Rosmarinic acid (RA) is a naturally occurring polyphenolic compound found in various plants. It belongs to the class of compounds known as phenolic acids, and is a derivative of caffeic acid and related to other bioactive compounds such as flavonoids. Polyphenolic compounds are highly effective against numerous diseases, such as peptic ulcers, carcinogenesis, ischaemic heart disease, tumour cell proliferation, hyperglycaemia, atherosclerosis, apoptosis, etc. Polyphenols demonstrate potent capabilities as anti-inflammatory, antiallergy, antioxidant, antimicrobial, antiviral, and anticancer agents. RA is a functional component of numerous medicinal plants. Various studies have demonstrated a wide range of biological activities associated with RA.
1. Role of RA in Diabetes Mellitus
Diabetes mellitus (DM) comprises a group of metabolic disorders characterized by chronic hyperglycaemia, which is the presence of elevated sugar levels in the bloodstream. This condition can result from either irregular insulin secretion, inappropriate insulin action, or a combination of both factors. DM has emerged as a substantial and escalating global concern within the public health sector. Currently, the available drugs for diabetes are efficacious to a limit and have some safety issues like hypoglycaemia, weight gain, and gastrointestinal side effects. RA has shown potential therapeutic effects in the context of DM treatment through various ways. Owing to RA’s antioxidant and anti-inflammatory activities, significant researchers have focused on investigating the potential of RA in DM. Various studies suggest that the extract of
Rosmarinus officinalis, along with its phenolic constituents, particularly RA, carnosic acid, and carnosol, regulate glucose metabolism, lipid metabolism, anti-inflammation, and anti-oxidation, which ratchet up the therapeutic value against DM [
46].
DM treatment encompasses more than managing appropriate blood sugar levels; it also involves addressing insulin resistance, improving insulin sensitivity, and ensuring the proper function of beta cells [
47]. A research study explored how RA influences the regulation of glucose levels and insulin in two animal models mimicking diabetes: one induced to resemble type 1 diabetes using streptozocin (STZ) in rats, and the other induced to resemble type 2 diabetes through a high-fat diet (HFD) in rats. The result suggested that RA exhibits a dose-dependent improvement in high glucose (HG) levels and insulin resistance by reducing the expression of phosphoenolpyruvate carboxykinase (PEPCK) in the liver and enhancing glucose transporter 4 (GLUT4) expression in muscle tissues [
34]. Inhibiting α-glucosidase is another highly effective approach to tackle DM as α-glucosidase plays a significant regulatory role in DM [
48]. There is evidence to suggest that RA has α-glucosidase inhibitory activity [
11,
49]. These studies signify the importance of RA in DM treatment; however, ongoing research is focused on further understanding the mechanisms of action of RA in diabetes and its potential applications.
DM is associated with the pathogenesis of various health conditions such as cardiovascular disorders, diabetic nephropathy, diabetic neuropathy (DN), hypertension, and inflammation [
50,
51]. Various studies have highlighted the role of RA in diabetes-associated secondary health disorders. The role of RA in diabetes-linked nephropathy was studied. HG-stimulated cultured human renal proximal tubular epithelial cells (HK-2) were exposed to RA. RA inhibited connective tissue growth factor (CTGF), which is pathogenic in diabetic nephropathy [
52]. Further, in vivo studies were conducted on diabetic rats randomized to receive intragastric doses of RA [
52]. RA showed a significant enhancement of renal function and increased body weight in diabetic rats [
52]. Peripheral neuropathy is expressed as hypersensitivity to painful stimuli. This complication of diabetes is common in a majority of patients [
53].
In a study, rats with DN were studied to understand RA’s role in ameliorating the disease’s pathology. Oral administration of RA elicited antihyperalgesic and antiallodynic effects, attributed to reduced oxidative stress and inflammation associated with DN [
54]. According to a study, in diabetic rats, RA application led to notable reductions in glomerular hypertrophy, loss of glomerular count, and glomerulosclerosis [
55]. RA is associated with preventing damage related to oxidative stress in the liver and kidneys of diabetic rats [
56]. DM is also associated with various cardiovascular disorders. Aortic endothelial function and structure are damaged as a consequence of DM. RA shows protective and anti-inflammatory effects against diabetes-induced damage by restoring vascular endothelial function [
12]. Another study reported that RA effectively inhibits lipid peroxidation, consequently preventing the elevation of acetylcholinesterase (AChE) activity in diabetic rats, thereby underscoring the ability of RA to regulate cholinergic neurotransmission and mitigate oxidative damage in the brain during diabetic conditions [
57].
2. RA in Neurodegenerative Diseases
Neurodegenerative diseases, or neurodegeneration, arise from the loss or death of neuronal cells in different brain regions. Reports suggest that RA shows neuroprotective effects by reducing oxidative stress and preventing brain cell deaths in vitro against various neurological diseases and neurotoxic molecules [
58,
59,
60,
61].
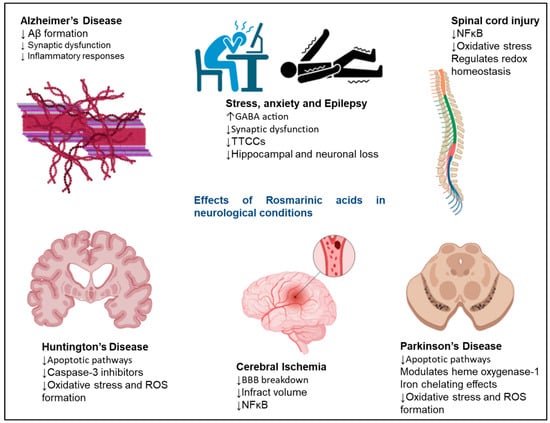
Figure 1 shows the therapeutic roles of RA in various neurological conditions. RA shows neuroprotective effects at the cellular level in various incidences, such as neurodegeneration, chemically induced neurotoxicity, oxidative stress, and neuroinflammation. The neuroprotective effects of RA were evaluated in N2A cells by H
2O
2-induced neuronal cell damage; it was observed that the RA treatment suppressed the H
2O
2-induced cytotoxicity in N2A cells [
38]. The results demonstrated that RA is highly effective in reducing the disturbance of lactate dehydrogenase, preserving mitochondrial membrane potential, and lowering intracellular ROS levels [
38]. Overall, the results stressed the fact that RA has the potential to serve as an agent for preventing various human neurodegenerative diseases that result from oxidative stress.
Figure 1. Neuroprotective effects exerted by RA in neurodegenerative disorders. The signalling molecules modulated in the presence of RA to exert neuroprotective effects. RA shows therapeutic effects in AD, Huntington’s disease, cerebral ischaemia, Parkinson’s disease, epilepsy, and stress. The upward arrows represent an increase in function, and the downwards arrows represent a decrease in function of various pathways on RA administration.
A study investigated the neuroprotective effects of RA on H
2O
2-induced neurotoxicity in the human dopaminergic cell line, SH-SY5Y [
62]. The results of the study indicated that RA played a significant role in mitigating the production of ROS induced by hydrogen peroxide (H
2O
2). RA also showed its effectiveness in suppressing the upregulation of the proapoptotic protein Bax while concurrently down-regulating the levels of the antiapoptotic protein Bcl-2. Additionally, RA exhibited a stimulatory effect on heme oxygenase-1 (HO-1), an antioxidant enzyme. Overall, the results unveiled the potential of RA to protect SH-SY5Y cells when exposed to oxidative stress [
62].
2.1. Alzheimer’s Disease
The crucial factor in developing Alzheimer’s disease (AD) is oxidative stress, which plays a vital role in free radical production, mitochondria dysfunction, cell death, amyloid beta peptide deposition, and mitochondrial interaction [
11]. In an investigation, when amyloid β
(25–35)-induced AD in rats was treated with RA, which mitigated the impairment of learning and memory disturbance by reducing oxidative stress [
63]. Additionally, research on memory impairment explored the potential protective effects of RA using an intracerebroventricular-administered A
(β25–35)-induced mice model. This study revealed that daily consumption of RA diminished the effect of neurotoxicity of Aβ
25–35 in mice models by scavenging peroxynitrite (ONOO
−), preventing memory impairments in AD [
64]. RA was evaluated on a cultured neuronal cell line, SH-SY5Y in vitro and ischaemic diabetic stroke in vivo, and the studies revealed that a 50 mg/kg dose of RA decreased high-mobility group box1 (HMGB1) expression, histopathological damage, brain oedema, oxygen-glucose deprivation-induced apoptosis, and cytotoxicity, and blocked TNF-α-induced NF-κB activation in SH-SY5Y cells [
65].
2.2. Epilepsy
Epilepsy is a central nervous system disorder regarded as a sudden, repetitive seizure because of abnormal behaviour of neuronal cells in the brain—the abnormal behaviour of neuronal cells in the brain results from irregular activities and extreme excitability. Gamma-aminobutyric acid (GABA) is key in maintaining the balance between neuronal excitation and inhibitory tone. Perturbance in this balance is the main factor in promoting epilepsy [
66]. RA doses of 100 μg/mL significantly inhibited the activity of gamma-aminobutyric acid transaminase (GABA-T) [
67]. Numerous reports showed the protective role of RA in animal models and in vitro studies against epilepsy [
68,
69]. A study investigated the potential advantages of RA in both in vitro and in vivo models of epileptiform activity induced by pilocarpine. This study revealed RA’s potential to reduce the acute neuromotor disturbances and oxidative damage triggered by status epilepticus (SE) in mice [
68]. Furthermore, it appears to offer favourable effects in in vitro models of epileptiform activity, as evidenced by a decrease in lactate release. In another study, two mouse models of acute seizure, 4-aminopyridine (4-AP) and picrotoxin (PTX)-induced seizures were used to investigate the effects of RA. The available drugs, diazepam and valproic acid, were used as positive controls. The outcomes of this study revealed that RA has the capacity to diminish cell damage induced by seizures triggered by 4-AP and PTX, making it a potential candidate for mitigating the pathophysiological processes associated with epilepsy. The outcomes of this study revealed that RA exhibited antioxidant activity, decreased reactive oxygen species production, superoxide dismutase activity, DNA damage, and neuroprotective effects [
69].
2.3. Huntington’s Disease
Huntington’s disease (HD) is a rare autosomal dominant inherited neurodegenerative disease of the central nervous system due to 36 or more repeats of CAG on the short arm of chromosome 4 in the
Huntingtin gene. Involuntary choreatic movements, dementia, and psychiatric and behavioural perturbation are the characteristic symptoms of HD [
70]. Expression of the
Huntingtin gene containing CAG repeats results in the production of Huntingtin protein with an expanded polyglutamine region (mutant Huntingtin protein). Mutant Huntingtin protein is related to various synaptic dysfunctions and is directly associated with impaired cellular mechanisms involved in synaptic transmission. Moreover, mutant Huntingtin protein also results in transcriptional dysregulation, glutamate excitotoxicity, and cell death [
71]. In an investigation, a rat model of HD, induced by 3-nitro propionic acid (3-Np), was administered RA-loaded solid lipid nanoparticles (SLN) through the nasal passage. The RA-loaded SNL caused resulted in a significant improvement in the behavioural abnormalities and a reduction in oxidative stress. The beneficial outcome of this study led to the conclusion that RA-loaded SLN formulation is an effective therapeutic strategy and can be used as a potential drug in managing HDs [
72].
2.4. Parkinson’s Disease
Degeneration of dopaminergic neurons in the substantia-nigra of the midbrain, increased iron levels, oxidative stress, and neuronal apoptosis are the main neuropathological features that promote Parkinson’s disease (PD). Thus, any compound that can work against these features of PD can be considered a potential therapeutic molecule [
73]. RA effectively mitigates oxidative stress by regulating the neuronal apoptotic process, making it an effective therapeutic agent against PD [
62].
The effect of RA was studied on a lesioned rat model of PD induced with 6-OHDA. RA prevented dopamine depletion in the striatum and showed a significant reduction in TH-Positive neurons in the substantia nigra. It also prevented the downregulation of Bcl-2/Bax ratio in 6-OHDA-induced PD rats [
59]. Therefore, RA is used in optimizing the mechanical perturbation and disturbances involved in neurodegenerative diseases.
This entry is adapted from the peer-reviewed paper 10.3390/nu15194297