Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Green & Sustainable Science & Technology
The bio-based solvent dihydrolevoglucosenone (Cyrene) is a green and sustainable alternative to petroleum-based dipolar aprotic solvents. Cyrene can be prepared from cellulose in a simple two-step process and can be produced in a variety of yields. Cyrene is compatible with a large number of reactions in the chemical industry and can be applied in organic chemistry, biocatalysis, materials chemistry, graphene and lignin processing, etc.
- Cyrene
- levoglucosanone
- green chemistry
1. Introduction
The preparation of Cyrene is carried out in two steps (Figure 1): the first step is the catalytic pyrolysis of cellulose or cellulose-containing substances (e.g., pine, poplar, kraft pulp, newspaper, sawdust, etc.) to generate levoglucosanone (LGO), and in the second step, the catalytic hydrogenation of levoglucosanone to obtain Cyrene. Because the catalytic pyrolysis of cellulose is a very complicated reaction process, and the yield of the second step of catalytic hydrogenation of pure LGO is generally higher and can reach more than 90%, the main limitation of the preparation is the first step.
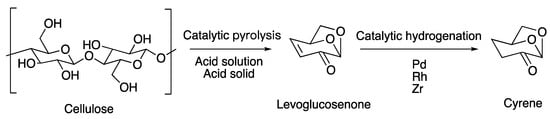
Figure 1. The process of preparing Cyrene with cellulose.
1.1. Preparation of LGO
The preparation process for the catalytic pyrolysis of cellulose is divided into two main categories depending on the type of catalyst used: one is catalytic pyrolysis using an acid solution as the catalyst, and the other is pyrolysis using an acidic solid catalyst. The main product of cellulose pyrolysis without a catalyst is levoglucosan (LGA) with a yield of around 70%. The yield of LGO can be increased in the presence of an acid catalyst. Pyrolysis using an acid solution as a catalyst is subdivided into two types: one in which the cellulose is pretreated by impregnation in an acid solution and then pyrolysed at atmospheric pressure, and the other in which the cellulose is pyrolysed in a solvent under high pressure. Kudo, through a comprehensive investigation of LGO preparation methods, classified LGO production strategies into three categories based on the catalytic technique employed (Figure 2): direct LGO production from cellulose pyrolysis after catalyst impregnation, catalytic pyrolysis in the gas phase using solid catalysts and catalytic pyrolysis in the liquid phase using liquid catalysts [9]. This is due to the fact that Lu et al. computed potential reaction pathways from β-D-glucopyranose and cellobiose to LGO using density functional theory (DFT) to derive the fact that LGA cannot be the essential intermediate of LGO [10]. Assary and Curtiss considered low yields or difficulties in the formation of LGO from LGA by quantitatively calculating the reaction energies and barriers [11]. They concluded that the reaction using catalyst impregnation followed by direct pyrolysis is a complex process for making LGO.
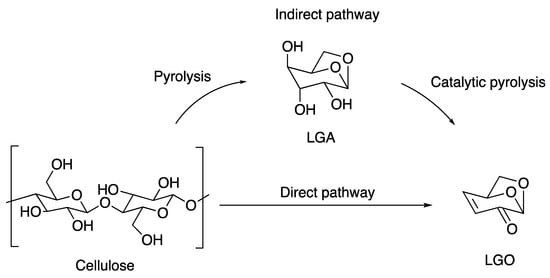
Figure 2. Cellulose conversion pathway to LGO.
1.1.1. Acid Impregnation Followed by Pyrolysis to Produce LGO Directly
As early as 1972, Halpern et al. obtained LGO by mixing cellulose with acidic additives and then pyrolysing it, isolating LGO in the product and determining its structure for the first time [12]. In 1998, Dobele et al. impregnated cellulose with phosphoric acid at different concentrations and then pyrolysed it, finding that depolymerisation began to take place in the amorphous region of the cellulose at 250–300 °C, and depolymerisation also began to take place in the crystalline region at 325 °C [13]. The presence of phosphoric acid inhibits the formation of LGA and facilitates the conversion of cellulose to glucopyranose, the precursor of LGO, at 200–250 °C. The presence of sulfuric acid also acts as a catalyst for the formation of LGO, but phosphoric acid is more effective in catalysing the pyrolysis of cellulose. It was found that 5 wt% phosphoric acid was the optimal concentration, although the higher the concentration of phosphoric acid, the better the dehydration effect, but with more than 5% phosphoric acid, impregnation of cellulose condensed structures are formed, which can no longer be degraded to volatile monomers. By comparing the yields of different reaction feedstocks, it was found that the crystallinity of cellulose affects the accessibility of phosphoric acid, which indirectly affects the yield of LGO: a more ordered supramolecular structure of cellulose should be chosen as the feedstock if a high LGO yield is to be pursued [14,15,16]. Pyrolysis of cellulose using microwave radiation can also generate LGO, but the yield of 7.5% is lower than the traditional pyrolysis method [17]. It was previously found that the addition of iron to the pretreatment would lead to ions entering the cellulose by ion exchange or adsorption, thus promoting depolymerisation and dehydration reactions [15], leading to a further increase in the yield of LGO. Kudo et al. pretreated the cellulose in this way and reacted it using 1-butyl-2,3-dimethylimidazolium triflate ionic liquid, which is thermally stable and strongly catalytic obtaining a yield of 22 wt%, which is higher than that obtained by using pyrolysis after conventional acid impregnation [18]. Zandersons et al. used birch and pine as pyrolysis feedstocks for LGO production and found that elevated lignin content and total or partial elimination of hemicellulose contributed to the selective generation of LGO using phosphoric acid catalysis at 375 °C [19]. Pyrolysis of birch and pine wood using aqueous phosphoric acid at concentrations of 3.7 wt% and 3 wt%, respectively, as a catalyst yielded the highest LGO yields of 21.8% and 29%, which, when converted to cellulose as a feedstock, was found to be more than 55% using pine as a feedstock. Hydrothermal treatment of the feedstock followed by impregnation can increase the yield of LGO. Analytical pyrolysis of birch wood impregnated with 5 per cent phosphoric acid after hydrothermal treatment at 180 °C at 350 °C resulted in an LGO yield of 21.08% [20]. A lignin-rich biorefinery waste stream can also be used as a feedstock for the production of LGO. Selectivity to LGO is over 90%. Microwave-assisted pyrolysis using sulphuric acid as a catalyst at 180 °C only gave LGO yields up to 8 wt% [21]. The hemicellulose of barley straw was removed using microwave-assisted acid hydrolysis, followed by a combination of microwave-assisted pyrolysis and steam distillation of the wet samples to obtain the LGO [22]. Acid concentration was the most important factor affecting the distribution of the products, with LGO and LGA being the main products at low acid concentrations and furfural and levulinic acid being the main products at high acid concentrations. In situ vaporisation of water produces a microwave-transparent vapour environment that prevents further degradation of the distillation products.
1.1.2. Indirect Generation of LGO Using Solid Catalysts
In catalytic pyrolysis using solid catalysts, the solid catalysts do not act directly on the cellulose feedstock but further the catalytic pyrolysis of the initial pyrolysis products, such as LGA [9]. The advantage of using solid catalysts compared with acid impregnation methods lies in the fact that yields are much higher, and also the catalysts can be recovered for reuse. In the process of selective generation of LGO, two indicators of the solid catalyst have the greatest influence on the yield, the acidity and the porosity [23]. In 2011, Wang et al. used sulfated zirconia as a catalyst for the rapid pyrolysis of cellulose for the preparation of LGO, and the highest yield of 8.14% was attained at 335 °C with a ratio of SO42−/ZrO2 of 3:3 [24]. The recycled catalyst still maintained the parent structure after recovery, but the activity decreased due to the leaching of SO42−. Wei et al. similarly used SO42−/ZrO2 and obtained a 7.25% yield of LGO: the pore size of the prepared catalysts was 10/40 nm. They also compared the effect of HZSM-5, ZrO2, TiO2 and Al2O3 as catalysts for the rapid pyrolysis of cellulose to generate LGO. The calcined sulphuric acid catalysts compared with uncalcined catalysts impregnated with sulphuric acid activity increased significantly [25]. Lu et al. prepared magnetic super acid (SO42−/TiO2-Fe3O4) for the catalytic fast pyrolysis of cellulose and poplar wood. The LGO yields of cellulose and poplar wood as feedstock were 15.43 wt% and 7.06 wt% at 300 °C with a 1:1 catalyst feedstock, respectively. Compared with the non-magnetic solid catalysts and phosphoric acid and sulphuric acid, the selective catalytic effect on LGO was better. And it was also found that the LGO yield from poplar wood was comparable to that from direct cellulose as feedstock, which indicated that other components in poplar wood (hemicellulose, lignin, etc.) had little effect on the inhibition of LGO pyrolysis formation [26]. Zhang et al. prepared solid phosphoric acid (SPA) catalysts with different carriers for the catalytic fast pyrolysis of poplar wood for the production of LGO. The highest LGO yield of 8.2 wt% (16.1 wt% when cellulose was used as a feedstock) was obtained at 300 °C with a biomass-to-catalyst ratio of 1 for the catalysts prepared based on SBA-15, which was higher than that when using phosphoric acid [27]. When Al was loaded on the ordered mesoporous catalyst MCM-41 by Casoni et al., the LGO yield reached 53 wt% using cellulose as the feedstock at 400 °C [23]. Characterisation of different catalysts in the literature and comparison in combination with yields revealed that mesoporous catalysts enable free diffusion of cellulose primary pyrolysis molecules compared with catalysts with larger pore volumes and pore sizes, resulting in higher bio-oil yield. Catalysts with smaller pore sizes slowed down the diffusion of the generated anhydrous sugars into the gas phase, leading to re-polymerisation and accompanying char formation, so the 3.8 nm pore size Al- MCM-41 presented the best selectivity. Li et al. prepared the Ni-P-MCM-41 catalysts at 350 °C with Ni/P at 5 and catalyst/feedstock at 3 [28]. The yield reached 27.34 wt% for cellulose as feedstock and up to 14.30 wt% when pine wood was used as feedstock. Although MCM-41 catalysts have shown excellent yields in laboratory-scale processes, it is difficult to achieve mass production due to their instability. The advantage of phosphorus-molybdenum-tin mixed metal oxide (P-Mo/SnO2) as a catalyst for the selective generation of LGO lies in its reusability, with a maximum LGO yield of 17.98% using P-Mo/SnO2 as a catalyst, and the yield of LGO remained above 10 wt% after five regenerations [29].
1.1.3. Indirect Generation of LGO by Pyrolysis in Liquid Phase
When cellulose is reacted in the liquid phase of aprotic solvent at high pressure, there are two main paths: one is in the presence of water in the system, where cellulose undergoes hydrolysis, with 5-HMF being the main product, and the second is in the absence of water, where cellulose undergoes pyrolysis to produce LGA, which is then converted to LGO [30]. Cellulose in sulfuric acid or phosphoric acid within a cyclic sulfone solution can also be used to obtain LGO. The further conversion of LGO to furfural requires water, so the removal of water generated under mild vacuum conditions can significantly improve LGO yield. Further conversion of LGO to furfural requires water, so removal of the resulting water under mild vacuum conditions can significantly increase the yield of LGO, which reached 38% using phosphoric acid as a catalyst for 2.5 min at 280 °C [31]. The use of GVL and THF as solvents also produced LGO selectively, but GVL promoted the conversion of LGO to HMF and LGO was degraded in GVL. LGO is stable in THF. The pyrolysis pathway of cellulose in THF is shown in Figure 3. A 51% yield of LGO was achieved after 30 min at 210 °C using THF sulphate solution as a catalyst [30]. Using high voltage alternating current (HVAC) as a plasma source in a polar aprotic solvent without external heating, in situ-generated hydrogen radicals contribute to cellulose depolymerisation and intermediate dehydration to form LGO. A 43% yield of levoglucosanone was obtained in GVL after 15 min of conversion using a voltage of 6 kV and a frequency of 6 kHz and in sulfone after 7 min using a voltage of 3.5 kV 40% yield. This method can be carried out at lower energy consumption compared with previous methods [32]. The preparation methods and associated yields of LGO are shown in Table 1.
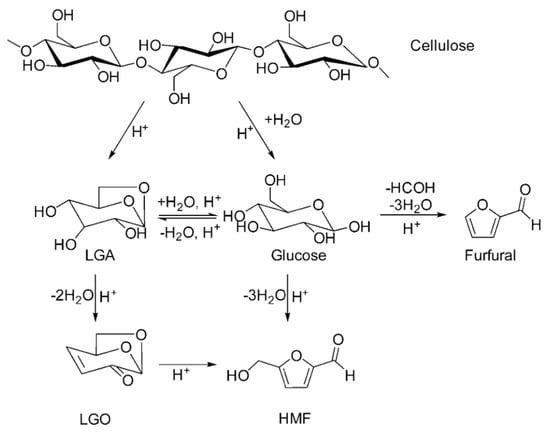
Figure 3. Cellulose pyrolysis pathway in THF [30].
Table 1. Methods for the preparation of LGO.
| Entry | Materials | Catalyst | Conditions | Reactor | Yield | Ref |
|---|---|---|---|---|---|---|
| 1 | Cellulose | NaHSO4 | Slow pyrolysis at 300 °C | Horizontal tube reactor | 6.9% | [12] |
| 2 | Cellulose | 5.4 wt% H3PO4 | Slow pyrolysis at 350 °C | vertical flow reactor | 22.3% | [13] |
| 3 | Cellulose | 2 wt% H3PO4 | Fast pyrolysis at 500 °C | CDS Pyroprobe 100 | 34% | [15] |
| 4 | Newsprint | 1 wt% H3PO4 | Fast pyrolysis at 500 °C | CDS Pyroprobe 100 | 21% | [15] |
| 5 | Kraft pulp | 2 wt% H3PO4 | Fast pyrolysis at 500 °C | CDS Pyroprobe 100 | 19% | [15] |
| 6 | Birchwood | 2.5 wt% H3PO4 | Fast pyrolysis at 500 °C | CDS Pyroprobe 100 | 17% | [15] |
| 7 | Cellulose | Ionic liquids | Slow pyrolysis at 300 °C | Horizontal reactor | 38.2% | [18] |
| 8 | Cellulose | SO42−/ZrO2 | Fast pyrolysis at 335 °C | Vertical reactor | 8.14% | [24] |
| 9 | Cellulose | HZSM-5 | Fast pyrolysis at 335 °C | Vertical reactor | 1.7% | [24] |
| 10 | Cellulose | TiO2 | Fast pyrolysis at 335 °C | Vertical reactor | 5% | [24] |
| 11 | Cellulose | SO42−/TiO2-Fe3O4 | Fast pyrolysis at 300 °C | CDS Pyroprobe 5200HP pyrolyser | 15.43% | [26] |
| 12 | Poplar wood | SO42−/TiO2-Fe3O4 | Fast pyrolysis at 300 °C | CDS Pyroprobe 5200HP pyrolyser | 7.06% | [26] |
| 13 | Cellulose | SPA | Fast pyrolysis at 280 °C | CDS Pyroprobe 5200HP pyrolyser | 16.1% | [27] |
| 14 | Cellulose | Al-MCM-41 | Fast pyrolysis at 400 °C | Vertical reactor | 53% | [23] |
| 15 | Cellulose | Ni-P-MCM-41 | Fast pyrolysis at 350 °C | Vertical reactor | 21.4% | [28] |
| 16 | Pine wood | Ni-P-MCM-41 | Fast pyrolysis at 350 °C | Vertical reactor | 10.7% | [28] |
| 17 | Cellulose | P-Mo/SnO2 | Fast pyrolysis at 300 °C | Micropyrolyser | 18% | [29] |
| 18 | Cellulose | P-Mo/SnO2 | Fast pyrolysis at 300 °C | Vertical reactor | 12.7% | [29] |
| 19 | Cellulose | 1 wt% H3PO4 | In sulfolane at 200–280 °C | Round flask | 38% | [31] |
| 20 | Cellulose | 20 mM H2SO4 | In THF at 210 °C | Hastelloy autoclave | 51% | [30] |
| 21 | Cellulose | 7 mM H2SO4 | Plasma electrolysis in GVL at 160 °C | Round-bottom flask | 43% | [32] |
| 22 | Brichwood | 5 wt% H3PO4 | Analytical pyrolysis at 350 °C | Micro Double-shot Pyrolyser | 21.08% | [20] |
| 23 | Crude waste softwood hydrolysis lignin | H2SO4 | Microwave-assisted pyrolysis at 180 °C | CEM ‘Discover’ MW generator | 8% | [21] |
2. Catalytic Hydrogenation of LGO to Produce Cyrene
Table 2 shows the method and yield for the preparation of Cyrene from LGO. In 2014, the Green Chemistry Centre of Excellence produced and named Cyrene as a solvent, and their catalytic hydrogenation of LGO was achieved under both low- and high-pressure conditions [1]. At low pressure, LGO is dissolved in ethyl acetate with 10 wt% Pd/C catalyst, and hydrogen is applied with a special Sigma Aldrich balloon for 96 h (8 days without solvent) to obtain Cyrene. At high pressure, the same solvent and catalyst are used to obtain Cyrene at room temperature for 2–48 h at pressures ranging from 3–80 bar. The reaction can be similarly completed in 2 h at 80 bar pressure without the addition of solvent [1]. Formic acid is a promising material for hydrogen storage and can be used as a substitute for hydrogen as a hydrogen source in the catalytic hydrogenation reaction of LGO [33]. In the paper, different catalysts (Ru/C, Rh/C, Pt/C, Pd/C, Ni) were also compared with different solvents (formic acid, cyclohexane, dimethylacetamide (DMA), 1,4-dioxane, 2-propanol and tetrahydrofuran), and it was finally found that the combination of THF and Pd/C showed the best performance in LGO hydrogenation, which could produce more than 99% yield at 66 °C. Pd/t-ZrO2 (tetragonal ZrO2) has also been shown to have good catalytic performance, with a very low catalyst loading (~3 wt%) and the use of water as a solvent, achieving 95% yields of Cyrene. Reusability is also a key feature, with 90% Cyrene yield being achieved after a single use and thorough washing [34]. The conversion of LGO to Cyrene using wild-type Old Yellow Enzyme 2.6 (OYE 2.6 Tyr78Tt) from Pichia yeast and its mutant (OYE 2.6 Tyr78Trp) was 99%, and the biocatalysis through the enzymatic process of enzyme olefin reductase is low-toxicity, environmentally friendly and green [35]. In the conversion process of LGO to Cyrene, taken together, the yields can basically reach more than 99%. In summary, when using chemical catalysis, Pd/C as a catalyst and THF as a solvent = more than 99% = Cyrene can be achieved. For the hydrogen source, it is recommended to choose the more promising and greener formic acid, taking into account the toxicity of THF. We can also use non-toxic TMO as a solvent to replace THF [36].
Table 2. Methods for the preparation of Cyrene.
| Entry | Catalyst | Solvent | Conditions | Reactor | Yield | Ref |
|---|---|---|---|---|---|---|
| 1 | 10% Pd/C | / | 1.1 atm H2, 8 days | / | >90% | [1] |
| 2 | 10% Pd/C | EtOAc | 1.1 atm H2, 96 h | / | >90% | [1] |
| 3 | 10% Pd/C | EtOAc | 3 to 80 bar, 2 to 48 h | High-pressure reactor | >90% | [1] |
| 4 | 10% Pd/C | / | 80 bar, less than 2 h | High-pressure reactor | >90% | [1] |
| 5 | 5% Pd/C | THF | Stirring at 66 °C, 2 h | Autoclave | >99% | [33] |
| 6 | Pd/t-ZrO2 | Water | 10 bar H2, 80 °C | Batch glass micro-reactor | >99% | [34] |
| 7 | OYE 2.6 Tyr78Trp | EtOH | Room temperature | / | >99% | [37] |
3. Furacell Process
Circa Group has achieved the large-scale mass production of Cyrene using the Furacell process. In this process (Figure 4) [37,38,39], the raw material is pine sawdust, the solvent is sulfone, and the catalyst is phosphoric acid. The pine sawdust was impregnated with a solution of sulfone containing phosphoric acid, and after mixing, it was put into a pyrolyser and heated rapidly to 330–350 °C under reduced pressure to separate the volatile products from the solid products and then separated the high purity LGO by several steps of distillation. Subsequently, compressed hydrogen is added to hydrogenate the LGO with a solid catalyst to obtain Cyrene. The purity of Cyrene generally depends on the purity of the separated LGO due to the 99% selectivity of the hydrogenation process. In this process, the separated biochar can be used as a fuel to provide energy for the pyrolysis process and can likewise be recycled as a by-product or sold as a fuel. Sulfone separated from LGO can be recycled many times, and the wastewater contains only water, acetic acid and furfural. Sulpholane can swell the cellulose so that the phosphoric acid can better penetrate into the cellulose, improving the process, although the recent classification by the EU of sulpholane as a suspected reproductive toxin makes its continued use problematic.
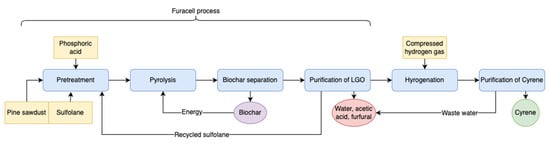
Figure 4. Potential pathways from LGA to LGO when pyrolysis in solvent.
This entry is adapted from the peer-reviewed paper 10.3390/chemistry5040154
This entry is offline, you can click here to edit this entry!
