, B.L.; Wang, J.-Y. Regulation of adherens junctions and epithelial paracellular permeability: A novel function for polyamines. Am. J. Physiol.-Cell Physiol. 2003, 285, C1174–C1187.
- Timmons, J.; Chang, E.T.; Wang, J.Y.; Rao, J.N. Polyamines and Gut Mucosal Homeostasis. J. Gastrointest. Dig. Syst. 2012, 2, 001.
- Zhu, H.; Jia, Z.; Misra, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: Updated experimental and clinical evidence. J. Dig. Dis. 2012, 13, 133–142.
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965.
- Matés, J.M.; Pérez-Gómez, C.; Núñez de Castro, I. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603.
- Liu, G.; Magnuson, A.D.; Sun, T.; Tolba, S.A.; Starkey, C.; Whelan, R.; Lei, X.G. Supplemental methionine exerted chemical form-dependent effects on antioxidant status, inflammation-related gene expression, and fatty acid profiles of broiler chicks raised at high ambient temperature1. J. Anim. Sci. 2019, 97, 4883–4894.
- Magnuson, A.D.; Liu, G.; Sun, T.; Tolba, S.A.; Xi, L.; Whelan, R.; Lei, X.G. Supplemental methionine and stocking density affect antioxidant status, fatty acid profiles, and growth performance of broiler chickens. J. Anim. Sci. 2020, 98, skaa092.
- Sharma, M.K.; Liu, G.; White, D.L.; Tompkins, Y.H.; Kim, W.K. Effects of mixed Eimeria challenge on performance, body composition, intestinal health, and expression of nutrient transporter genes of Hy-Line W-36 pullets (0–6 wks of age). Poult. Sci. 2022, 101, 102083.
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. J. Virtual Libr. 2008, 13, 7210–7226.
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019, 6, 60.
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248.
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018, 98, 4471–4478.
- Burkholder, K.; Thompson, K.; Einstein, M.; Applegate, T.; Patterson, J. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult. Sci. 2008, 87, 1734–1741.
- Lozano-Sepulveda, S.A.; Bautista-Osorio, E.; Merino-Mascorro, J.A.; Varela-Rey, M.; Muñoz-Espinosa, L.E.; Cordero-Perez, P.; Martinez-Chantar, M.L.; Rivas-Estilla, A.M. S-adenosyl-L-methionine modifies antioxidant-enzymes, glutathione-biosynthesis and methionine adenosyltransferases-1/2 in hepatitis C virus-expressing cells. World J. Gastroenterol. 2016, 22, 3746–3757.
- Kachungwa Lugata, J.; Ortega, A.D.S.V.; Szabó, C. The Role of Methionine Supplementation on Oxidative Stress and Antioxidant Status of Poultry-A Review. Agriculture 2022, 12, 1701.
- Del Vesco, A.P.; Gasparino, E.; Grieser, D.O.; Zancanela, V.; Gasparin, F.R.; Constantin, J.; Oliveira Neto, A.R. Effects of methionine supplementation on the redox state of acute heat stress-exposed quails. J. Anim. Sci. 2014, 92, 806–815.
- Del Vesco, A.P.; Gasparino, E.; de Oliveira Grieser, D.; Zancanela, V.; Soares, M.A.M.; de Oliveira Neto, A.R. Effects of methionine supplementation on the expression of oxidative stress-related genes in acute heat stress-exposed broilers. Br. J. Nutr. 2015, 113, 549–559.
- Kalvandi, O.; Sadeghi, A.; Karimi, A. Methionine supplementation improves reproductive performance, antioxidant status, immunity and maternal antibody transmission in breeder Japanese quail under heat stress conditions. Arch. Anim. Breed. 2019, 62, 275–286.
- Reda, F.M.; Swelum, A.A.; Hussein, E.O.; Elnesr, S.S.; Alhimaidi, A.R.; Alagawany, M. Effects of varying dietary DL-methionine levels on productive and reproductive performance, egg quality, and blood biochemical parameters of quail breeders. Animals 2020, 10, 1839.
- Chen, Y.; Chen, X.; Zhang, H.; Zhou, Y. Effects of dietary concentrations of methionine on growth performance and oxidative status of broiler chickens with different hatching weight. Br. Poult. Sci. 2013, 54, 531–537.
- Ruan, T.; Li, L.; Lyu, Y.; Luo, Q.; Wu, B. Effect of methionine deficiency on oxidative stress and apoptosis in the small intestine of broilers. Acta Vet. Hung. 2018, 66, 52–65.
- Chang, Y.; Tang, H.; Zhang, Z.; Yang, T.; Wu, B.; Zhao, H.; Liu, G.; Chen, X.; Tian, G.; Cai, J. Zinc methionine improves the growth performance of meat ducks by enhancing the antioxidant capacity and intestinal barrier function. Front. Vet. Sci. 2022, 9, 774160.
- Sasaki, J.; Nakashima, N. Methionine-independent initiation of translation in the capsid protein of an insect RNA virus. Proc. Natl. Acad. Sci. USA 2000, 97, 1512–1515.
- Levine, R.L.; Moskovitz, J.; Stadtman, E.R. Oxidation of methionine in proteins: Roles in antioxidant defense and cellular regulation. IUBMB Life 2000, 50, 301–307.
- Shin, S.H.; Yoon, H.; Chun, Y.S.; Shin, H.W.; Lee, M.N.; Oh, G.T.; Park, J.W. Arrest defective 1 regulates the oxidative stress response in human cells and mice by acetylating methionine sulfoxide reductase A. Cell Death Dis. 2014, 5, e1490.
- Moskovitz, J. Methionine sulfoxide reductases: Ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim. Biophys. Acta 2005, 1703, 213–219.
- Miao, Z.Q.; Dong, Y.Y.; Qin, X.; Yuan, J.M.; Han, M.M.; Zhang, K.K.; Shi, S.R.; Song, X.Y.; Zhang, J.Z.; Li, J.H. Dietary supplementation of methionine mitigates oxidative stress in broilers under high stocking density. Poult. Sci. 2021, 100, 101231.
- Del Vesco, A.P.; Khatlab, A.d.S.; Santana, T.P.; Pozza, P.C.; Menck Soares, M.A.; Brito, C.O.; Barbosa, L.T.; Gasparino, E. Heat stress effect on the intestinal epithelial function of broilers fed methionine supplementation. Livest. Sci. 2020, 240, 104152.
- Shen, Y.B.; Ferket, P.; Park, I.; Malheiros, R.D.; Kim, S.W. Effects of feed grade L-methionine on intestinal redox status, intestinal development, and growth performance of young chickens compared with conventional DL-methionine. J. Anim. Sci. 2015, 93, 2977–2986.
- Zhang, H.; Zhang, Y.; Liu, X.; Elsabagh, M.; Yu, Y.; Peng, A.; Dai, S.; Wang, H. L-Arginine inhibits hydrogen peroxide-induced oxidative damage and inflammatory response by regulating antioxidant capacity in ovine intestinal epithelial cells. Ital. J. Anim. Sci. 2021, 20, 1620–1632.
- Duan, X.; Li, F.; Mou, S.; Feng, J.; Liu, P.; Xu, L. Effects of dietary L-arginine on laying performance and anti-oxidant capacity of broiler breeder hens, eggs, and offspring during the late laying period. Poult. Sci. 2015, 94, 2938–2943.
- El-Sheikh, N.M.; Khalil, F.A. l-Arginine and l-glutamine as immunonutrients and modulating agents for oxidative stress and toxicity induced by sodium nitrite in rats. Food Chem. Toxicol. 2011, 49, 758–762.
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328.
- Wang, Z.; Liang, M.; Li, H.; Cai, L.; He, H.; Wu, Q.; Yang, L. l-Methionine activates Nrf2-ARE pathway to induce endogenous antioxidant activity for depressing ROS-derived oxidative stress in growing rats. J. Sci. Food Agric. 2019, 99, 4849–4862.
- Kasahara, Y. Intraepithelial lymphocytes in birds. Adv. Host Def. Mech. 1994, 37, 163–174.
- Smith, A.L.; Powers, C.; Beal, R.K. Chapter 13—The Avian Enteric Immune System in Health and Disease. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 227–250.
- Ruan, T.; Li, L.; Peng, X.; Wu, B. Effects of methionine on the immune function in animals. Health 2017, 9, 857–869.
- Takahashi, K.; Konashi, S.; Akiba, Y.; Horiguchi, M. Effects of marginal excess or deficiency of dietary methionine on antibody production in growing broilers. Anim. Sci. Technol. 1993, 64, 13–19.
- Zhao, T.; Lum, J.J. Methionine cycle-dependent regulation of T cells in cancer immunity. Front. Oncol. 2022, 12, 969563.
- Sinclair, L.V.; Howden, A.J.M.; Brenes, A.; Spinelli, L.; Hukelmann, J.L.; Macintyre, A.N.; Liu, X.; Thomson, S.; Taylor, P.M.; Rathmell, J.C.; et al. Antigen receptor control of methionine metabolism in T cells. eLife 2019, 8, e44210.
- Klein Geltink, R.I.; Pearce, E.L. The importance of methionine metabolism. eLife 2019, 8, e47221.
- Swain, B.K.; Johri, T.S. Effect of supplemental methionine, choline and their combinations on the performance and immune response of broilers. Br. Poult. Sci. 2000, 41, 83–88.
- Mirzaaghatabar, F.; Saki, A.A.; Zamani, P.; Aliarabi, H.; Hemati Matin, H.R. Effect of different levels of diet methionine and metabolisable energy on broiler performance and immune system. Food Agric. Immunol. 2011, 22, 93–103.
- Bouyeh, M. Effect of Excess Lysine and Methionine on Immune system and Performance of Broilers. Ann. Biol. Res. 2012, 3, 3218–3224.
- Wu, B.; Cui, H.; Peng, X.; Fang, J.; Cui, W.; Liu, X. Pathology of bursae of Fabricius in methionine-deficient broiler chickens. Nutrients 2013, 5, 877–886.
- Wu, B.-y.; Cui, H.-m.; Peng, X.; Fang, J.; Cui, W.; Liu, X.-d. Effect of Methionine Deficiency on the Thymus and the Subsets and Proliferation of Peripheral Blood T-Cell, and Serum IL-2 Contents in Broilers. J. Integr. Agric. 2012, 11, 1009–1019.
- Li, P.; Yin, Y.-L.; Li, D.; Woo Kim, S.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252.
- Durante, W.; Johnson, F.K.; Johnson, R.A. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007, 34, 906–911.
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916.
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178.
- James, S.L. Role of nitric oxide in parasitic infections. Microbiol. Rev. 1995, 59, 533–547.
- Gradoni, L.; Ascenzi, P. Nitric oxide and anti-protozoan chemotherapy. Parassitologia 2004, 46, 101–103.
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of t cell receptor cd3ζ chain expression byl-arginine. J. Biol. Chem. 2002, 277, 21123–21129.
- Popovic, P.J.; Zeh, H.J.; Ochoa, J.B. Arginine and Immunity123. J. Nutr. 2007, 137, 1681S–1686S.
- Liu, G.; Ajao, A.M.; Shanmugasundaram, R.; Taylor, J.; Ball, E.; Applegate, T.J.; Selvaraj, R.; Kyriazakis, I.; Olukosi, O.A.; Kim, W.K. The effects of arginine and branched-chain amino acid supplementation to reduced-protein diet on intestinal health, cecal short-chain fatty acid profiles, and immune response in broiler chickens challenged with Eimeria spp. Poult. Sci. 2023, 102, 102773.
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112.
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, gut health and chicken productivity: What is the connection? Microorganisms 2019, 7, 374.
- Dunkley, K.; Dunkley, C.; Njongmeta, N.; Callaway, T.; Hume, M.; Kubena, L.; Nisbet, D.; Ricke, S. Comparison of in vitro fermentation and molecular microbial profiles of high-fiber feed substrates incubated with chicken cecal inocula. Poult. Sci. 2007, 86, 801–810.
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135.
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119.
- Zhang, B.; Lv, Z.; Li, Z.; Wang, W.; Li, G.; Guo, Y. Dietary l-arginine Supplementation Alleviates the Intestinal Injury and Modulates the Gut Microbiota in Broiler Chickens Challenged by Clostridium perfringens. Front. Microbiol. 2018, 9, 1716.
- Ruan, D.; Fouad, A.M.; Fan, Q.L.; Huo, X.H.; Kuang, Z.X.; Wang, H.; Guo, C.Y.; Deng, Y.F.; Zhang, C.; Zhang, J.H.; et al. Dietary L-arginine supplementation enhances growth performance, intestinal antioxidative capacity, immunity and modulates gut microbiota in yellow-feathered chickens. Poult. Sci. 2020, 99, 6935–6945.
- Zhang, B.; Li, G.; Shahid, M.S.; Gan, L.; Fan, H.; Lv, Z.; Yan, S.; Guo, Y. Dietary l-arginine supplementation ameliorates inflammatory response and alters gut microbiota composition in broiler chickens infected with Salmonella enterica serovar Typhimurium. Poult. Sci. 2020, 99, 1862–1874.
- Brugaletta, G.; Zampiga, M.; Laghi, L.; Indio, V.; Oliveri, C.; De Cesare, A.; Sirri, F. Feeding broiler chickens with arginine above recommended levels: Effects on growth performance, metabolism, and intestinal microbiota. J. Anim. Sci. Biotechnol. 2023, 14, 33.
- Kumar, S.; Adhikari, P.; Oakley, B.; Kim, W.K. Changes in cecum microbial community in response to total sulfur amino acid (TSAA: DL-methionine) in antibiotic-free and supplemented poultry birds. Poult. Sci. 2019, 98, 5809–5819.
- Wu, X.; Han, Z.; Liu, B.; Yu, D.; Sun, J.; Ge, L.; Tang, W.; Liu, S. Gut microbiota contributes to the methionine metabolism in host. Front. Microbiol. 2022, 13, 1065668.
This entry is offline, you can click here to edit this entry!
