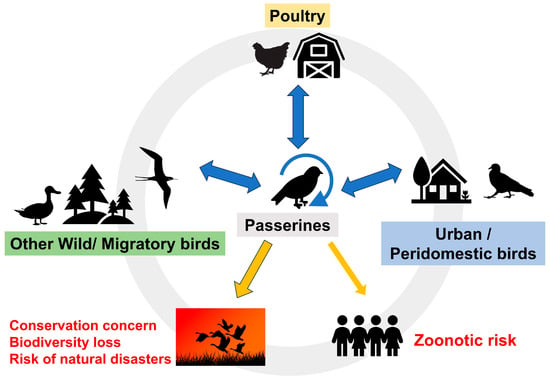
Interest in emerging viruses is growing because some can cause serious or lethal disease in humans and animals. The number of cloacal virome studies is also growing, however, these usually focus on poultry and other domestic birds, These studies reveal a wide variety of viruses, although the pathogenic significance of most newly discovered viruses is uncertain. Analysis of viruses detected in wild birds is complex and often biased towards waterfowl because of the obvious interest in avian influenza or other zoonotic viruses. Less is known about the viruses present in the order Passeriformes, which comprises approximately 60% of extant bird species. This review aims to compile the most significant contributions, from traditional and metagenomic studies, on the viruses that affect passerines. It highlights most passerine species have never been sampled. Some viruses, especially Flaviviridae, Orthomyxoviridae, Poxviridae and Togaviridae, and arguably others, are considered emerging because of increased incidence or avian mortality/morbidity, spread to new geographical areas or hosts and their zoonotic risk. However, many of these viruses have only recently been described in passerines using metagenomics and their role in the ecosystem is unknown.
- biodiversity
- DNA viruses
- emergence
- metagenomic studies
- passeriformes
- RNAviruses
- spillover
- zoonoses
Introduction
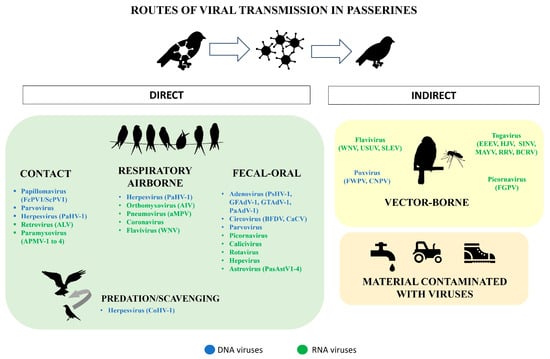
2. Families of Viruses Affecting Passeriformes
| Viral Family | Genus | Viral Taxa | Abbreviations | Nomenclature | Bird Family | Clinical Signs | Mortality Rate | References |
|---|---|---|---|---|---|---|---|---|
| dsDNA VIRUSES | ||||||||
| Herpesviridae | Iltovirus | Gallid herpesvirus 1 | GaHV-1 | Iltovirus gallidalpha1 | Turdidae, Estrildidae, Sturnidae | Subclinical infection/ Respiratory signs |
High | [7,26,34,35,37] |
| Psittacid herpesvirus 1 | PsHV-1 | Iltovirus psittacidalpha1 | Sturnidae | |||||
| passerid herpesvirus 1 | not recognized | Fringillidae | ||||||
| Mardivirus | Columbid herpesvirus 1 | CoHV-1 | Mardivirus columbidalpha1 | Corvidae, Turdidae | Subclinical infection | Probably not | [40] | |
| Poxviridae | Avipoxvirus | Fowlpox virus (Lineage A1) | FWPV | same | Fringillidae, Passeridae | Cutaneous lesions/Upper respiratory and digestive tract lesions | High | [42,43,44,45,46,47,48,49,50,51,52,53,54] |
| Pigeonpox virus (Lineage A2) | PGPV | same | Fringillidae | High/Medium | ||||
| Accipitriformespox virus (Lineage A7) | same | Lanidae | High/Medium | |||||
| Canarypox virus (Lineage B1) | CNPV | same | Fringillidae, Paridae, Corvidae (and 11 other families) | High/Medium | ||||
| Starlingpox virus (Lineage B2) | same | Sturnidae, Passeridae | High/Medium | |||||
| Lineage B3 poxvirus | not recognized | Corvidae (and eight other mostly Passeriformes families) | High | |||||
| Adenoviridae | Atadenovirus | European robin adenovirus | not recognized | Meliphagidae, Fringillidae, Ploeceidae, Estrildidae, Turdidae, Ptilonorhynchidae, Sturnidae, Zosteropidae, Maluridae, Petroicidae | Subclinical infection/ Not known |
Probably not | [57,58] | |
| European greenfinch adenovirus | not recognized | |||||||
| Eurasian siskin adenovirus | not recognized | |||||||
| Eurasian bullfinch adenovirus | not recognized | |||||||
| common chaffinch adenovirus | not recognized | |||||||
| passerine adenovirus 1 | not recognized | |||||||
| white plumed honeyeater adenovirus 1, -2 | not recognized | |||||||
| vitelline masked weaver adenovirus strains 37869 and 38132 | not recognized | |||||||
| Aviadenovirus | great tit adenovirus strain 47292 | not recognized | Fringillidae, Estrildidae, Paridae, Ploceidae, Sylvidae, Falcunculidae, Petroicidae | Subclinical infection/ Not known |
Probably not | |||
| european greenfinch adenovirus | not recognized | |||||||
| goldfinch adenovirus | not recognized | |||||||
| vitelline masked weaver adenovirus strain 39658 | not recognized | |||||||
| Siadenovirus | double-barred finch adenovirus | not recognized | Estrildidae, Paridae, Sylvidae, Thraupidae, Meliphagidae, Pardalotidae, Rhipiduridae, Sturnidae | Subclinical infection/ Probable renal lesions |
Probably not | [56,60] | ||
| eurasian blackcap adenovirus | not recognized | |||||||
| gouldian finch adenovirus 1 | GFAdV-1 | not recognized | ||||||
| great tit adenovirus strain 47292 | GTAdV-1 | Great tit siadenovirus A | ||||||
| zebra finch adenovirus strain 47535 | not recognized | |||||||
| Papillomaviridae | Etapapillomavirus | Fringilla coelebs Papillomavirus 1 | FcPV1 | Etapapillomavirus 1 | Fringillidae | Cutaneous lesions | Low | [66,67,69,70,71,72] |
| Serinus canaria papillomavirus 1 | ScPV1 | not recognized | Fringillidae | Not known | Not known | [68] | ||
| ssDNA VIRUSES | ||||||||
| Circoviridae | Circovirus | Beak and feather disease virus | BFDV | Circovirus parrot | Artamidae, Corvidae, Estrildidae, Fringillidae, Petoicidae, Sturnidae, Thamnophilidae | Feather lesions | Probably not | [76,77,78] |
| Circovirus canary | CaCV | same | Feather diseases and immunosupression/ Subclinical infection |
Yes/Not known | [25,79,80,81,82] | |||
| Circovirus finch | FiCV | same | ||||||
| Circovirus raven | RaCV | same | ||||||
| Circovirus starling | StCV | same | ||||||
| Circovirus zebrafinch | ZfiCV | same | ||||||
| Cyclovirus | Robinz virus RP_736 | RobinzV736 | Cyclovirus cervienka | Petroicidae | Not known | Not known | [17] | |
| Robinz virus RP_1170 | RobinzV1170 | Cyclovirus liepsnele | ||||||
| Robinz virus RP_493 | RobinzV493 | Cyclovirus pettirosso | ||||||
| Robinz virus RP_620 | RobinzV620 | Cyclovirus prihor | ||||||
| Robinz virus RP_526 | RobinzV526 | Cyclovirus punarinta | ||||||
| Robinz virus RP_584 | RobinzV584 | Cyclovirus rudzik | ||||||
| Robinz virus RP_259 | RobinzV259 | Cyclovirus totoi | ||||||
| Probably new genus | Gyrovirus 11 | not recognized | Thamnophilidae | Not known | Not known | [89] | ||
| Genomoviridae | Gemycirculavirus | new species | not recognized | Petroicidae, Aegithalidae, Corvidae, Emberizidae, Fringillidae, Muscicapidae, Paridae, Sylviidae, Turdidae, Zosteropidae | Not known | Not known | [88] | |
| Chickadee associated gemycircularvirus 1 | Gemycircularvirus chickad1 | Paridae | [85] | |||||
| Finch associated genomovirus 5 | Gemycircularvirus haeme1 | Fringillidae | [86] | |||||
| Finch associated genomovirus 6 | Gemycircularvirus haeme2 | [86] | ||||||
| Gemykibivirus | Blackbird associated gemykibivirus 1 | Gemykibivirus blabi1 | Turdidae | [87] | ||||
| Black robin associated gemykibivirus 1 | Gemykibivirus blaro1 | Petroicidae | ||||||
| Finch associated genomovirus 3 | Gemykibivirus haeme1 | Fringillidae | [86] | |||||
| Finch associated genomovirus 2 | Gemykibivirus haeme3 | |||||||
| Finch associated genomovirus 4 | Gemykibivirus haeme4 | |||||||
| Finch associated genomovirus 1 | Gemykibivirus haeme5 | |||||||
| Gemykroznavirus | Finch associated genomovirus 8 | Gemykronzavirus haeme1 | Fringillidae | |||||
| Gemygorvirus | Starling associated gemygorvirus 1 | Gemygorvirus stara1 | Sturnidae | [87] | ||||
| Parvoviridae | Aveparvovirus | Aveparvovirus passeriform1 | PfPV | same | Thraupidae | Not known | Not known | [95] |
| ssRNA VIRUSES | ||||||||
| Orthomyxoviridae | Alphainfluenzavirus 1 | Highly Pathogenic Avian Influenza H5N1 | FLUAV/(H5N1) | Alphainfluenzavirus influenzae | Passeridae, Turdidae, Ploceidae, Corvidae, Hirundidae, Icteridae (and many other families) | Mild/Severe disease | High | [101,102,103,104,105,106,107,108,110,116] |
| Paramyxoviridae | Orthoavulavirus | Avian paramyxovirus 1 (NDV, Newcastle Disease virus) | APMV-1 | Orthoavulavirus javaense | Passeridae, Motacillidae, Passarellidae | Not known/Subclinical disease | High/Not known | [113,123,124,126,127] |
| Metaavulavirus | Avian paramyxovirus 2 | APMV-2 | Metaavulavirus yucaipaense | Corvidae, Motacillidae, Troglodytidae, Muscicapidae, Emberizidae, Estrildidae | Mild/Severe disease | [128,129,130,131,132,133,134] | ||
| Paraavulavirus | Avian paramyxovirus 3 | APMV-3 | Paraavulavirus wisconsinense | [135,136] | ||||
| Paraavulavirus | Avian paramyxovirus 4 | APMV-4 | Paraavulavirus hongkongense | [138] | ||||
| Pneumoviridae | Metapneumovirus | Avian metapneumovirus | AMPV | Metapneumovirus avis | Passeridae, Sturnidae | Not known | Not known | [141,142] |
| Bornaviridae | Orthobornavirus | Estrildid finch bornavirus 1 | EsBV-1 | Orthobornavirus estrildidae | Estrildidae, Corvidae, Fringillidae | Not known | Not known | [146,147,148,149] |
| Canary bornavirus 1 | CnBV-1 | Orthobornavirus serini | ||||||
| Canary bornavirus 2 | CnBV-2 | |||||||
| Canary bornavirus 3 | CnBV-3 | |||||||
| Flaviviridae | Orthoflavivirus 1 | Japanese encephalitis virus | JEV | Orthoflavivirus japonicum | Passeridae, Corvidae, Fringillidae, Turdidae (and >23 more families) | Not known | High | [183] |
| Saint Louis encephalitis virus | SLEV | Orthoflavivirus louisense | [191,192,193,194] | |||||
| Murray Valley Encephalitis virus | MVEV | Orthoflavivirus murrayense | [186] | |||||
| Usutu virus | USUV | Orthoflavivirus usutuense | Severe disease | [174,175,176] | ||||
| West Nile virus | WNV | Orthoflavivirus nilense | Mild/Severe disease | [158,159,160,161,162,169] | ||||
| Togaviridae | Alphavirus 1 | Eastern equine encephalitis virus | EEEV | same | Turdidae, Hirundidae, Icteridae, Petroicidae, Estrildidae, Monarchidae | Neurological disease | Yes | [206,207,208] |
| Highlands J virus | HJV | same | Mild disease | [210] | ||||
| Sindbis virus | SINV | same | Severe disease | [215,216] | ||||
| Ross River virus | RRV | same | Not known | [219,220] | ||||
| Mayaro virus | MAYV | same | [225,226,227,228] | |||||
| Buggy Creek virus | BUCGV | Fort Morgan virus | Neurological disease | [230,231,232] | ||||
| Deltacoronavirus 2 | Bulbul coronavirus HKU11 | BulCV_HKU11 | same | Turdidae, Estrildidae, Pycnonotidae, Passeridae, Zosteropidae, Fringillidae, Emeberizidae | Severe disease | Probably high | [239,240,241] | |
| Munia coronavirus HKU13 | MunCV_HKU13 | same | ||||||
| Thrush coronavirus | not recognized | |||||||
| White-eye coronavirus HKU16 | WECoV_HKU16 | same | ||||||
| Coronavirus HKU15 | PoCoV_HKU15 | same | ||||||
| Picornaviridae | Oscivirus | Oscivirus A1 and A2 (turdivirus 2 and 3) | OsV-A1/OsV-A2 | Oscivirus A | Turdidae, Muscicapidae | Severe disease | Not known | [247,248] |
| Passerivirus | Passerivirus A and B | PasV-A/PasV-B | same | Turdidae, Estrildidae | High | |||
| Poecivirus | Poecivirus A1 | PoeV-A | Poecivirus A | Paridae | Yes | [251,252] | ||
| Probably new genus | French Guiana Picornavirus | FGPV | not recognized | Thamnophilidae | Not known | Not known | [13] | |
| Astroviridae | Avastovirus | novel avastrovirus clades 4 and 5 | not recognized | Fringillidae, Monarchidae Parulidae, Passeridae | Subclinical infectionMild disease | Not known | [258,259,260] | |
| Caliciviridae | Probably new genera | new calicivirus | not recognized | Prunellidae, Turdidae, Emberizidae, Fringillidae, Muscicapidae, Petroicidae | Subclinical infection | Not known | [7,15,275,276] | |
| Hepeviridae | Probably new genera | new hepe-like viruses | not recognized | Thamnophilidae, Turdidae, Zosteropidae | Not known | Not known | [7,13,15] | |
| dsRNA VIRUSES | ||||||||
| Reoviridae | Orthoreovirus 2 | new orthoreovirus | not recognized | Emberizidae, Turdidae, Fringillidae | Not known | High mortality | [283,284] | |
| RNA REVERSE TRANSCRIBING VIRUS | ||||||||
| Retroviridae | Alpharetrovirus | Avian leukosis virus | ALV | same | Emberizidae, Paridae, Corvidae, Muscicapidae, Thraupidae, Phylloscopidae | Subclinical infection | Possibly | [291,292,293] |
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11092355
