1. Linear Peptides and Lipopeptides
Cyanobacteria are a rich source of multiple classes of linear peptides and lipopeptides. Whilst species growing in all environments produce these compounds, the peptide and lipopeptide composition of marine cyanobacteria have been particularly well reported
[1]. Therefore, this research has a greater emphasis on linear peptides and lipopeptides identified in marine cyanobacteria. The marine cyanobacterium
Lyngbya majuscula has been particularly well studied and has yielded a wealth of bioactive structurally diverse secondary metabolites. Whilst the research emphasises the identification of marine cyanobacterial compounds, similar compounds are also produced by freshwater and terrestrial cyanobacterial species, and these are also discussed where relevant in the following sections.
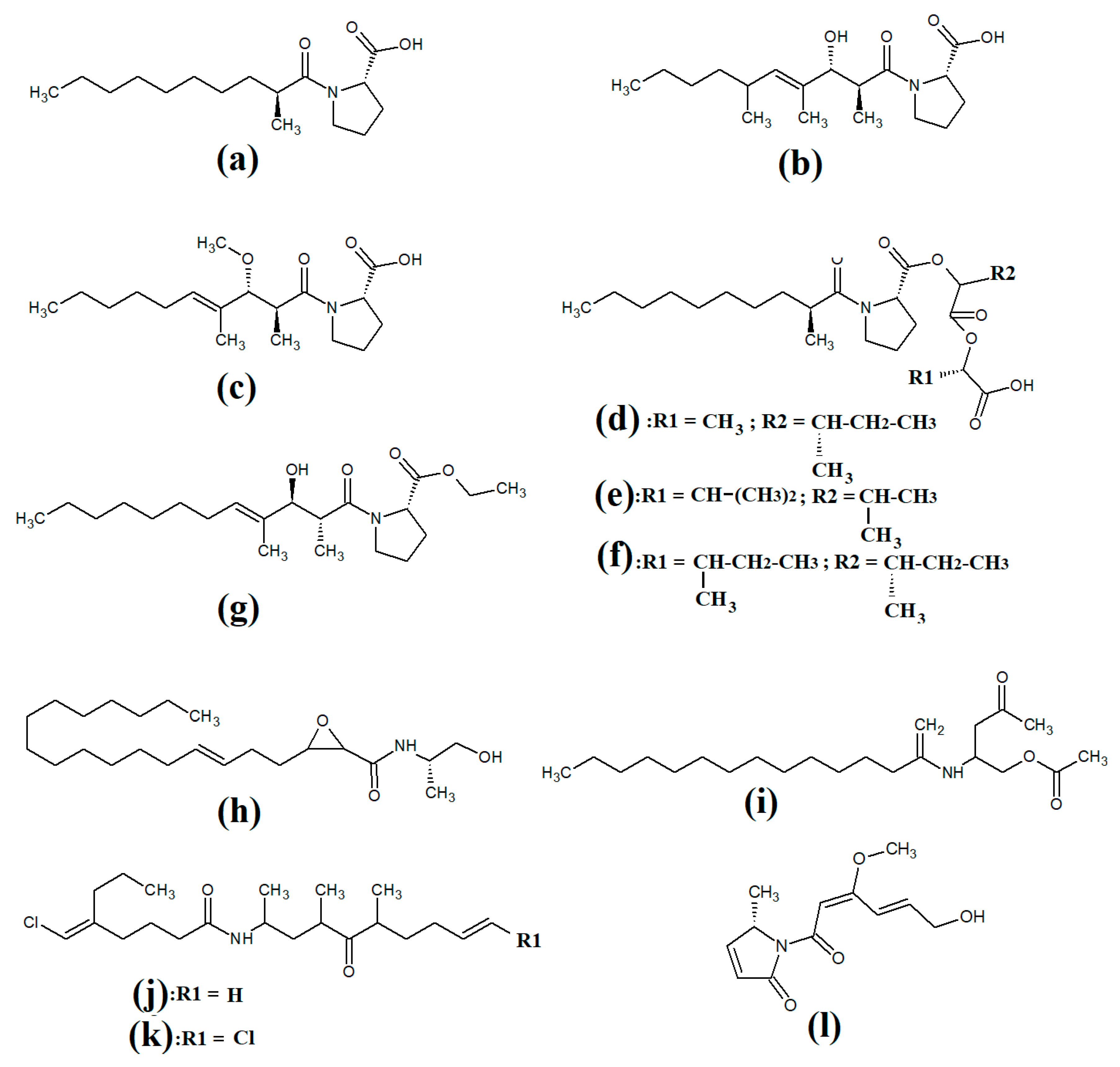
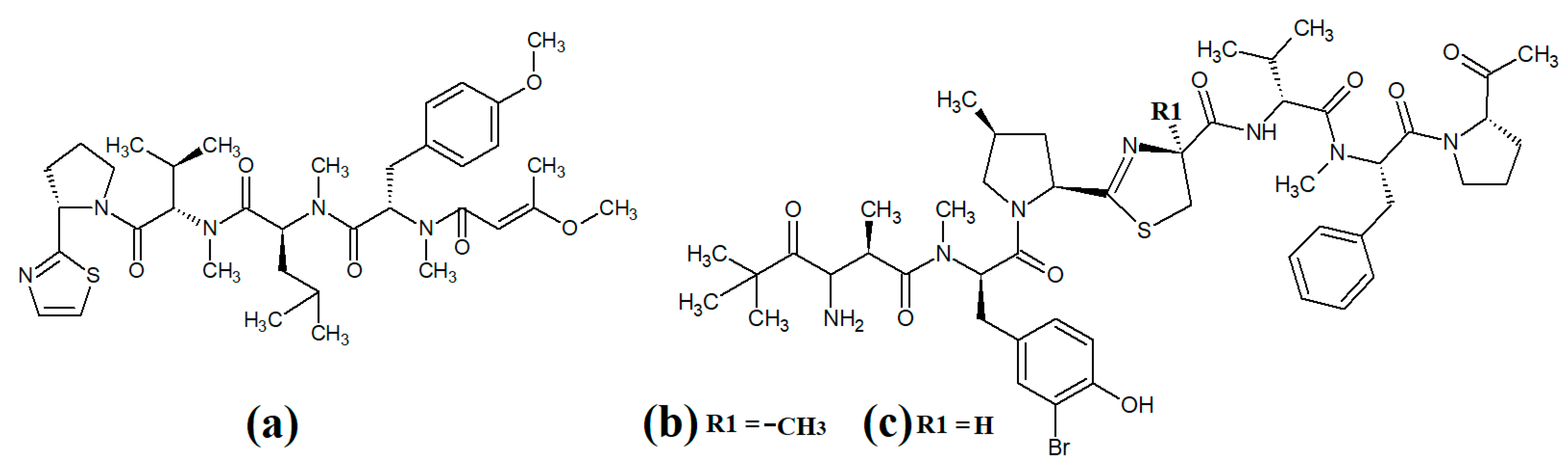
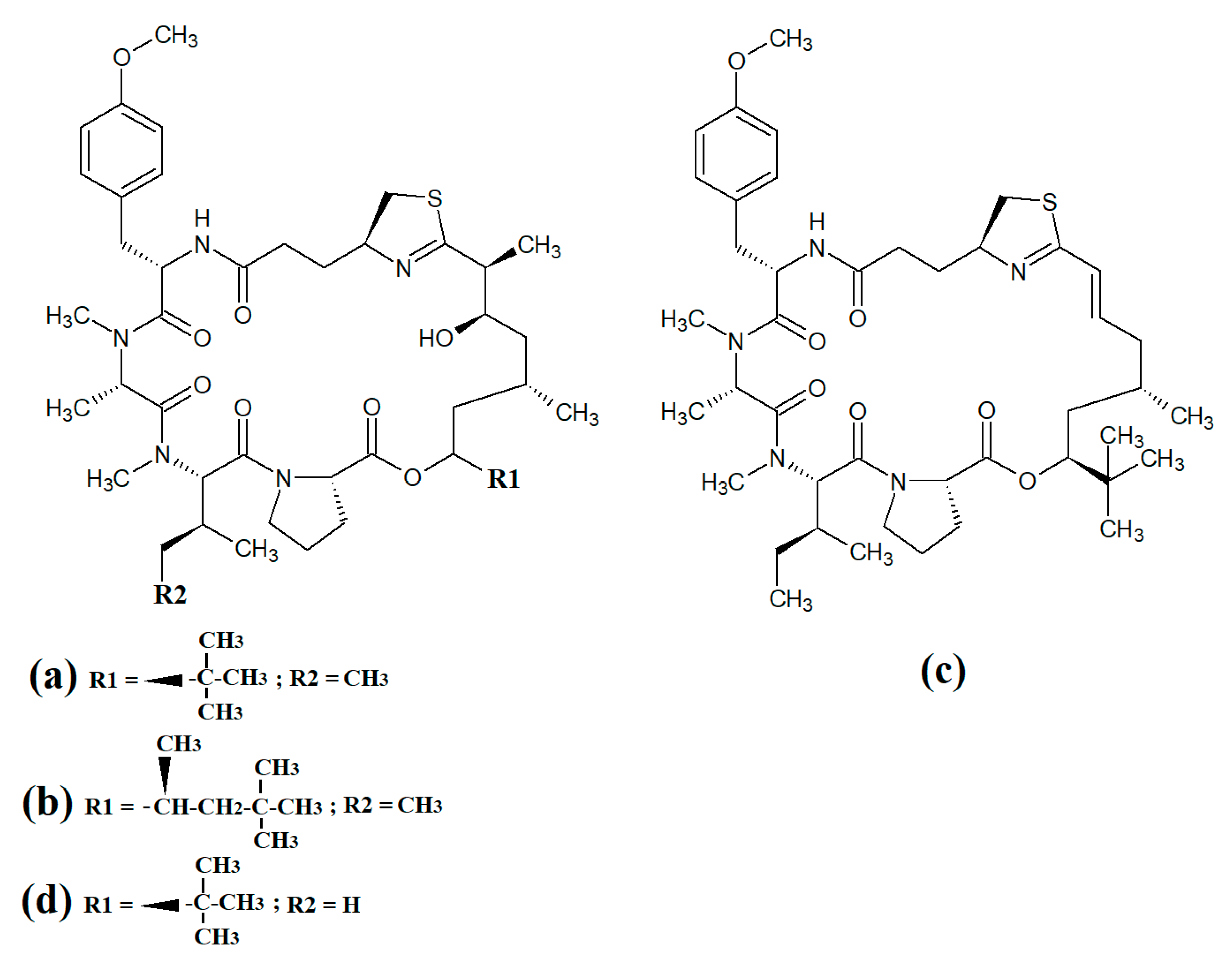
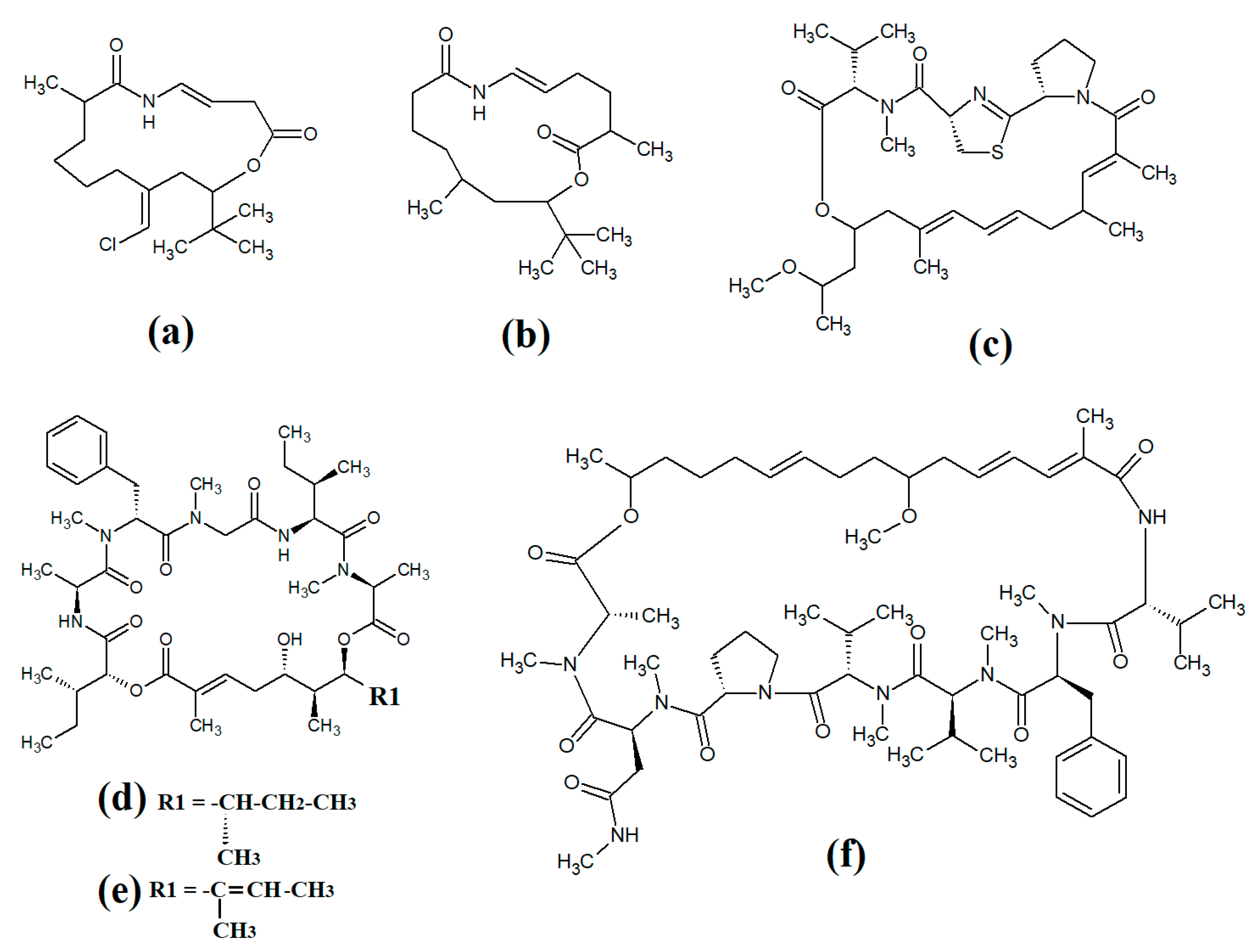
Tumonoic acid and its derivatives are some of the most abundant linear peptides in many cyanobacteria. Three tumonoic acids (A–C) were initially identified in the marine cyanobacterial species
Lyngbya majuscula and
Schizothrix calcicola in 1999
[2]. Since that time, tumonic acid D (
Figure 1a), tumonic acid E (
Figure 1b), tumonic acid F (
Figure 1c), tumonic acid G (
Figure 1d), tumonic acid H (
Figure 1e) and tumonic acid I (
Figure 1f) were identified in
Blennothrix cantharidomum [3]. Because the tumonoic acids share structural characteristics with homoserine lactones (which function as bacterial signalling molecules), the authors of that study tested their ability to inhibit quorum sensing in
Vibrio harveyi. Inhibitory activity was reported for all of the tumonoic acids, although tumonoic acid F was particularly potent, with an IC
50 of 62 μM. That study also reported that tumonoic acid I had noteworthy antimalarial activity (IC
50 = 2 μM). Subsequently, the tumonoic acid derivative ethyl tumonoate A (
Figure 1g) was identified in
Oscillatoria margaritefera and shown to have anti-inflammatory activity against RAW264.7 murine macrophages in a nitric oxide inhibition assay (IC
50 = 9.8 μM)
[4]. Besarhanamide A (
Figure 1h) and besarhanamide B (
Figure 1i) were also isolated from
Lyngbya majuscula extracts and their structures were reported
[5]. The therapeutic potential of the besarhanamides remain to be rigorously explored, although the authors of that study reported that they had moderate toxicity in an
Artemia nauplii lethality assay.
Figure 1. Cyanobacterial tumonoic acid-, besarhananmide-, grenadamine- and palmyrroline-class linear lipopeptides: (a) tumonic acid D; (b) tumonic acid E; (c) tumonic acid F; (d) tumonic acid G; (e) tumonic acid H, (f) tumonic acid I; (g) ethyl tumonoate A; (h) besarhanamide A, (i) besarhanamide B; (j) grenadamine B; (k) grenadamine C; (l) palmyrrolinone.
In another study, grenadamide B (
Figure 1j) and grenadamide C were isolated from lipophilic
Lyngbya majuscula extracts
[6]. Both compounds have planar fatty acid amide structures. Grenadamide C (
Figure 1k) was determined to have a substituted vinyl chloride group. The same study also reported that both of these compounds have weak insecticidal properties against armyworms, with mortality rates 38–50% at 1 mg/mL. Other biological activities remain relatively unexplored for these compounds and further studies are required.
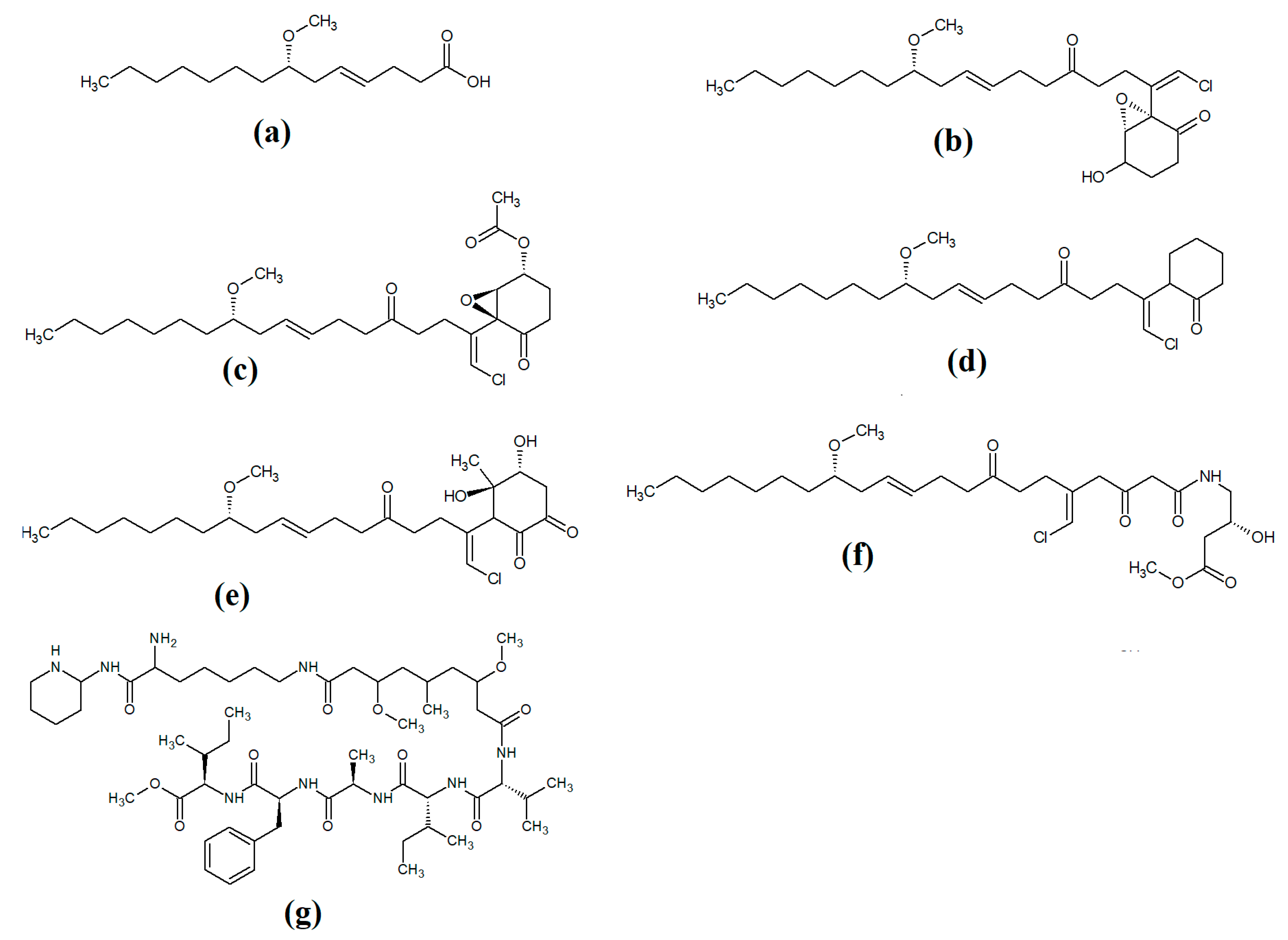
Lipopeptides of the malyngamide class are also common across multiple cyanobacteria of the genus
Lyngbya. Indeed, more than 30 malyngamide class lipopeptides have been reported in multiple cyanobacterial species, with the majority of these identified in
Lyngbya majuscula [7][8]. Whilst these compounds vary considerably, all contain a 7
S-methoxytetradec-4(
E)-enoic acid (commonly known as lyngbic fatty acid;
Figure 2a) chain, or a lyngbic acid derivative. Several of the malyngamides have been reported to have noteworthy bioactivities. In particular, the malyngamide stereoisomers 8-epi-malyngamide C (
Figure 2b) and 8-
O-acetyl-8-epi-malyngamide C (
Figure 2c) are strongly cytotoxic against NCI-H460, Neuro-2a and HT29 cells, with IC
50 values ranging from 3.1 to 23.9 μM
[7][8]. Additionally, 8-epi-malyngamide C also inhibits bacterial quorum sensing in transformed
Escherichia coli [8]. Isomalyngamide K (
Figure 2d) was isolated from
Lyngbya majuscula extracts in another study, although no bioactivities were reported for this compound
[9] and further studies are required to evaluate its therapeutic potential.
Figure 2. Cyanobacterial malyngamide-class linear lipopeptides: (a) lyngbic acid; (b) 8-epi-malyngamide C; (c) 8-O-acetyl-8-epi-malyngamide C; (d) isomalyngamide K; (e) malyngamide 2; (f) malyngamide 3; (g) mitosoamide A.
Another study isolated malyngamide 2 (
Figure 2e) from
Lyngbya sordida and determined that it contains a trihydroxy cyclohexanone ring structure
[10]. The authors reported noteworthy nitric oxide inhibitory activity against LPS-stimulated RAW264.7 murine macrophages (IC
50 = 8 μM). They also reported that malyngamide 2 had moderate cytotoxic activity against H-460 human lung cancer cells (IC
50 = 8 μM). However, the antimicrobial properties of this compound are yet to be rigorously evaluated. Another study reported the isolation and identification of malyngamide 3 (
Figure 2f) from
Lyngbya majuscula and reported that it has weak cytotoxicity against MCF-7 breast cancer cells (IC
50 = 29 μM) and HT-29 colon cancer cells (IC
50 = 48 μM)
[11]. Additionally, mitsoamide A (
Figure 2g) was isolated from a marine cyanobacterium of the
Geitlerinema genus and was reported to have several unusual structural features
[12]. The structure contains a homolysine, 3,7-dimethoxy-5-methyl-nonanedioic acid and a piperidine moiety, linked by an alanine, isoleucine, N-methyl-isoleucine, phenylalanine and valine peptide. The authors of that study reported that mitsoamide A was cytotoxic to human NCI-H460 lung cells (IC
50 = 0.46 μM).
The lipodepsipeptide gallinamide A (
Figure 3a) was isolated from
Schizithrix spp., although the specific species was not identified
[13]. Interestingly, gallinamide A had moderate antiprotozoal activity against
Plasmodium falciparum (IC
50 = 8.4 μM) and
Leishmania donovani (IC
50 = 9.3 μM). The same study also reported moderate cytotoxicity against human NCI-H460 lung cancer cells and neuro-2a mouse neuroblastoma cells. A different study isolated and identified the structural isomer symplostatin 4 (
Figure 3b) from
Symploca spp., although the specific species was not identified
[14]. The authors of that study reported that symplostatin 4 had noteworthy cytotoxicity against HT-29 human colon cancer cells (IC
50 = 53 μM) and HeLa cervical cancer cells (IC
50 = 12 μM) due to its antimitotic activity. Symplostatin 4 disrupted intracellular microtubule formation at 50 μM, and completely depolymerised microtubules at 100 μM. Additionally, the authors also reported that symplostatin 4 and largazole (which was also produced by the
Symploca spp. cells) synergised the cytotoxicity of the combination against HT-29 cells, although fractional IC
50 values of the combination components were not reported. Largazole functions via a different cytotoxic mechanism, through inhibition of histone deacetylases
[15], possibly accounting for these effects.
Figure 3. Cyanobacterial gallinamide-, symplostatin- and dragonamide-class linear lipopeptides: (a) gallinamide A; (b) symplostatin 4; (c) dragomabin; (d) dragomabin B; (e) dragomabin D; (f) dragomabin E; (g) dragomabin C; (h) almiramide A; (i) almiramide B; (j) almiramide C; (k) viridamide A; (l) viridamide B.
Dragomabin (
Figure 3c) and the dragonamides B (
Figure 3d), D (
Figure 3e), E (
Figure 3f) and C (
Figure 3g), as well as the almiramides A (
Figure 3h), B (
Figure 3i) and C (
Figure 3j) and viridamides A (
Figure 3k) and B (
Figure 3l), are a group of structurally related linear lipopeptides. All contain an eight-carbon polyketide moiety linked to amino acids via amide bonds, with all of these compounds except the viridamides terminating in a primary amide functional group
[16]. Dragomabin and dragonamides A and B were isolated from
Lyngbya majuscula and screened against a chloroquine-resistant
Plasmodium falciparum strain
[17]. The authors reported IC
50 values between 4.3 and 7.7 μM for all compounds except dragonamide B, which was completely inactive against the chloroquine-resistant
Plasmodium falciparum strain, indicating that the aromatic moiety is required for antimalarial activity. Additionally, the same study also determined the cytotoxicity of these compounds against Vero cells, with IC
50 values ≤ 182 μM for all of the tested compounds. Dragonamides C and D were isolated from
Lyngbya majuscula and
Lyngbya polychroa in another study and reported to have noteworthy inhibitory activity against
Leishmania donovani (IC
50 = 5.1 μM)
[18] and U2OS osteocarcoma cells (IC
50 = 56–59 μM).
Almiramides A–C were isolated from
Lyngbya majuscula, identified and screened for antiprotozoal activity against
Leishmania donovani [18]. The inhibitory activity of almiramides B and C were particularly noteworthy (IC
50 values of 2.4 and 1.9 μM, respectively). Another study screened the almiramides against a chloroquine-resistant
Plasmodium falciparum strain and reported good antimalarial activity for dramonamide A (IC50 = 7.7 μM)
[19]. Another study isolated viridamide A (
Figure 3k) and viridamide B (
Figure 3l) from the marine cyanobacteria
Oscillatoria nigro-viridis and determined that the structures consisted of N-methylated amino acids, hydroxyl acids and a 5-methoxydec-9-ynoic acid moiety
[20]. The authors of that study reported noteworthy antiparasitic activity for viridamide A against
Leishmania mexicana,
Plasmodium falciparum and
Trypanosoma cruzi (IC
50 values 1.1–5.8 μM). Contrastingly, the effects of all of these compounds against bacteria, fungi, viruses and other protozoal pathogens are yet to be rigorously examined.
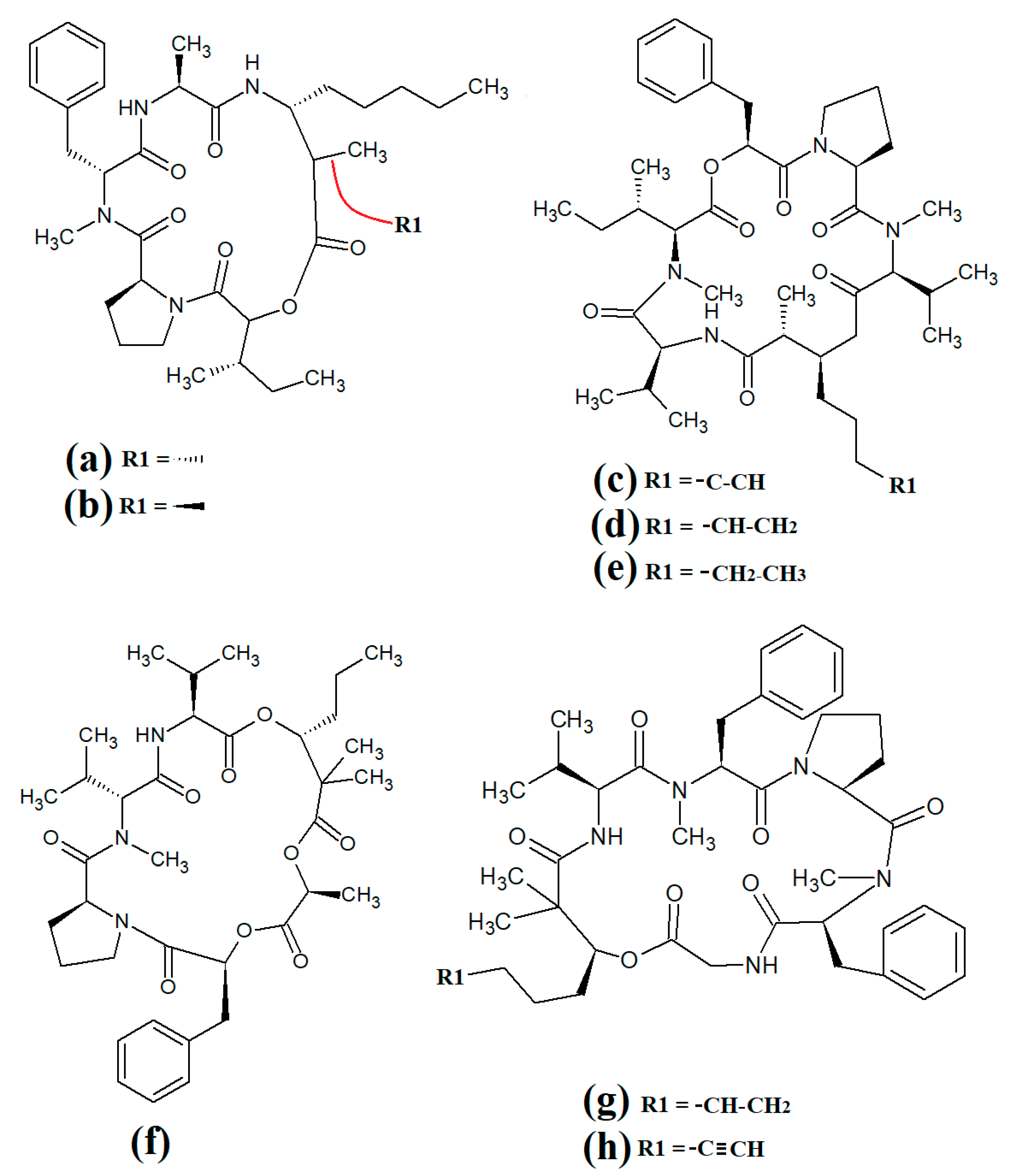
Grassystatins are linear decadapsipeptides that are potent inhibitors of cathepsin E enzyme
[21]. Grassystatins A (
Figure 4a), B (
Figure 4b) and C (
Figure 4c) were isolated and identified from the marine cyanobacterium
Lyngbya conferoides [22]. That study reported that these compounds are potent inhibitors of both cathepsin D (IC
50 = 16.9 nM) and E (IC
50 = 0.62 nM). As cathepsins D and E are involved in antigen processing via the HHC class II pathway and bioactive protein degradation
[23], it is likely that grassystatins may block pathogen activation mechanisms, and thereby modulate the course of multiple infections. Notably, grassystatins have profound effects on the course of viral diseases. By inhibiting cathepsin enzymes, grassystatins block viral attachment glycoprotein activation and therefore inhibit the entry of virus into the target cells, as well as directly inhibiting viral replication
[24].
Figure 4. Cyanobacterial grassystatin linear lipopeptides: (a) grassystatin A; (b) grassystatin B; (c) grassystatin C.
2. Linear Lipopeptides and Peptides Containing Heterocyclic Moieties
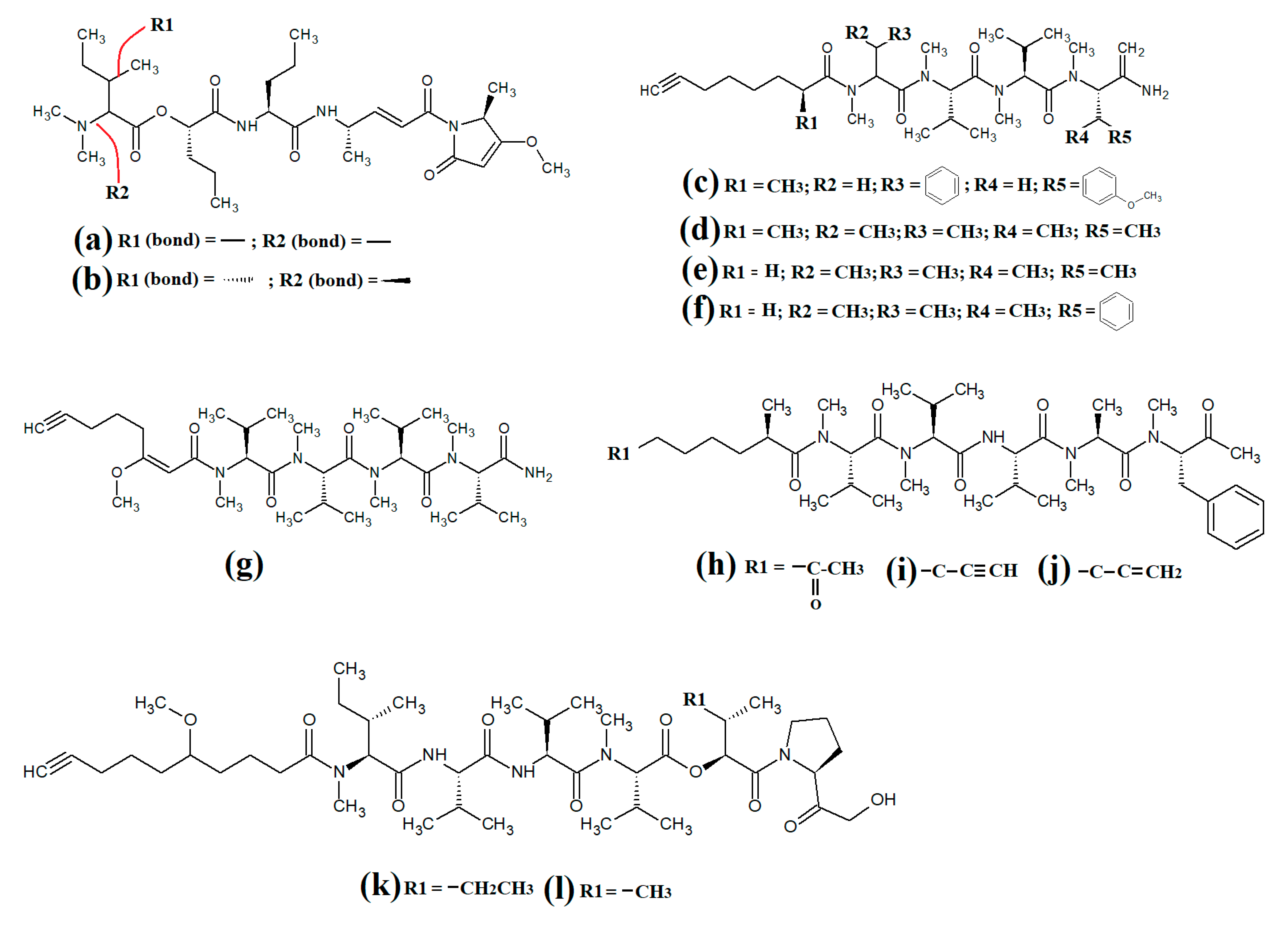
Lyngbyapeptin D (
Figure 4a) and three structural analogues (27-deoxylyngbyabellin A, lyngbyabellin J and laingolide B) have been identified in
Lyngbya boullonii extracts
[25]. That study reported that all of these compounds were moderately cytotoxic towards HT29 colorectal adenocarcinoma and HeLa cervical cancer cell lines, and therefore may have potential as cancer therapeutics. Notably, the effects of lyngbyapeptin D and its analogues have yet to be tested against bacterial, fungal, protozoal and viral pathogens and substantially more work is required.
The cytotoxic linear peptide bisebromoamide (
Figure 5) was isolated from an unidentified
Lyngbya spp. in 2009
[26]. A subsequent study also identified the structural analog norbisebromamide (
Figure 5c)
[27]. Bisebromoamide is highly cytotoxic against an extensive panel of human cancer cells with an average GI
50 value of 40 nM
[28]. That study reported that bisebromoamide exerts its anticancer effects via modulation of extracellular signal-regulated protein kinase (ERK) pathways. Another study reported that bisebromoamide also destabilises actin filaments
[29]. However, the effects of bisebromoamide and norbisebromoamide are yet to be elucidated.
Figure 5. Cyanobacterial lyngbypeptin- and bisebromoamide-class heterocycle containing linear lipopeptides: (a) lyngbyapeptin D; (b) bisebromoamide; and (c) norbisebromoamide.
3. Cyclic Depsipeptides and Peptides
Cyclic depsipeptides are a diverse group of compounds containing ring structures composed of amino and hydroxyl acid moieties linked by amide and ester bonds. Differences in the ring structure and the nature of the side chains provides substantial diversity in the structure and function of this class of compounds
[30].
4. Cyclic Depsipeptides Containing Heterocyclic Moieties
The epimeric cyclic depsipeptides porpoisamide A (
Figure 6a) and porpoisamide B (
Figure 6b) were isolated from an unidentified marine
Lyngbya spp. cyanobacterium and the structure was elucidated
[31]. Both compounds contained alanine, N-methyl-phenylalanine, 2-hydroxy-3-methylpentanoic acid and 3-amino-2-methyl-octanoic acid moieties and differed only in the stereochemistry of the amino acid at C2 of the ring structure. That study reported that both porpoisamides have weak cytotoxic activity against human HCT-116 colorectal (IC
50 = 21 μM) and U2OS osteosarcoma cell lines (IC
50 = 28 μM).
Figure 6. Cyclic cyanobacterial lipopeptides: (a) porpoisamide A; (b) porpoisamide B; (c) hantupeptin A; (d) hantupeptin B; (e) hantupeptin C; (f) palmyramide A; (g) cocosamide A; (h) cocosamide B.
Hantupeptin A (
Figure 6c) and its structural analogues hantupeptin B (
Figure 6d) and hantupeptin C (
Figure 6e) have been isolated from
Lyngbya majuscula extracts
[32]. These compounds each contain a cyclic structure consisting of four amino acids and two hydroxy acid residues, one of which is a PKS-derived 3-hydroxy-2-methyloctynoic acid. The hantupeptins differ only in the nature of a chain extension that occurs at C2 of this residue. Subsequent studies by the same group reported significant cytotoxic activity against MOLT-4 leukaemia and MCF-7 breast cancer cell lines, with IC
50 values between 32 and 3000 nM
[33]. Hantupeptin A was particularly potent against MOLT-4 cells (32 nM). However, the potential of these compounds in the treatment and inhibition of pathogenic diseases remains to be examined.
The 19-membered cyclodepsipeptide palmyramide A (
Figure 6f) has been reported in
Lyngbya majuscula extracts
[34]. Its structure was reported to consist of five amino acid moieties, including three valine residues, as well as one residue each of
N-methyl-valine and proline. It also contains the three hydroxy acids 2,2-dimethyl-3-hydroxyhexanoic acid, lactic acid and 3-phenyllactic acid. The authors of that study tested palmyramide A in sodium channel blocking assays and reported noteworthy inhibition of veratridine and ouabain-induced sodium channel overload, resulting in considerable cytotoxicity in neuro-2a murine neuroblast cells (IC
50~17 μM) and in H-460 human lung cancer cells (IC
50~40 μM).
The cyclodepsipeptides cocosamide A (
Figure 6g) and cocosamide B (
Figure 6h) have also been isolated from
Lyngbya majuscula extracts in another study
[11]. Both compounds contain two
N-methyl-phenylalanine residues, proline, glycine, valine, and either 2,2-dimethyl-3-hydroxy-7-octynoic acid or 2,2-dimethyl-3-hydroxy-7-octenoic acid. Both compounds were moderately cytotoxic against HT-29 and MCF-7 breast cancer cells, with IC
50 values ranging from 11 to 39 μM. The effects of these compounds against bacterial, fungal, protozoal and viral pathogens remain to be investigated.
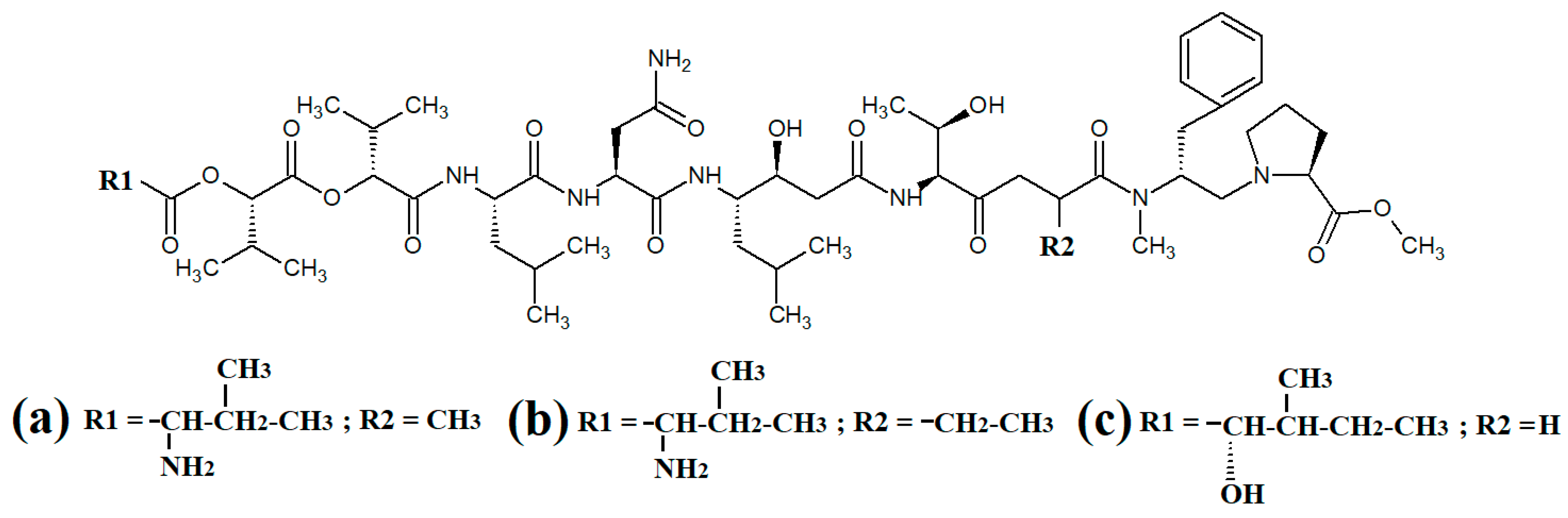
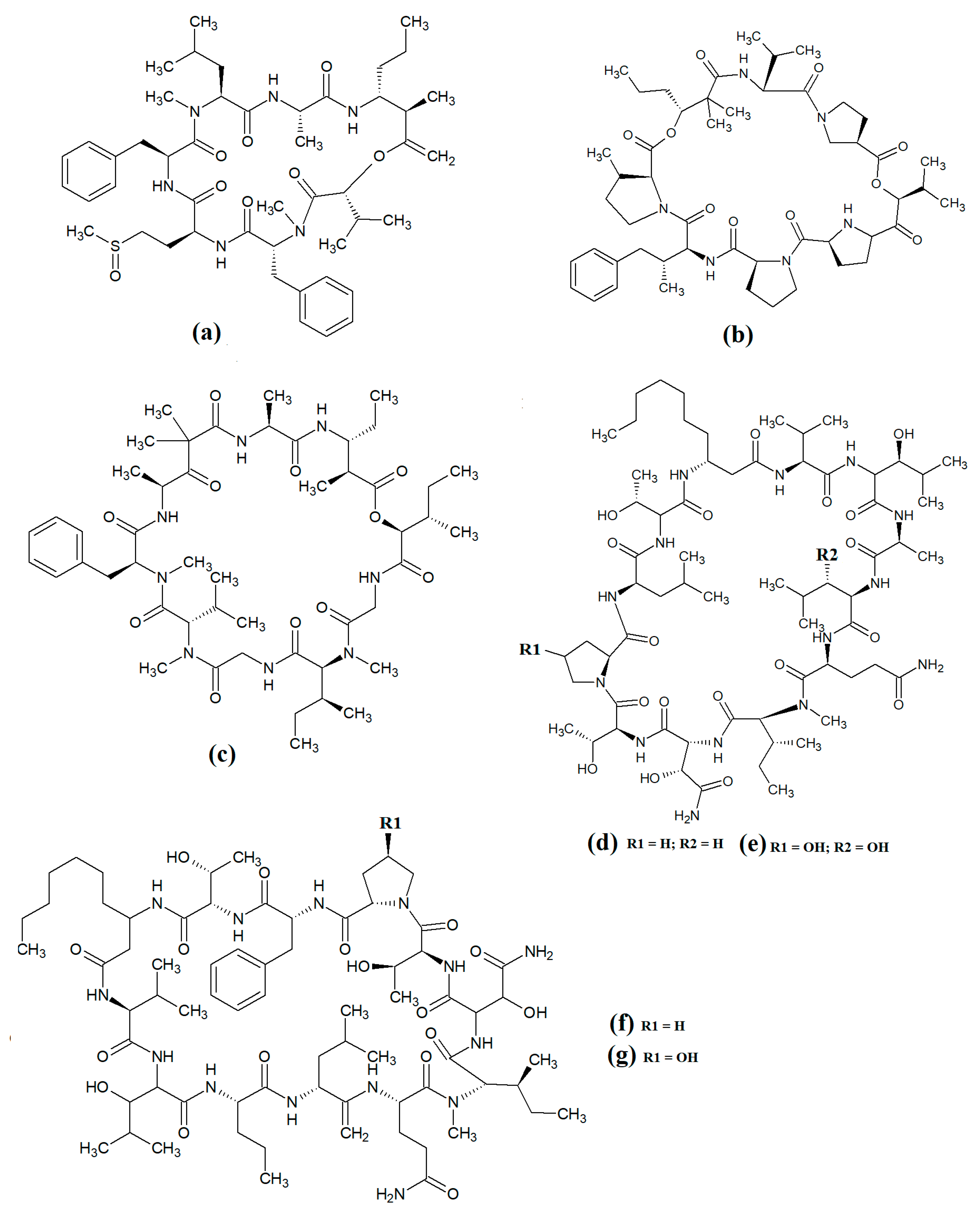
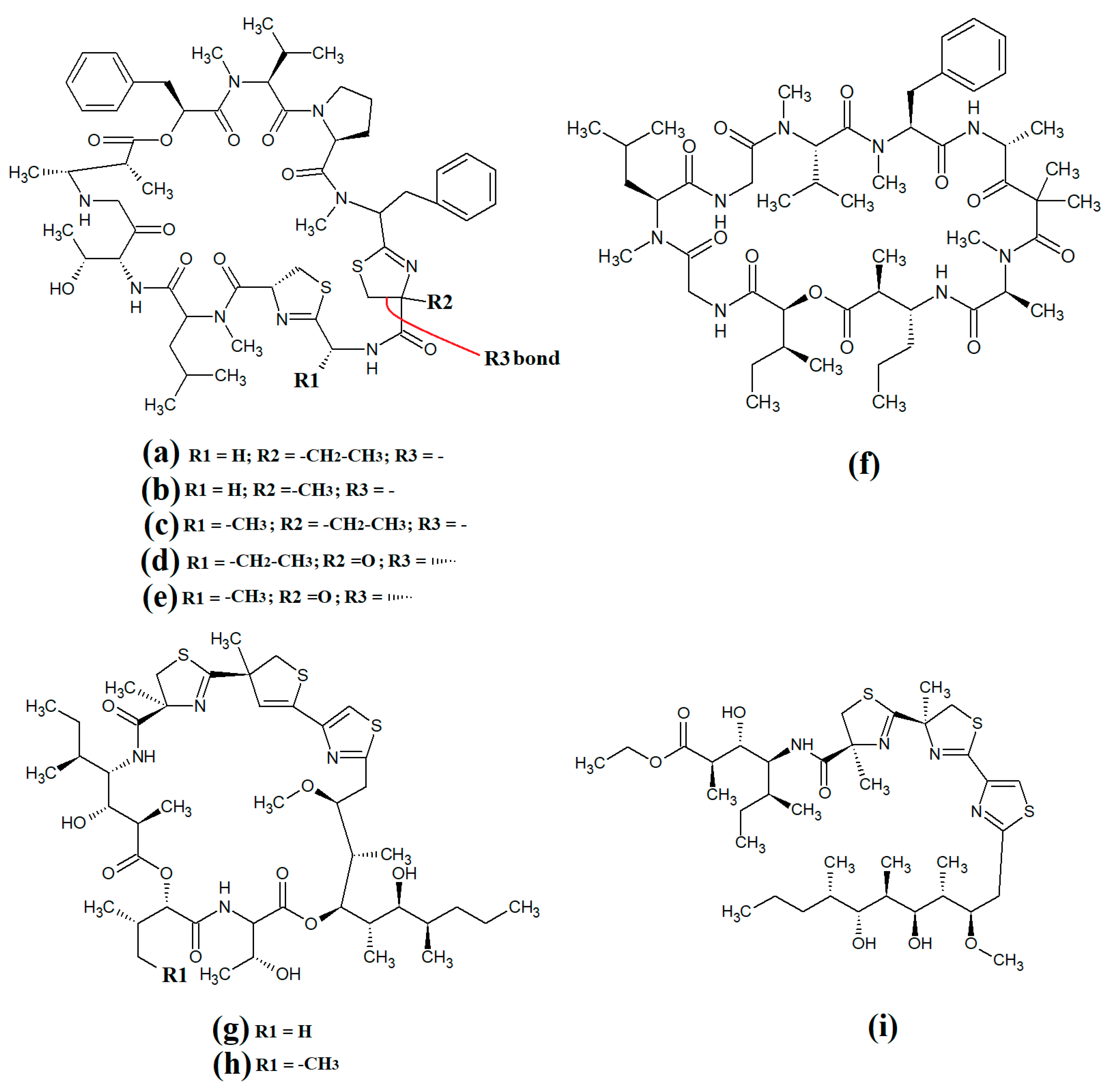
Carriebowmide (
Figure 7a) was isolated from
Lyngbya majuscula extracts and the structure was determined to consist of a 21-membered cyclic depsipeptide structure, which consists of alanine,
N-methyl-leucine, phenylalanine, methionine,
N-methyl-phrnylanine and a 2-hydroxy-3-methylbutyric acid moiety
[35]. It also contains the two rare amino acids 3-amino-2-methyl-hexanoic acid and methionine sulfoxide. Another study reported that carriebowmide is moderately cytotoxic towards human HEK-293 embryonic kidney cells, with an IC
50 of approximately 50 μM
[6].
Figure 7. Cyclic cyanobacterial lipopeptides: (a) carriebowmide; (b) pitiprolamide; (c) desmethoxymajusculamide C; (d) laxaphycin B2; (e) laxaphycin B3; (f) lynbyacyclamide A; (g) lynbyacyclamide B.
Pitiprolamide (
Figure 7b) was also identified in
Lyngbya majuscula extracts
[36]. Its structure was reported to be similar to that of dolastatin, although it contains four proline residues, as well as a valine, dolaphenvaline and 2-hydroxy-isovaleric acid moieties. The authors of that study reported that pitiprolamide was mildly cytotoxic towards human MVC7 breast cancer and HCT116 colorectal cancer cell lines (IC
50 = 33 μM for both). Of further interest, pitiprolamide also showed antibacterial activity against
Mycobacterium tuberculosis in a disc diffusion assay at 50 μg. However, the MIC was not determined, making it impossible to compare with the potency of other compounds tested in other studies. Additionally, the disc diffusion assay was perhaps not the most appropriate assay system to test antibacterial activity of this compound. Pitiprolamide has a molecular mass of 905.54 Da and its size would hinder its diffusion in agar gels, thereby providing falsely low results. Furthermore, dolastatin (which is structurally similar) has low water solubility, also hampering its diffusion in agar. Therefore, liquid-based assay systems may have been more appropriate for testing the antibacterial potency of this compound. Despite that, the reported mycobacterial activity is promising, and future studies should focus on testing pitiprolamide against other pathogens using more appropriate assays.
Another cyclic depsipeptide was isolated from the marine cyanobacterium
Lyngbya majuscula and was identified as desmethoxymajusculamide C (
Figure 7c)
[37]. Notably, desmethoxymajusculamide C has highly potent and selective cytotoxicity, with an IC
50 value of 20 nM against human HCT-116 colorectal cells, although it is substantially less potent against other cell lines. The authors of that study determined the HCT-116 cytotoxicity to be mediated through inhibition of microfilament production.
Laxaphycin B2 (
Figure 7d) and laxaphycin B3 (
Figure 7e) were detected in
Annabaena torulosa extracts, albeit in low abundance
[38]. Both compounds were reported to have noteworthy anti-proliferative activity towards a panel of drug-resistant and drug-sensitive solid lymphoblastic cancer cells, with IC
50 values generally between 1 and 20 μM. Interestingly, these compounds substantially potentiated each other’s anti-proliferative activity when tested in combination, with at least a two-fold increase in potency against all of the cell lines tested. Lynbyacyclamide A (
Figure 7f) and lynbyacyclamide B (
Figure 7g) are structurally related to the laxaphycins, which were originally isolated from
Lyngbya majuscula extracts
[39]. Structurally, they contain 37-member rings consisting of 11 α-amino acids and 1 β-amino acid (β-amino-decanoic acid). Similar to laxaphycin B2 and laxaphycin B3, the lynbyacyclamides are strongly cytotoxic, both with IC
50 values of 0.7 μM against mouse B16 melanoma cancer cells.
Several recent studies have focused on cyanobacterial compounds for antiprotozoal activity, particularly against malaria-, leishmaniasis- and schistomiasis-causing pathogen species. Several promising compounds have been identified with noteworthy antiprotozoal activities. In particular, ventutramide A (
Figure 8a) and ventutramide B (
Figure 8b) that were isolated from
Oscillatoria spp. are reported to have good antimalarial activity. Ventutramide A was a particularly good inhibitor of
Plasmodium falciparium growth, with an IC
50 of 8.2 μM
[40]. Both compounds also inhibited
Trypanasoma cruzi and
Leishmania donovani, albeit with substantially higher IC
50 values. The ventutramides are structurally unusual, containing two thiazole and one methyl-oxazole ring moieties, which may contribute to the antiprotozoal activity of these compounds. The effects of these compounds on non-protozoal pathogens have been less extensively examined and substantially more work is required to determine the therapeutic potential of these molecules.
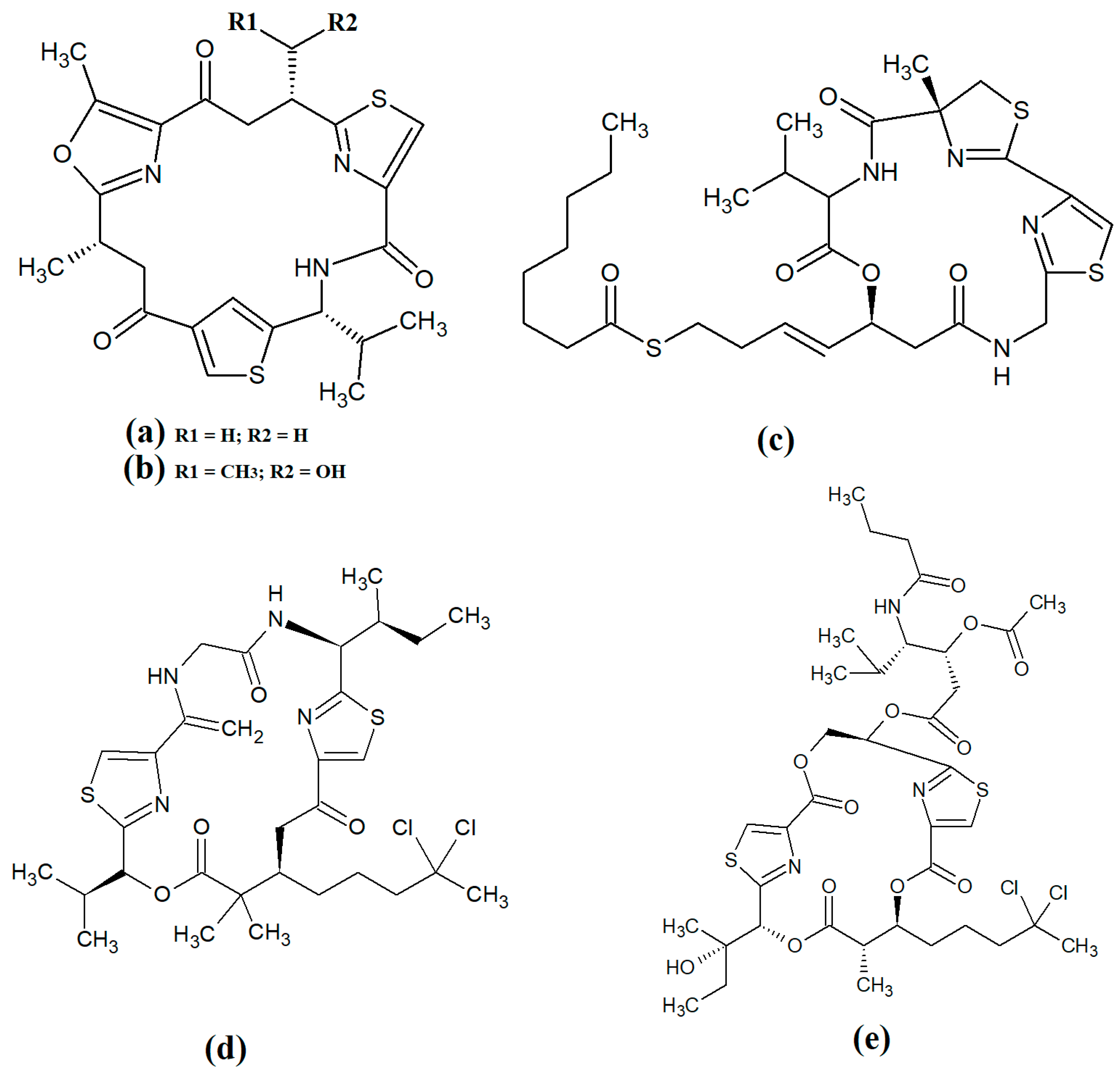
Figure 8. Cyclic cyanobacterial depsipeptides/peptides: (a) ventutramide A; (b) ventutramide B; (c) largazole; (d) 27-deoxylyngbyaybellin A; (e) lyngbyabellin J.
Largazole (
Figure 8c) was initially isolated from marine cyanobacteria of the genus
Symploca in 2008
[15]. This compound has attracted substantial interest since that time as it is a potent class I histone deacetylase (HDAC) inhibitor. As HDACs regulate HIV latency
[41], largazole has potential as an anti-retroviral therapy for use in HIV-AIDS. Additionally, the HDAC inhibitory activity of largazole also makes it a promising target for anticancer drug development. Notably, largazole (as well as its analogues) has anti-proliferative activity towards the NCI 60 panel of human cancer cell lines
[42]. Further studies demonstrated that largazole also has cytostatic effects in a human HCT116 xenograft mouse model via stimulation of histone hyperacetylation, as well cytotoxic effects by inducing apoptosis
[42]. Notably, these compounds are yet to be tested for anti-infective properties against pathogens of interest to human health.
Lyngbyabellins are a group of cyclic lipopeptides that are characterised by the presence of thiazole/thaizoline moieties and dichlorination of a polyketide moiety. The structural lyngbyabellin analogues 27-deoxylyngbyaybellin A (
Figure 8d) and lyngbyabellin J (
Figure 8e) were isolated and characterised from
Lyngbya bouillonii extracts and tested for cytotoxic activity against human HT29 colorectal and HeLa cervical carcinoma cell lines
[25]. Whilst both compounds displayed good cytotoxic activity, 27-deoxylyngbyaybellin A was particularly potent, with IC
50 values of 12 and 7.3 nM against the HT29 and HeLa cells, respectively. Lyngbyabellin J also had noteworthy activity, albeit with significantly higher IC
50 values (54 and 41 nM, respectively).
Multiple members of the apratoxin class of compounds have also been identified in cyanobacteria. These compounds have cyclic structures that contain a thiazoline unit, as well as an extensive polyketide moiety. Apratoxin A (
Figure 9a) was reported over twenty years ago in the marine cyanobacterium
Lyngbya majuscula [43]. Since that time, apratoxin D (
Figure 9b), apratoxin E (
Figure 9c) and apratoxin G (
Figure 9d) have also been isolated from
Lyngbya majuscula, as well as from
Lyngbya sordida [44]. Apratoxin D was reported to have potent cytotoxic activity against human H-460 lung cancer cells (IC
50 = 2.6 nM)
[44]. Apratoxin E also has potent cytotoxicity against HT29, HeLa and U2OS cell lines (IC
50s of 21, 72 and 59 nM, respectively)
[45]. Similarly, apratoxin G was cytotoxic to human H-460 cells (IC
50 = 14 nM)
[46]. However, these compounds are yet to be rigorously tested against pathogens.
Figure 9. Cyclic cyanobacterial apratoxins: (a) apratoxin A; (b) apratoxin D; (c) apratoxin E; (d) apratoxin G.
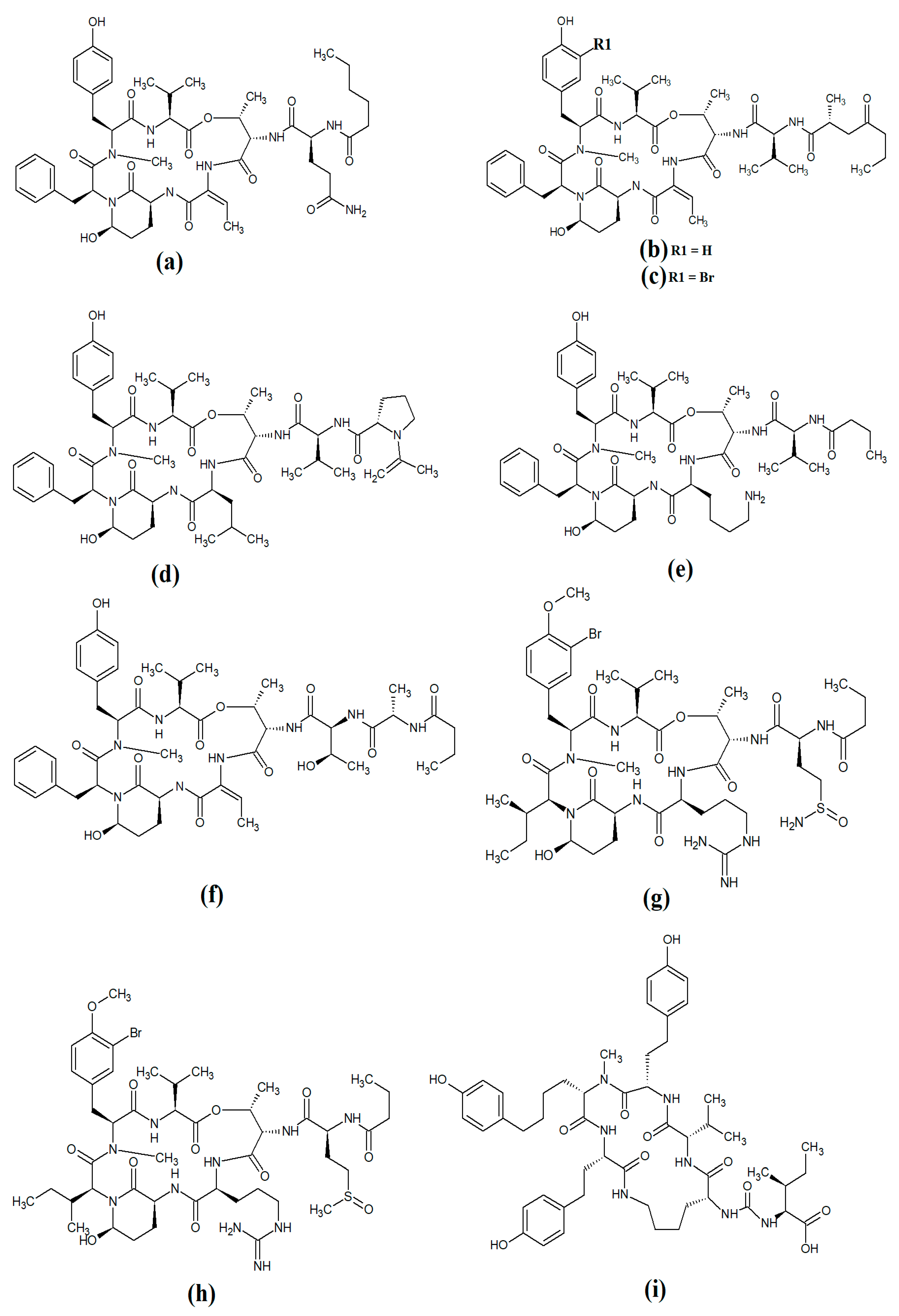
Grassypeptolide A (
Figure 10a) and grassypeptolide B (
Figure 10b) were isolated and characterised from
Lyngbya confervoides [47][48]. The authors of those studies also screened these compounds and reported noteworthy cytotoxic activity for grassypeptolide A against human U2OS osteosarcoma, HeLa cervical cancer, HT29 colorectal and IMR-32 neuroblastoma cells, with IC
50 values of 2.2, 1, 1.5 and 4.2 μM, respectively
[47]. Subsequent studies demonstrated that grassypeptolides A and C inhibit cell cycle progression at G1 phase in low concentrations, and also at the G2/M phase when tested at higher concentrations
[48]. More recently, grassypeptolide D (
Figure 10c), grassypeptolide F (
Figure 10d) and grassypeptolide G (
Figure 10e) were isolated from the marine cyanobacteria
Leptolyngbya spp. and
Lyngbya majuscula and tested for cytotoxicity
[49][50]. Grassypeptolides D and E were particularly cytotoxic against HeLa (IC
50 = 335 and 192 nM, respectively) and mouse neuro-2a-blastoma cells (IC
50 = 599 and 407 nM, respectively)
[49]. In contrast, grassypeptolides F and G were only moderate inhibitors of transcription factor AP-1 (IC
50 = 5.2 and 6 μM, respectively)
[50].
Figure 10. Cyanobacterial grassypeptolide- and hoiamide-class depsipeptides/peptides: (a) grassypeptolide A; (b) grassypeptolide B; (c) grassypeptolide D; (d) grassypeptolide F; (e) grassypeptolide G; (f) ibu-epidemethoxylyngbyastatin; (g) hoiamide A; (h) hoiamide B; (i) hoiamide C.
The hoiamides are a class of cyclic depsipeptides that have been isolated from several filamentous marine cyanobacteria, including
Lyngbya majuscula and
Phormidium gracile [51]. Hoiamide A (
Figure 10g) contains an extended isoleucine moiety, two methylated thiazoles, a thiazole moiety and a highly methylated and oxygenated polyketide group
[51]. The same study also reported that hoiamide A is a potent inhibitor of [3H]-batrachotoxin binding to voltage-gated sodium channels (VGSCs), and therefore increases sodium influx in neural cells (IC
50 = 93 nM; EC
50 = 2.3 μM). A subsequent study isolated and identified hoiamide B (
Figure 10h) and hoiamide C (
Figure 10i) from other marine cyanobacteria
[52]. Notably, that study reported that hoiamide B also promotes sodium influx into neocortical neural cells (EC
50 = 3.9 μM)
5. Lariat-Type Cyclic Depsipeptides
Lariat-type cyclic depsipeptides are a class of compounds with a wide range of ring sizes and tail lengths. What they all have in common is that they contain non-peptide polyketide regions within their structures
[53]. Many of these compounds have relatively low polarity and therefore have good membrane permeability, allowing them to affect intracellular targets. Additionally, many compounds of this class have good serine protease inhibitory activity. The lyngbyastatins are one such class of molecules with noteworthy biological properties. Cyanobacterial-derived lynbyastatins include lynbyastatin 7 (
Figure 11a), bouillomide A (
Figure 11b), bouillomide B (
Figure 11c), kempopeptin A (
Figure 11d), kempopeptin B (
Figure 11e), molassamide (
Figure 11f), symplocamide A (
Figure 11g), pompanopeptin A (
Figure 11h) and pompanopeptin B (
Figure 11i). As serine proteases are involved in multiple important processes in human health, including immunological responses and blood clotting, compounds that modulate serine protease activities may have profound effects on inflammation, cancer, cardiac health, etc.
Figure 11. Cyanobacterial lynbyastatins and kempopeptins: (a) lynbyastatin 7; (b) bouillomide A; (c) bouillomide B; (d) kempopeptin A; (e) kempopeptin B; (f) molassamide; (g) symplocamide A; (h) pompanopeptin A; (i) pompanopeptin B.
Lynbyastatin 7 was originally isolated and characterised from
Lyngbya confervoides [54]. The authors of that study also reported strong elastase inhibitory activity for lynbyastatin 7 (IC
50 = 8.3 nM), as well as similar activity for the structural analogues lynbyastatins 5 and 6. In another study, bouillomide A and bouillomide B were isolated from
Lyngbya bouillonii and their structures were elucidated
[55]. These compounds were reported to be selective inhibitors of serine proteases, with substantial inhibitory effects against elastase (IC
50 = 1.2 μM for both compounds) and chymotrypsin (IC
50 values of 2.6 and 0.32 μM for bouillomides A and B, respectively). Notably, their inhibitory activity was substantially lower against the other serine proteases tested.
Kemopopetins A and B were isolated from an unidentified marine cyanobacterium of the genus
Lyngbya in 2008
[56]. These compounds were noteworthy inhibitors of elastase and chymotrypsin activity, although kemopopetin A had the most potent inhibitory activity (IC
50 values of 0.32 and 2.6 μM against elastase and chymotrypsin, respectively). In a different study, molassamide was isolated from the cyanobacterium
Dichothrix utahensis, making it the first natural product identified in any species from the genus
Dichothrix [57]. Molassamide was also a good inhibitor of elastase and chymotrypsin activity (IC
50 values of 32 and 234 nM, respectively).
In another study, symplocamide A was identified in an unidentified marine bacterium of the
Symploca genus
[58]. The structure was unusual amongst this class of compounds as it contained citrilline and N,O-dimethyl-bromo-tyrosine residues within the cyclic backbone structure. The authors of that study reported that symplocamide A was a good inhibitor of chymotrypsin and trypsin protease activity (IC
50 = 0.38 and 80.2 μM, respectively). Additionally, this compound had potent cytotoxicity towards NCI-H460 human lung cancer cells (IC
50 = 40 nM) and murine neuro-2a neuroblastoma cells (IC
50 = 29 nM). Symplocamide A was also a noteworthy inhibitor of
Plasmodium falciparum growth (IC
50 = 950 nM).
Pompanopeptins A (
Figure 11h) and B (
Figure 11i) were isolated and characterised from
Lyngbya confervoides [59]. Despite their similar names, these compounds are not structurally related, due to the presence of a
N-methyl-2-amino-6-(4′-hydroxy-phenyl) hexanoic acid moiety in the cyclic backbone of pompanopeptin B. Pompanopeptin A (but not pompanopeptin B) was determined to be a selective inhibitor of trypsin, with an IC
50 of 2.4 μM. Notably, with the exception of the antiprotozoal activities of some compounds discussed above, the anti-pathogenic properties of all lyngbyastatins have been largely neglected and substantially more work is required to understand their therapeutic potential.
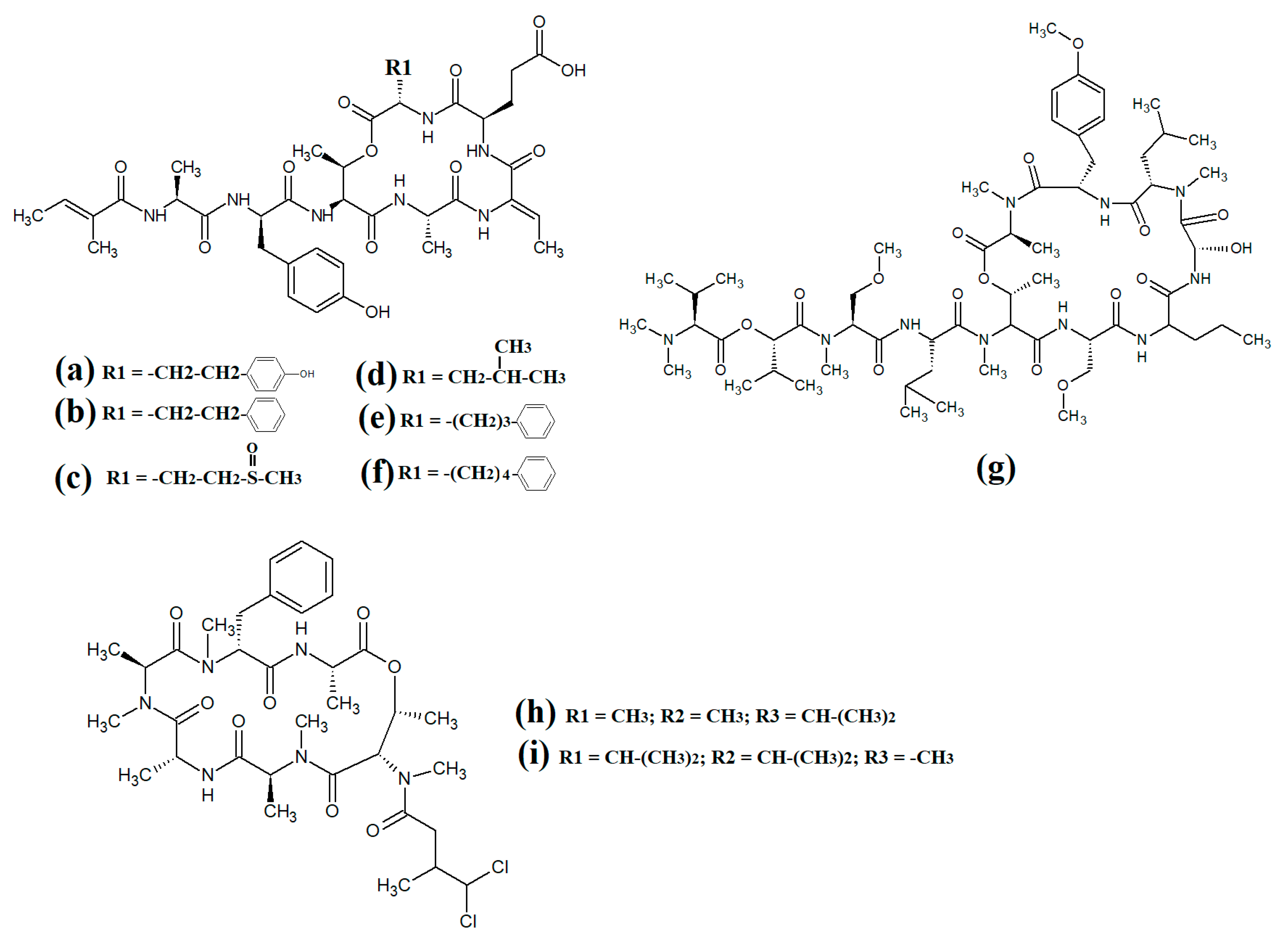
Further lariat-type cyclic depsipeptides have been isolated from
Lyngbya confervoides. In particular, the structural analogues tiglicamide A (
Figure 12a), tiglicamide B (
Figure 12b), tiglicamide C (
Figure 12c), largamide A (
Figure 12d), largamide B (
Figure 12e) and largamide C (
Figure 12f) were identified and their structures were determined to differ by a single amino acid residue in the cyclic backbone
[60]. The authors reported that these compounds were moderate inhibitors of elastase activity, with IC
50 values ranging from 0.53 to 7.3 μM.
Figure 12. Lariat-type cyanobacterial compounds: (a) tiglicamide A; (b) tiglicamide B; (c) tiglicamide C; (d) largamide A; (e) largamide B; (f) largamide C; (g) coibamide A; (h) itralamide A; (i) itralamide B.
Another study reported the isolation and characterisation of coibamide A (
Figure 12g)
[61]. The authors reported that the compound was highly
N-methylated, with eight of the eleven amino acids in the macrocyclic group containing
N-methyl moieties. Interestingly, coibamide A displayed potent cytotoxicity against human NCI-H460 lung cancer cells and murine neuro-2a cells (LC
50 values < 23 nM against both cell lines). Additionally, coibamide A was screened against the 60 human cancer cell lines of the NCI panel, and the authors reported substantial toxicity against MDA-MB-231 (IC
50 = 2.8 nM), LOX IMVI (IC
50 = 7.4 nM), HL-60(TB) (IC
50 = 7.4 nM) and SNB-75 cells (IC
50 = 7.6 nM)
[61].
Itralamide A (
Figure 12h) and itralamide B (
Figure 12i) were isolated from
Lyngbya majuscula and their structures were reported
[6]. Interestingly, both compounds contained a branched chlorinated group (4,4-dichloro-3-methylbutanoic acid) linked to N-methyl-threonine. The itralamides are both toxic, although itralamide B was particularly toxic towards human HEK-293 embryonic kidney cells (IC
50 = 6 μM).
6. Cyclic Depsipeptides with Extensive Polyketide Chain
Laingolide B (
Figure 13a) was isolated from the marine cyanobacteria
Lyngbya bouillonii and structurally characterised
[25]. Unfortunately, this compound is highly labile and biological activity testing was not undertaken in that study. Palmyrolide (
Figure 13b) contains a similar cyclic structure, with extensive polyketone groups, and was isolated from mixed cyanobacterial cultures rich in
Oscillatoria spp.
[62]. Interestingly, palmyrolide A was a moderate inhibitor of calcium influx in cerebrocortical neurons (IC
50 = 3.7 μM) via blocking of sodium channels (IC
50 = 5.2 μM). In another study, alotamide A (
Figure 13c) was isolated from
Lyngbya bouillonii and it was determined to consist of a macrocyclic structure containing seven acetate units, as well as N-methylvaline, a thiazoline moiety and a proline, linked by a polyketide group
[63]. Interestingly, alotamide A was reported to activate calcium influx in cerebrocortical neurons (IC
50 = 4.2 μM).
Figure 13. Cyclic cyanobacterial depsipeptides with extensive polyketone chains: (a) laingolide B; (b) palmyrolide A (c) alotamide A; (d) lagunamide A; (e) lagunamide B; (f) malevamide E.
A related class of compounds (lagunamides) were also isolated from
Lyngbya majuscula extracts and their structures were elucidated
[64]. The authors of that study reported that both lagunamide A (
Figure 13d) and langunamide B (
Figure 13e) were strongly cytotoxic towards murine P338 leukaemia cells (IC
50 values of 6.4 and 20.5 nM, respectively). These compounds also had significant antimalarial potential, with IC
50 values of 0.2 and 0.9 μM against
Plasmodium falciparium. Additionally, lagunamide A and lagunamide B had substantial anti-swarming activity against
Pseudomonas aeruginosa, although IC
50 values were not reported in that study, making comparisons difficult. Despite this wide range of promising bioactivities, most pathogens of human importance are yet to be tested using these compounds.
Malevamide E (
Figure 13f) is a structural analogue of dolastatin that was isolated from the marine cyanobacterium
Symploca laete-viridis [65]. This compound is a noteworthy inhibitor of calcium entry into immortalised human HEK embryonic kidney cells treated with thapsigargin. However, the mechanism of inhibition was not reported.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28207127