Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Ophthalmology
The growing incidence of myopia worldwide justifies the search for efficient methods of myopia prevention. Numerous pharmacological, optical, and lifestyle measures have already been utilized, but there remains a need to explore more practical and predictable methods for myopia control.
- myopia control
- myopia prevention
- defocus-incorporated multiple-segment spectacle lenses (DIMSsl)
1. Introduction
Myopia control is a topic of widespread discussion among ophthalmologists, optometrists, and other eye care specialists. There is a growing incidence of myopia observed worldwide. Considering geographical prevalence, myopia is particularly prevalent in industrialized East Asian regions [1].
A meta-analysis conducted by Holden et al. estimated that, by the year 2050, nearly 50% of the global population will have myopia, with approximately 10% developing high myopia [2]. Myopia can lead to complications such as staphyloma, myopic maculopathy, myopic choroidal neovascularization (CNV), peripheral retinal degenerative changes, rhegmatogenous retinal detachment, optic disc changes, and glaucoma [3]. Thus, preventing the progression of myopia also helps prevent these associated comorbidities.
Numerous medical and environmental risk factors for myopia have been identified. The association between myopia prevalence and parental myopia is well documented [4]. Additionally, several twin-eye studies support the importance of genetic factors in myopia occurrence [5]. Researchers have discussed a multitude of other potential risk factors for myopia, for example, the level of education, time spent near work, accommodation and convergence function, and time spent outdoors. An interesting question is how does the time spent outdoors protect against myopia? Lingham G. et al. reviewed the evidence for and against eight features of spending time outdoors: brighter light, reduced peripheral defocus, higher vitamin D levels, differing chromatic spectrum of light, higher physical activity, entrained circadian rhythms, less time spent on near work, and greater high spatial frequency (SF) energies [6].
In the pathomechanism of myopia, we understand that not only the axial length of the eye but also the structure of the anterior segment of the eye plays a significant role. Nevertheless, it is the axial length that is a key parameter for the progression of myopia [7]. A large number of laboratory and clinical studies support the theory of the biochemical transformation of retinal image defocus, resulting in axial elongation of the eye [8]. As our knowledge of the risk factors as well as pathophysiological and biological mechanisms of myopia advances, new insights are emerging for the prevention of myopia progression.
Studies in the literature describe various methods for myopia control. A network meta-analysis by Huang et al. [9] lists several interventions for myopia control in children, including high-dose atropine (1% or 0.5%), low-dose atropine (0.01%), moderate-dose atropine (0.1%), bifocal spectacle lenses, cyclopentolate, increased outdoor activities, orthokeratology, progressive addition spectacle lenses, prismatic bifocal spectacle lenses, peripheral defocus modifying contact lenses, peripheral defocus-modifying spectacle lenses, pirenzepine, rigid gas-permeable contact lenses, soft contact lenses, single-vision spectacle lenses, timolol, and under-corrected single-vision spectacle lenses. The aforementioned network meta-analysis included randomized controlled trials with a minimum duration of one year, spanning from inception to August 2014. More recent research describes additional interventions for myopia control, such as defocus-incorporated multiple-segment spectacle lenses [10,11,12,13,14,15,16], repeated low-level red-light therapy [17,18,19,20,21,22,23], and a combination of orthokeratology and low-dose atropine (0.01%) [24,25,26,27,28,29,30,31,32].
Defocus-incorporated multiple-segment spectacle lenses (DIMSsl) are designed to correct refractive errors while simultaneously preventing the progression of myopia. Each DIMSsl consists of a central zone for distance refractive correction and a surrounding zone comprising approximately 400 multiple defocus segments that create myopic defocus. Animal studies have demonstrated that imposed myopic defocus inhibits eye elongation, whereas hyperopic defocus promotes eye elongation [33].
Repeated low-level red-light therapy involves using a device that emits low-level red light with a wavelength of 650+/−10 nm. The intervention requires looking into the device twice a day for 3 min, with a minimum 4-h interval between sessions. Parental supervision is necessary during the therapy [21].
2. The Efficacy and Safety of Defocus-Incorporated Multiple-Segment Spectacle Lenses in the Prevention of Myopia Progression
The first double-masked clinical trial assessing the efficacy of defocus-incorporated spectacle lenses (DIMSsl) was published in 2020 [12]. The study included 183 Chinese children with myopia ranging from −1.0 to −5.0 diopters and astigmatism below 1.5 diopters, aged between 8 and 13 years. Following randomization, 93 children received DIMSsl, while 90 children received single-vision spectacle lenses (SVsl). The trial lasted for 2 years, and assessments were conducted at 6-month intervals, including cycloplegic refraction and axial length of the eye. Of the initial participants, 160 completed the study. Over the course of two years, the changes in cycloplegic refraction for both groups were as follows: 0.41 ± 0.06 D in the DIMSsl group and 0.85 ± 0.08 D in the SVsl group. The mean axial elongation was 0.21 ± 0.02 mm in the DIMSsl group and 0.55 ± 0.02 mm in the SVsl group. In comparison, the myopia progression in the DIMSsl group was 52% slower than in the SVsl group, and the DIMSsl group had 62% less axial elongation than the SVsl group. Myopia control with spectacle lenses is considered a noninvasive approach. No adverse events related to the intervention were reported in this study. The authors also assessed the visual performance of DIMSsl and SVsl users in terms of parameters such as visual acuity (near and distant), the amplitude of accommodation (monocular and binocular), lag of accommodation, and stereopsis. Only the difference in stereopsis was statistically significant, but the clinical significance was negligible (5 s of arc).
In another publication from 2020, [16] documented the changes in relative peripheral refraction associated with myopia progression in the same group of patients. Central refraction and peripheral refraction at six retinal points (10°, 20°, and 30° nasally and temporally) were measured every 6 months, along with axial length measurements after cycloplegia. In the SVsl group, asymmetry between nasal and temporal retina myopic shifts was observed, while the DIMSsl group exhibited a constant and symmetrical relative peripheral refraction profile.
The visual function of the same group of patients was described in detail in a paper published in 2020 by Lam et al. [11]. The study included 160 participants who completed a 2-year trial, with 79 wearing DIMSsl and 81 wearing SVsl. Visual function was assessed by measuring distance and near best-corrected visual acuity measured monocularly, distance and near phoria, the monocular and binocular amplitude of accommodation, lag of accommodation, and stereopsis at baseline and every 6-month interval over 2 years. After two years, both groups showed a slight improvement in high contrast visual acuity: −0.09 ± 0.07 logMAR for DIMSsl wearers and −0.07 ± 0.06 logMAR for SVsl wearers. Accommodative lag was significantly reduced in both groups, and stereo-acuity improved. Distance and near phoria showed no significant changes from baseline in either group.
In 2022, Lam et al. published the results of a 3-year follow-up study [10]. Their study included 128 children who had participated in the previous 2-year trial. Those who wore DIMSsl in the previous trial continued with DIMSsl, while the SVsl group switched to DIMSsl. Refraction after cycloplegia and axial length were assessed at 6-month intervals. Both groups were compared to a new historical control group, obtained by reviewing the clinical records of the optometry clinic. Over 3 years, the mean changes in spherical equivalent refraction (SER) were −0.52 ± 0.69 D for the DIMS group and −0.92 ± 0.81 D for the SVsl group switched to DIMSsl. The mean changes in axial length over 3 years were 0.31 ± 0.26 mm for the DIMSsl group and 0.57 ± 0.33 mm for the SVsl group switched to DIMSsl.
In 2023, Zhang et al. published the results of the continued observation of changes in relative peripheral refraction associated with myopia progression [16]. The study included 128 children who continued to wear DIMScl (n = 65) for 1 year after the previous 2-year trial and those who switched to DIMSsl after 2 years of using SVsl (n = 55). The authors observed a constant and symmetrical peripheral refraction profile in the DIMSsl group. Within the SVsl group in the first 2 years, significant increases in hyperopic relative peripheral refraction (RPR) were noted at 20° nasal. After switching to DIMSsl in the third year, there were significant reductions in hyperopic RPR at 20° nasal (mean difference: −1.14 ± 1.93 D, p < 0.0001) and 30° nasal (mean difference: −1.07 ± 1.17 D, p < 0.0001).
A clinical trial conducted by Lam et al. and published in 2023 followed 90 Chinese children for a period of 6 years [13]. The mean age of the participants at enrollment was approximately 10 years old. The study involved four different groups:
-
Group 1 (36 children) wore defocus-incorporated multiple-segment spectacle lenses (DIMSsl) for the entire 6-year duration;
-
Group 2 (14 children) wore DIMSsl for the first 3.5 years and then switched to single-vision lenses (SVsl);
-
Group 3 (22 children) wore SVsl for the first 2 years and then switched to DIMSsl for the remaining 4 years;
-
Group 4 (18 children) wore SVsl for the first 2 years, then DIMSsl for 1.5 years, and finally switched back to SVsl for the last 2.5 years.
The main measured outcomes were changes in axial length (AXL) and cycloplegic refraction. The spherical equivalent refraction (SER) at baseline and the 6-year follow-up for each group were as follows:
-
Group 1: SER −3.04 ± 0.89 D/−3.69 ± 1.42 D;
-
Group 2: SER −2.98 ± 1.13 D/−4.28 ± 1.15 D;
-
Group 3: SER −2.68 ± 0.88 D/−3.92 ± 1.18 D;
-
Group 4: SER −2.65 ± 1.18 D/−3.87 ± 1.53 D.
The AXL measurements at baseline and the 6-year follow-up for each group were as follows:
-
Group 1: AXL 24.68 ± 0.76 mm/25.28 ± 0.81 mm;
-
Group 2: AXL 25.00 ± 0.80 mm/25.71 ± 0.69 mm;
-
Group 3: AXL 24.62 ± 0.79 mm/25.43 ± 1.01 mm;
-
Group 4: AXL 24.42 ± 0.86 mm/25.14 ± 0.87 mm.
These results indicate that there was no rebound effect after stopping the use of DIMSsl.
A retrospective study conducted by [14], which aimed to assess the effectiveness of DIMSsl in clinical settings, involved children aged 6 to 16 years old. After propensity score matching, data from 2240 pairs (1-year observation) and 735 pairs (2-year observation) were analyzed. The results confirmed the effectiveness of DIMSsl in slowing the progression of myopia compared to SVsl in clinical settings. The spherical equivalent progression in the first year was −0.50 ± 0.43 D for DIMSsl and −0.77 ± 0.58 D for SVsl (p < 0.001). In the second year, the spherical equivalent progression was −0.88 ± 0.62 D for DIMSsl and −1.23 ± 0.76 D for SVsl (p < 0.001). These findings provide further evidence of the effectiveness of DIMSsl in slowing myopia progression.
Figure 1 depicts the mechanism of action of defocus-incorporated multiple-segments spectacle lens.
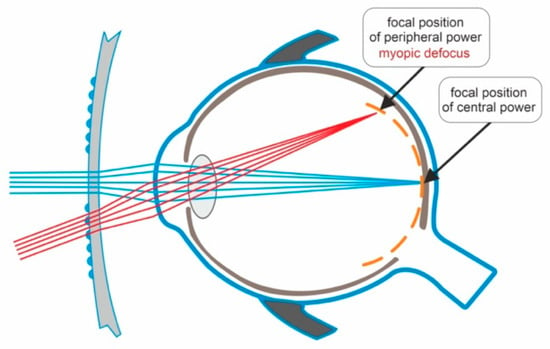
Figure 1. Light passing through the central zone of the DIMSsl creates a clear image on the retina. Light passing through the peripheral part of the DIMSsl creates myopic defocus on the peripheral retina.
3. The Efficacy and Safety of Low-Intensity Red-Light Therapy in the Prevention of Myopia Progression
In 2022, Jian Y. et al. published a multicenter, randomized, parallel-group, single-blind clinical trial [21]. The study included 264 children aged 8–13 years with myopia ranging from −1.0 to −5.0 D cycloplegic refraction. After randomization, the sample size was as follows: 117 children in the repeated low-level red-light (RLRL) therapy group and 129 children in the single-vision spectacle lenses (SVsl) group. Cycloplegic refraction and axial length were measured at baseline and at 1-, 3-, 6-, and 12-month follow-up visits. In the RLRL group, the intervention involved repeated low-level red-light therapy using a desktop light therapy device at home, with parental supervision required. Each session lasted 3 min and was repeated twice a day, five days per week. The main results of the study were as follows: The adjusted 12-month axial elongation for the RLRL group was 0.13 mm (95% CI, 0.09–0.17 mm), and for the SVsl group, it was 0.38 mm (95% CI, 0.34–0.42 mm). The adjusted 12-month spherical equivalent refraction (SER) for the RLRL group was −0.20 D (95% CI, −0.29 to −0.11 D), and for the SVsl group, it was −0.79 D (95% CI, −0.88 to −0.69 D).
In 2023, Dong J. et al. conducted a double-masked clinical trial on repeated low-level red-light (RLRL) therapy involving 112 Chinese myopic children aged 7 to 12 years [19]. The children were divided into two groups: The RLRL group with 56 children and the sham device control group with 55 children. In the control group, a sham device with 10% of the power of the original device was used. Each session of RLRL therapy lasted 3 min and was repeated twice a day for a duration of 6 months. The mean change in spherical equivalent refraction (SER) over 6 months was as follows: −0.06 ± 0.03 D for the RLRL group and −0.11 ± 0.33 D for the sham device control group. The mean change in axial length (AL) over 6 months was −0.02 ± 0.11 D for the RLRL group and −0.13 ± 0.10 D for the sham device control group. No treatment-related adverse events were reported during the study.
In 2022, Xiong R. et al. published a prospective, post-trial follow-up study or real-world study (RWS) [22]. After completing a 1-year randomized controlled trial (RCT), the participants were invited to voluntarily participate in the real-world study. A total of 114 participants were enrolled and divided into four groups: SVS-SVS group (n = 41), SVS-RLRL group (n = 10), RLRL-SVS group (n = 52), and RLRL-RLRL group (n = 11). Cycloplegic refraction and axial length were measured at the 24-month mark from the beginning of the RCT. Over the 2-year period, the mean change in axial length (AXL) and spherical equivalent refraction (SER) were found to be the smallest in the RLRL-RLRL group. However, a modest rebound effect was observed after the cessation of treatment.
In a secondary analysis of data from a multicenter randomized controlled trial (RCT), Xiong R. et al. investigated the measurements of macular choroidal thickness (mCT) using swept-source optical coherence tomography (SS-OCT) and its associations with myopia control [23]. The study also assessed other variables at 1, 3, 6, and 12 months, including visual acuity, axial length, spherical equivalent refraction (SER), and treatment compliance. The authors aimed to determine the predictive value of different covariates for myopia control. They constructed models that included only changes in mCT at 3 months and evaluated their ability to predict good myopia control over a 12-month period. These models demonstrated acceptable predictive discrimination for myopia control.
In 2022, Chen Y. et al. conducted an RCT comparing the efficacy of repeated low-level red-light (RLRL) therapy with low-dose atropine for myopia control [18]. The study included 62 children aged 7 to 15 years who were randomly assigned to receive RLRL therapy or atropine 0.01%. Each group consisted of 31 participants. Axial length and cycloplegic spherical equivalent refraction were monitored at 1, 3, 6, and 12 months. The results indicated that RLRL therapy was effective in controlling myopia progression over one year compared to low-dose atropine eye drops.
Another RCT published in 2023 by Chen H. et al. analyzed the efficacy of low-intensity red light (LRL) therapy compared to single-focus spectacles (SFSs) for myopia control [17]. The study included 51 children in the LRL group and 51 children in the SFS group, aged 6 to 13 years, with myopia ranging from −0.75 to −6.0 diopters of cycloplegic spherical equivalent refraction. The treatment phase lasted for 12 months, followed by a 3-month washout phase. LRL therapy was administered twice a day, with each session lasting 3 min. Ophthalmic examinations, including assessments of axial length (AL), spherical equivalent refraction (SER), subfoveal choroidal thickness (SFCT), and accommodative function, were conducted at 3, 6, 9, 12, and 15 months. At the end of the 12-month trial, 46 children in the LRL group and 40 children in the SFS group completed the study. The AXL elongation at 12 months for the LRL group was 0.01 mm (95% CI 0.05–0.07 mm), while for the SFS group, it was 0.39 mm (95% CI 0.33–0.45 mm). The SER progression at 12 months for the LRL group was 0.05 D (95% CI 0.08–0.19 D), whereas for the SFS group, it was 0.64 D (95% CI 0.78–0.51 D). Changes in SFCT showed thickening in the first 3 months for the LRL group, followed by relative stability in the subsequent months, while the SFS group exhibited progressive thinning of SFCT. Accommodative function was assessed through measurements of the amplitude of accommodation (AA), accommodative response (AR), accommodative facility (AF), positive relative accommodation (PRA), and negative relative accommodation (NRA). The LRL group demonstrated more negative accommodative response and positive relative accommodation than the SFS group.
He X. et al. conducted a study to assess the effectiveness of repeated low-level red-light (RLRL) therapy in children with premyopia, defined as a cycloplegic spherical equivalent refraction of −0.5 D to 0.5 D in the more myopic eye. The inclusion criteria also required at least one parent to have a spherical equivalent refraction of −3.0 diopters or less in either eye [20]. The study enrolled pupils in grades 1–4 from 10 primary schools in Shanghai. Participants assigned to the treatment group received two RLRL therapy sessions lasting 3 min each, daily for 5 days per week. The sessions were conducted at school, except during winter and summer vacations when they took place at home. The main outcome measure was the incidence of myopia after 12 months, which was 40.8% (49 out of 120) in the RLRL group and 61.3% (68 out of 111) in the control group. Additional results showed that RLRL intervention significantly reduced spherical equivalent refraction (SER) and axial length (myopic shifts). No adverse effects on visual acuity or structural damage were observed on optical coherence tomography (OCT) in the intervention group.
Figure 2 depicts the mechanism of action of low-level red light therapy device.
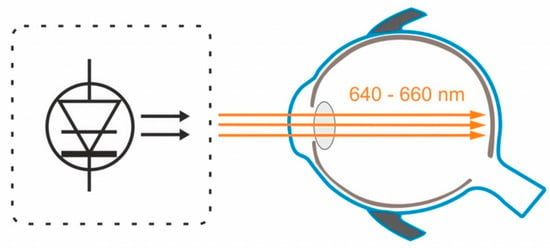
Figure 2. The semiconductor laser diode delivers low-level red light through the pupil to the fundus.
This entry is adapted from the peer-reviewed paper 10.3390/medicina59101859
This entry is offline, you can click here to edit this entry!
