Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Biomimicry can take lessons regarding the secret laws that govern the perfect machines of our biological systems. Biomimetic materials are developed, thus, to emulate and replicate one or more attributes of a living organism, to restore a natural function, or to sustain an environment in terms of chemistry, processing, and structure of materials.
- biomimetics
- biocompatibility
- tissue engineering
- mechanical properties
- biomimetic hydrogels
1. Introduction
Can science imitate life? Nature has always inspired researchers to assess principles for the design of sophisticated models and architectures of human life [1]. During 3.8 billion years, evolution has refined living organisms, systems, materials, and processes to create the most efficient arrays of products [2]. Therefore, biomimicry can take lessons regarding the secret laws that govern the perfect machines of our biological systems. Biomimetic materials are developed, thus, to emulate and replicate one or more attributes of a living organism, to restore a natural function, or to sustain an environment in terms of chemistry, processing, and structure of materials [3]. Biomimetic science is not restricted to biomaterials engineering but instead, is referred to a wide variety of applications. Functional biomimicry, process biomimicry, molecular biomimicry, and structure biomimicry identify the context of the benefit. Restoring and maintaining the normal functions of a damaged tissue or organ is the aim of modern tissue engineering. Nevertheless, due to its complexity, this field involves the knowledge of multidisciplinary areas, including chemistry, physics, materials science, biology, medicine, bioengineering, and biotechnology [4]. An enormous clinical need exists for the development of biomaterials to provide structural support in terms of mechanical properties and tissue growth (i.e., cell attachment) and proliferation, to present biologically active signals (e.g., growth factors), and to allow in vivo cell migration in a transplanted organ. The key element of the rationale for building an efficient and usable biomimetic material lies in the emulation of the extracellular matrix (ECM) and the related local microenvironment. Due to this, ideal materials for biological use resembling ECM microenvironments are continuously studied. Despite significant progress in bioengineering, clinical translation faced limitations due to the inevitable variability in patients. To achieve the required goals, scientists must consider minimizing the invasive techniques, as well as involve the use of cell populations and bioactive factors and to develop tailor-made and patient-specific biomaterials [5]. The important role of mechanical properties, as a key point for the design of tissue-engineering constructs, demands strengthened attention, and very little success has been achieved [6]. Hydrogels, 3D-polymeric substances, can act as a scaffold and can the mimic properties of ECM and tissues [7]. Their programmable reactivity to specific stimuli (pH, temperature, ionic strength, electromagnetic field, and light) make hydrogels suitable for the development of biomimetics. In the class of hydrogels, self-assembling peptides (SAPs) hold a large space in tissue engineering and biomedical applications [8].
2. Rationale for Biomimetic Materials Design
2.1. The Role of Mechanical Properties
The studies on biological systems considered as engineering structures date back to the 1970s with works from D.W. Thompson [9] and J.D. Currey [10]. In many biological systems, it is possible to find mechanical properties that are hard to identically reproduce in synthetic materials [11]. This is quite curious considering that biological architectures are made of polymers with simple elements, such as C, N, H, and O.
Mechanical properties of the tissues, as of all other materials, are characterized by the relationship between the force applied to the material and the related extension, measured in terms of resistance to deformation and shape changes. The elastic modulus (E, or Young’s modulus) represents the stiffness of a material, and it is the slope of the stress–strain curve [12]. A stiffer material (higher elastic modulus, i.e., bones) is less deformable than one characterized by a lower elastic modulus (i.e., soft biological tissues, such as skin, cartilage, and heart valves) [13]. Table 1 lists measured elastic moduli of tissues and materials, which can be obtained mainly using atomic force microscopy (AFM) and rheological experiments (Figure 1) [14].
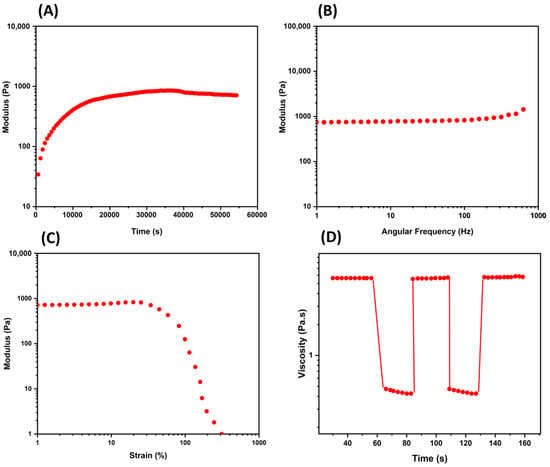
Figure 1. Mechanical property characterization using common rheological experiments. (A) The time sweep test (oscillatory) provides information regarding the change in a hydrogel over the time at constant oscillation; (B) the frequency sweep test (oscillatory) relates the frequency and the storage and loss moduli in a range of frequency; (C) the strain sweep test is conducted in an increasing oscillatory strain at constant frequency to examine the linear viscoelastic region (Newtonian behavior); (D) a thixotropy graph is used to study non-Newtonian behavior with continuous oscillatory strains and indicating the self-healing of a hydrogel. Other rheological evaluations are given by temperature ramps, creep and recovery tests, and hysteresis loops.
Table 1. Elastic moduli, E, of tissues and materials.
| Tissue | Young’s Elastic Modulus | Reference |
|---|---|---|
| Human epithelial cells (normal) | 1.60 kPa | [14] |
| Human epithelial cells (cancer) | 1.40 kPa | [14] |
| Human platelets | 1–50 kPa | [15] |
| Kidney | 180 kPa | [16] |
| Skin (different species) | 20–40 Mpa | [17] |
| Muscle | 480 Mpa | [18] |
| Tendon | 43–1660 Mpa | [19] |
| Elastin-free tendon | 1.2 Gpa | [20] |
| Cartilage | 100–500 kPa | [21] |
| Human proximal tibia | 11–14 Gpa | [22] |
| Biomaterial | Young’s Elastic Modulus | Ref. |
| Gelatin (Ge) 1 | 0 Pa < E < 75 kPa | [23] |
| Collagen mimetic peptide | ~8 kPa | [24] |
| SAPs | 0.1 kPa < E < 10 kPa | [25] |
| Hyaluronic acid (HA) hydrogel 2 | 1 kPa | [26] |
| HA-Ge | 22 kPa | [27] |
| Fmoc-Phe-Phe-OH | 200 kPa | [28] |
| Alginate hydrogel 3 | 117 kPa | [29] |
| Cross-SAPs | 200 kPa < E < 850 kPa | [25][30] |
| Chitosan | ~ 7 Mpa | [31] |
| Amphiphile polymer conetworks | 5 Mpa < E < 200 Mpa | [32] |
| H-Phe-Phe-OH nanotubes | 20 Gpa | [28] |
| Gelatin methacryloyl (GelMA) | 3 kPa < E < 184 kPa | [33] |
| Collagen type I (in rat) | 5–11.5 Gpa | [34] |
| Collagen (mammalian tendon) | 1.2 Gpa | [12] |
1 Measured at 20 °C (gelatin is very sensitive to the temperature); 0 Pa means that the solution is simply liquid at low concentration. 2 HA crosslinked with 5% w/t 1,4-butanediol diglycidyl ether (BDDE). 3 Alginate hydrogel crosslinked with Ca2+.
Above all, human tissues are elastic: this irreplaceable property is given by elastin [35], an ECM protein that confers not only extendibility and resiliency but also some sort of resistance [36]. Human native elastin biosynthesis takes place during the 17th week of pregnancy and continues into the early neonatal stage [37]. During its long half-life (about 70 years), elastin can be extended and relaxed billions of times without losing its functions [38]. After an injury, elastin is produced under the influence of exogen factors (i.e., tumor necrosis factor-a, interleukin 1b, insulin-like growth factor 1, and transforming growth factor), but the resulting synthesized elastin is not the same as the native as it appears more disorganized [37].
The never-ending quest for the elastomeric property is pushing researchers to confer this physical performance to polypeptide sequences in order to emulate living systems, and a vast number of studies in the literature agree on the potential use of elastin-like peptides (ELPs) for biomedical applications [21][39][40][41].
An important property of elastin fibers is extendibility, which consists of the ability to become more than twice as long before rupture and to return to the original dimensions when tension is released. On the other hand, elastic hysteresis (repetitive cyclic loading) occurs with a viscoelastic material. A more resilient elastomeric tissue will exhibit a lower hysteresis [12]. Collagen, the structural protein found in connective tissue, is the second element of elastic tissues. While elastin provides elasticity, collagen fibers confer strength and structural support. Its biological function is related to its mechanical properties [34]. Despite collagen being extensively characterized, a question regarding the comprehension of structural models for fibrils and fibers remains open.
Peptide-based materials were designed to mimic the properties of elastin and collagen, so they can be used in regenerative medicine and tissue engineering applications [21][42]. As they are formed of amino acid residues, they possess the considerable advantages of low immunogenicity and biodegradability in non-toxic metabolites [43].
ELPs are polymers made on the sequence of tropoelastin, the precursor of elastin, which contains alternating hydrophobic and hydrophilic residues [21]. ELP sequences are usually composed of hydrophobic repeats of Val-Pro-Gly-X-Gly (where X is any amino acid except Pro). They are faced with thermal responsivity, resulting in being soluble below a characteristic transition temperature [44]. An interesting point is that ELPs can be fine-tuned on the basis of requested physical properties, chemical reactivity, self-assembly behavior, and biological activity; thus, they can be conjugated with drugs, ligands, imaging agents, and crosslinkers. It was reported that ELPs support the formation of ECM in articular cartilage by other cell types and in different cell culture conditions, such as with chondrocyte differentiation of human adipose-derived adult stem (hADAS) cells [45]. Due to the poor mechanical properties of non-crosslinked ELPs compared with cartilage, which make ELPs unsuitable for implantation in a cartilage defect, crosslinked ELPs were designed to provide stiffer materials. Trabbic-Carlson et al. produced a genetically engineered ELP with Val-Pro-Gly-X-Gly repeats, where X was Val or Lys (every 7 or 17 pentapeptides) [46]. Then, they chemically crosslinked this ELP with tris-succinimidyl aminotriacetate, affording a hydrogel with a stiffness of 1.6 to 15 kPa at 37 °C (based on ELP concentration and Lys content). In 2007, another work demonstrated that Lys-rich ELPs can quickly form hydrogel thanks to the crosslinking with β-[tris(hydroxymethyl)phosphino]propionic acid (THPP) under physiological conditions [47]. In rabbits, genipin was crosslinked with an ELP before implantation to provide a stiff gel with similar aggregation to that of cartilage [48]. In 2018, Mozhdehi and co-workers developed an ELP functionalized with a C14 fatty-acid molecule in a post-translational modification [49]. The resulting hybrid biomaterial exhibited temperature-triggered hierarchical self-assembly with tuning properties typical of amphiphilic peptides. Further and extensive ELP modifications are described by Varanko et al. in an excellent review [21].
Improvements in the stiffness of materials for biomedical applications can be achieved with the use of chemical or physical crosslinkers [25][30][50][51][52][53][54][55] as well as enzymatic techniques [56]. Nevertheless, their application is sometimes limited due to the toxicity of the crosslinker. Drawing inspiration from natural bone and tissues, in 2022, Gelain and co-workers developed a double crosslinking based on 4-(N-Maleimidomethyl) cyclohexane-1-carboxylic acid 3-sulpho-N-hydroxysuccinimide ester (Sulfo-SMCC) and genipin with cysteine and lysine-rich LKLK12 SAPs [25]. The resulting hydrogels reached values higher than 800 kPa, an impressive achievement in the field of SAPs. Recently, Ciulla et al. reported a sustainable and cell-compatible crosslinking of SAPs with the use of low-power microwave (MW) irradiation, reaching modest to high values of stiffness and viscoelasticity [57]. Despite some steps forward that have been made, significant efforts are yet necessary to reach stiff and viscoelastic biomaterials.
2.2. Extracellular Matrix Mimicry
An artificial biomimetic scaffold for tissue engineering should provide properties of the natural ECM in order to allow cell regeneration and support cell adhesion, proliferation, differentiation, and neo-tissue formation [58][59][60][61]. Cell adhesion is a prerequisite for the regulation of fundamental cellular processes. Hence, it is a crucial task to control the adhesion of cells on biomaterials, which can be achieved with surface modification as the only intimate contact with the biological environment. As a dynamic component of all tissues, the desired ECM-like scaffold architecture should have a 3D geometry with a specific porosity necessary for migration and cell proliferation, as well as for nutrition and degradation product transition. The ability of a tissue’s mechanical strength depends, above all, on the matrix composition related to tissue function [12]. Vascularization and perfusion are other fundamental prerequisites for cell engraftment. In this regard, endothelial and vascular endothelial growth factor (VEGF) may be added to the new scaffold.
On the other hand, the assembly of tailored fibrous structural proteins is wired by the polymerization of ECM monomers into fibers (fibrils in the case of collagen) [62]. Fibrillar collagens assemble from triple helices of tropocollagen in fibrils (10–300 nm diameter) and then in fibers (1–20 µm). The tensile strength of tissues depends on a peculiar arrangement of fibrils, formed by different types of collagens in a single fibril [62]. In tendons, for example, fibers assemble into bundles (~500 µm). The interaction between fibrils and microenvironments is dominated by the dense heterogeneity in other specific macromolecules, including proteoglycans (i.e., hyaluronic acid and glycosaminoglycans), amino acids (mainly Pro and Gly), and elastin. As described by the Hodge and Petruska model [63], individual collagen monomers are aligned in a fibril, followed by a pseudo-periodicity made of a characteristic overlap gap. Collagen distribution provides strength, yielding bio-mechanical requirements typical of a tissue [64]: while collagen type I confers stiffness, collagen type III is more flexible. In summary, extracellular networks dictate the rules for the rational design of a resilient biomimetic material. ECM composition and assembly allow tissues to comply with different and sophisticated biological functions. Abune et al. recently reported the development of a dual aptamer-functionalized hydrogel able to bind cells and sequester growth factors with the use of a vascular endothelial growth factor binding aptamer (VEGF aptamer) and a c-MET receptor binding aptamer (c-MET aptamer), providing a synergistic effect on cell survival and proliferation [65].
In bioartificial biomimetic materials, several strategies can be adopted to build a scaffold able to direct cell responses, including the incorporation of cell binding motifs (e.g., RGD motif), the functionalization with polysaccharides (to enhance cell-material interactions), and the incorporation of decellularized ECM into the scaffold (to mimic a very close microenvironment to natural niches).
This entry is adapted from the peer-reviewed paper 10.3390/gels9100833
References
- Naik, R.R.; Singamaneni, S. Introduction: Bioinspired and Biomimetic Materials. Chem. Rev. 2017, 117, 12581–12583.
- Rowley, T. Science imitates life. Lab Anim. 2013, 42, 271–272.
- Glaser, D.E.; Viney, C. Biomimetic Materials. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 349–360.
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering Complex Tissues. Sci. Transl. Med. 2012, 4, 160rv12.
- Lavik, E.B.; Zheng, G. (Eds.) Biomimetic Materials. Bioconjug. Chem. 2018, 29, 825.
- Liebschner, M.; Bucklen, B.; Wettergreen, M. Mechanical Aspects of Tissue Engineering. Semin. Plast. Surg. 2005, 19, 217–228.
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323.
- Koutsopoulos, S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: Progress, design guidelines, and applications. J. Biomed. Mater. Res. Part A 2016, 104, 1002–1016.
- Thomson, J.A. On Growth and Form, 2nd ed.; Cambridge University Press: Cambridge, UK, 1968.
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 9780691128047.
- Vincent, J.F.V. Structural Biomaterials; Princeton University Press: Princeton, NJ, USA; Available online: https://press.princeton.edu/books/paperback/9780691154008/structural-biomaterials (accessed on 29 July 2012).
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 866–875.
- Coenen, A.M.J.; Bernaerts, K.V.; Harings, J.A.W.; Jockenhoevel, S.; Ghazanfari, S. Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers. Acta Biomater. 2018, 79, 60–82.
- Guz, N.; Dokukin, M.; Kalaparthi, V.; Sokolov, I. If Cell Mechanics Can Be Described by Elastic Modulus: Study of Different Models and Probes Used in Indentation Experiments. Biophys. J. 2014, 107, 564–575.
- Radmacher, M.; Fritz, M.; Kacher, C.M.; Cleveland, J.P.; Hansma, P.K. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 1996, 70, 556–567.
- Karimi, A.; Shojaei, A. Measurement of the Mechanical Properties of the Human Kidney. IRBM 2017, 38, 292–297.
- Vogel, H. Influence of maturation and aging on mechanical and biochemical properties of connective tissue in rats. Mech. Ageing Dev. 1980, 14, 283–292.
- Buchanan, C.I.; Marsh, R.L. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J. Appl. Physiol. 2001, 90, 164–171.
- Wren, T.A.; Yerby, S.A.; Beaupré, G.S.; Carter, D.R. Mechanical properties of the human achilles tendon. Clin. Biomech. 2001, 16, 245–251.
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological roles and mechanical properties. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002, 357, 121–132.
- Varanko, A.K.; Su, J.C.; Chilkoti, A. Elastin-Like Polypeptides for Biomedical Applications. Annu. Rev. Biomed. Eng. 2020, 22, 343–369.
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s modulus of trabecular bone at the tissue level: A review. Acta Biomater. 2018, 78, 1–12.
- Taberlet, N.; Ferrand, J.; Camus, É.; Lachaud, L.; Plihon, N. How tall can gelatin towers be? An introduction to elasticity and buckling. Am. J. Phys. 2017, 85, 908–914.
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.-P.; Horejs, C.-M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.M.; Stevens, M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225.
- Ciulla, M.G.; Pugliese, R.; Gelain, F. Boosted Cross-Linking and Characterization of High-Performing Self-Assembling Peptides. Nanomaterials 2022, 12, 320.
- Kaya, G.; Oytun, F. Rheological Properties of İnjectable Hyaluronic Acid Hydrogels for Soft Tissue Engineering Applications. Biointerface Res. Appl. Chem. 2020, 11, 8424–8430.
- Heris, H.K.; Rahmat, M.; Mongeau, L. Characterization of a Hierarchical Network of Hyaluronic Acid/Gelatin Composite for use as a Smart Injectable Biomaterial. Macromol. Biosci. 2012, 12, 202–210.
- Adler-Abramovich, L.; Gazit, E. The physical properties of supramolecular peptide assemblies: From building block association to technological applications. Chem. Soc. Rev. 2014, 43, 6881–6893.
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012.
- Pugliese, R.; Marchini, A.; Saracino, G.A.A.; Zuckermann, R.N.; Gelain, F. Cross-linked self-assembling peptide scaffolds. Nano Res. 2018, 11, 586–602.
- Le, H.R.; Qu, S.; Mackay, R.E.; Rothwell, R. Fabrication and mechanical properties of chitosan composite membrane containing hydroxyapatite particles. J. Adv. Ceram. 2012, 1, 66–71.
- Velasquez, S.T.R.; Jang, D.; Jenkins, P.; Liu, P.; Yang, L.; Korley, L.T.J.; Bruns, N. Peptide-Reinforced Amphiphilic Polymer Conetworks. Adv. Funct. Mater. 2022, 32, 2207317.
- Wu, Y.; Xiang, Y.; Fang, J.; Li, X.; Lin, Z.; Dai, G.; Yin, J.; Wei, P.; Zhang, D. The influence of the stiffness of GelMA substrate on the outgrowth of PC12 cells. Biosci. Rep. 2019, 39, BSR20181748.
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical Properties of Collagen Fibrils. Biophys. J. 2007, 93, 1255–1263.
- Hoeve, C.A.J.; Flory, P.J. The elastic properties of elastin. Biopolymers 1974, 13, 677–686.
- Trębacz, H.; Barzycka, A. Mechanical Properties and Functions of Elastin: An Overview. Biomolecules 2023, 13, 574.
- Saitow, C.B.; Wise, S.G.; Weiss, A.S.; Castellot, J.J.; Kaplan, D.L. Elastin biology and tissue engineering with adult cells. Biomol. Concepts 2013, 4, 173–185.
- Daamen, W.; Veerkamp, J.; Vanhest, J.; Vankuppevelt, T. Elastin as a biomaterial for tissue engineering. Biomaterials 2007, 28, 4378–4398.
- Aaron, B.B.; Gosline, J.M. Elastin as a random-network elastomer: A mechanical and optical analysis of single elastin fibers. Biopolymers 1981, 20, 1247–1260.
- Debelle, L.; Tamburro, A.M. Elastin: Molecular description and function. Int. J. Biochem. Cell Biol. 1999, 31, 261–272.
- Li, B.; Alonso, D.O.V.; Bennion, B.J.; Daggett, V. Hydrophobic Hydration Is an Important Source of Elasticity in Elastin-Based Biopolymers. J. Am. Chem. Soc. 2001, 123, 11991–11998.
- Luo, T.; Kiick, K.L. Collagen-like peptides and peptide–polymer conjugates in the design of assembled materials. Eur. Polym. J. 2013, 49, 2998–3009.
- Ciulla, M.G.; Civera, M.; Sattin, S.; Kumar, K. Nature-inspired and medicinally relevant short peptides. Explor. Drug Sci. 2023, 1, 140–171.
- Zhao, B.; Li, N.K.; Yingling, Y.G.; Hall, C.K. LCST Behavior is Manifested in a Single Molecule: Elastin-Like polypeptide (VPGVG) n. Biomacromolecules 2016, 17, 111–118.
- Betre, H.; Ong, S.R.; Guilak, F.; Chilkoti, A.; Fermor, B.; Setton, L.A. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials 2006, 27, 91–99.
- Trabbic-Carlson, K.; Setton, L.A.; Chilkoti, A. Swelling and Mechanical Behaviors of Chemically Cross-Linked Hydrogels of Elastin-like Polypeptides. Biomacromolecules 2003, 4, 572–580.
- Lim, D.W.; Nettles, D.L.; Setton, L.A.; Chilkoti, A. Rapid Cross-Linking of Elastin-like Polypeptides with (Hydroxymethyl)phosphines in Aqueous Solution. Biomacromolecules 2007, 8, 1463–1470.
- Hrabchak, C.; Rouleau, J.; Moss, I.; Woodhouse, K.; Akens, M.; Bellingham, C.; Keeley, F.; Dennis, M.; Yee, A. Assessment of biocompatibility and initial evaluation of genipin cross-linked elastin-like polypeptides in the treatment of an osteochondral knee defect in rabbits. Acta Biomater. 2010, 6, 2108–2115.
- Mozhdehi, D.; Luginbuhl, K.M.; Simon, J.R.; Dzuricky, M.; Berger, R.; Varol, H.S.; Huang, F.C.; Buehne, K.L.; Mayne, N.R.; Weitzhandler, I.; et al. Genetically encoded lipid–polypeptide hybrid biomaterials that exhibit temperature-triggered hierarchical self-assembly. Nat. Chem. 2018, 10, 496–505.
- Pugliese, R.; Maleki, M.; Zuckermann, R.N.; Gelain, F. Self-assembling peptides cross-linked with genipin: Resilient hydrogels and self-standing electrospun scaffolds for tissue engineering applications. Biomater. Sci. 2019, 7, 76–91.
- Pugliese, R.; Fontana, F.; Marchini, A.; Gelain, F. Branched peptides integrate into self-assembled nanostructures and enhance biomechanics of peptidic hydrogels. Acta Biomater. 2018, 66, 258–271.
- Pugliese, R.; Montuori, M.; Gelain, F. Bioinspired photo-crosslinkable self-assembling peptides with pH-switchable “on–off” luminescence. Nanoscale Adv. 2022, 4, 447–456.
- Chronopoulou, L.; Margheritelli, S.; Toumia, Y.; Paradossi, G.; Bordi, F.; Sennato, S.; Palocci, C. Biosynthesis and Characterization of Cross-Linked Fmoc Peptide-Based Hydrogels for Drug Delivery Applications. Gels 2015, 1, 179–193.
- Chronopoulou, L.; Toumia, Y.; Cerroni, B.; Pandolfi, D.; Paradossi, G.; Palocci, C. Biofabrication of genipin-crosslinked peptide hydrogels and their use in the controlled delivery of naproxen. New Biotechnol. 2017, 37, 138–143.
- Pugliese, R. Supramolecular-Covalent Peptides Self-Assembly: From Design to Regenerative Medicine and Beyond. Biophysica 2022, 2, 324–339.
- Gaar, J.; Naffa, R.; Brimble, M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814.
- Ciulla, M.G.; Marchini, A.; Gazzola, J.; Sambrotta, M.; Gelain, F. Low-Power Microwaves: A Cell-Compatible Physical Treatment to Enhance Self-Assembling Peptides Mechanical Propertie. Nanoscale 2023, 15, 15840–15854.
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198.
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40.
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479.
- Montaseri, Z.; Abolmaali, S.S.; Tamaddon, A.M.; Farvadi, F. Composite silk fibroin hydrogel scaffolds for cartilage tissue regeneration. J. Drug Deliv. Sci. Technol. 2023, 79, 104018.
- Wess, T.J. Collagen Fibril Form and Function. Adv. Protein Chem. 2005, 70, 341–374.
- Hodge, A.J.; Petruska, J.A. Recent studies with the electron microscope on ordered aggregates of the tropocollagen molecules. In Aspects of Protein Structure; Ramachandran, G.N., Ed.; Academic Press: New York, NY, USA, 1963; pp. 289–300.
- Ottani, V.; Raspanti, M.; Ruggeri, A. Collagen structure and functional implications. Micron 2001, 32, 251–260.
- Abune, L.; Lee, K.; Wang, Y. Development of a Biomimetic Extracellular Matrix with Functions of Protein Sequestration and Cell Attachment Using Dual Aptamer-Functionalized Hydrogels. ACS Biomater. Sci. Eng. 2022, 8, 1279–1289.
This entry is offline, you can click here to edit this entry!
