Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
肿瘤疫苗代表了肿瘤学的突破,利用免疫治疗原理刺激人体免疫系统对抗癌症,与手术、放疗和化疗等传统治疗相比,提供了一种更有针对性的方法,副作用更少。
- TME
- cancer immunology
- oncology vaccines
- combination therapies
1. 简介
根据2020年进行的一项全面的流行病学调查,恶性肿瘤作为全球发病率和死亡率的主要诱因一直占据首位,令人震惊的是,该年内新发癌症发病率约为19万例,死亡近3万例[10]。卫生和医疗系统面临着巨大的挑战。然而,癌症治疗的新希望以肿瘤疫苗的形式出现。肿瘤疫苗代表了肿瘤学的突破,它利用免疫治疗原理刺激人体免疫系统对抗癌症,与手术、放疗和化疗等常规治疗相比,提供了一种更具针对性的方法,副作用更少[1]。
癌症疫苗的演变已经展开了几十年,每个时代都以关键的进步为标志(图1)。这段旅程始于19世纪末的威廉·科利(William Coley),他在注意到感染经常导致癌症患者的肿瘤缩小后,产生了科利毒素[3]。这种方法利用免疫系统来对抗癌症——刘易斯·托马斯和弗兰克·麦克法兰·伯内特在20世纪中叶进一步扩展了这一概念,他们引入了癌症免疫编辑和肿瘤免疫的思想[4]。临床批准的癌症疫苗的曙光于2010年到来,FDA批准了sipuleucel-T,这是第一种针对前列腺癌的治疗性癌症疫苗[5]。随后的时代一直持续到今天,其特点是现代癌症疫苗和检查点抑制剂的开发,例如2021年批准用于治疗淋巴瘤的联合疗法[6]。目前,癌症疫苗的开发强调个性化,正在研究多种策略,包括新抗原疫苗、DNA/RNA疫苗和病毒载体,旨在针对个体患者的肿瘤量身定制治疗方法。
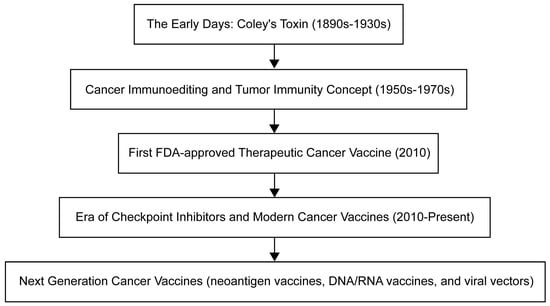
图1.肿瘤疫苗开发时间表。
深入研究机制复杂性,肿瘤在其中存在和进化的肿瘤微环境(TME)在肿瘤进展中起着关键作用。因此,TME已成为抗癌治疗药物进化领域的科学研究中心[7]。作为当代医学创新的证明,肿瘤疫苗经过精心设计,以利用宿主免疫系统的先天和适应性来靶向异常细胞。这些开创性的配方战略性地调节TME,从而明智地最大限度地减少对健康组织造成的附带破坏[8]。
随着各种肿瘤疫苗的开发,癌症免疫治疗领域正在经历重大进展,每种疫苗都有其独特的优势和挑战。肽疫苗虽然可有效引发免疫应答,但通常需要使用佐剂[9]。基于DNA/RNA的疫苗允许抗原的内源性产生,但在递送和表达方面面临障碍[10,11]。基于病毒载体的疫苗可以刺激强大的免疫应答,但受到安全问题和预先存在的免疫力的限制[12]。虽然基于树突状细胞的疫苗可以诱导强大的反应,但它们需要复杂的生产过程和不同的患者反应[13]。基于全细胞的疫苗在利用整个肿瘤细胞触发免疫反应的同时,努力解决生产和标准化问题[14]。深入了解这些疫苗及其局限性对于其在癌症免疫治疗中的有效部署至关重要[15]。目前的研究认识到肿瘤疫苗的独立疗效有限,正在通过与其他治疗方法(如免疫检查点抑制剂、化疗、放疗、靶向治疗和溶瘤病毒疗法)联合使用来寻求增强治疗效果[16,17,18,19]。同时,个性化癌症疫苗的创新领域正在获得牵引力,为个体特异性肿瘤提供量身定制的免疫反应[8]。尽管存在一些挑战,包括新抗原鉴定的复杂性和资源密集型生产,但技术进步有望为个性化癌症疫苗带来更快、更实惠的未来[20,21,22,23]。
2. 肿瘤微环境
肿瘤微环境(TME)对癌症的发作和进展至关重要,是科学家头疼的问题。它不仅是动态的,而且非常复杂,塑造了肿瘤的异质性。TME是多种细胞和非细胞成分(如癌细胞、基质细胞、免疫细胞、ECM)和信号分子(如生长因子和细胞因子)的混合物[24,25]。ECM是蛋白质,糖蛋白和蛋白聚糖的纠结网络,通过生化和生物力学信号提供结构并塑造细胞行为。当癌症改变ECM的组成时,它可以促进血管生成、免疫逃避和治疗抵抗[26,27]。例如,如赖氨氧化酶(LOX)过表达所见,ECM硬度增加,通过FAK激活等机制促进细胞增殖和存活,同时也增强肿瘤侵袭性[28]。同样,透明质酸的积累通常与预后不良有关,可以促进肿瘤生长并促进免疫逃逸[29]。基质金属蛋白酶(MMP)表达的改变可以重塑ECM,通过释放生长因子有利于肿瘤侵袭[30,31]。此外,当过度表达时,弹性蛋白、层粘连蛋白、tenascin-C和骨膜蛋白等特异性ECM蛋白支持肿瘤细胞迁移和存活[32]。值得注意的是,胶原取向的变化导致纤维排列,为增强肿瘤细胞迁移提供了途径,这种排列通常表明转移的风险更高[33]。ECM还参与各种生长因子的分泌,如转化生长因子-β(TGF-β),白细胞介素-1β(IL-1β),IL-6,肿瘤坏死因子-α(TNF-α)和血管内皮生长因子(VEGF),这些因子由TME的各个细胞分泌,可以启动肿瘤细胞的生长,存活,迁移,血管生成和上皮-间充质转化(EMT)。这是通过调节其特异性受体和刺激信号通路来实现的[34](图2)。ECM和肿瘤细胞之间这种错综复杂的相互作用不仅推动了癌症的进展,而且由于药物渗透屏障和细胞存活途径的激活等因素,也给治疗带来了挑战。
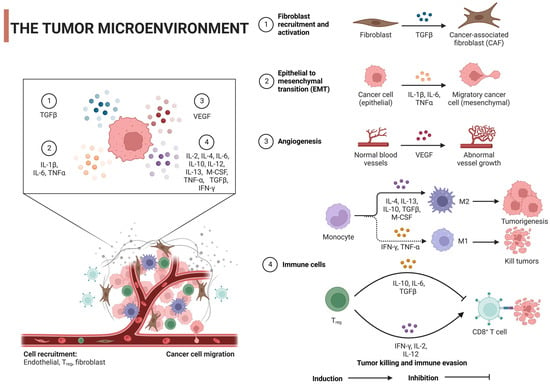
图2.肿瘤微环境对肿瘤细胞的调控机制:TGF-β,转化生长因子-β;IL,白细胞介素;VEGF,血管内皮生长因子;TNF-α,肿瘤坏死因子α;IFN-γ,干扰素γ;M-脑脊液,巨噬细胞集落刺激因子;Tregs,调节性T细胞。用 BioRender.com 创建。
同样,免疫细胞功能是由多种元素混合形成的。肿瘤细胞可分泌免疫抑制细胞因子和检查点配体,改变免疫应答[35,36],而肿瘤代谢变化引起的缺氧和酸性pH等疾病可抑制免疫活性[37,38]。同时,肿瘤消耗葡萄糖、细胞外基质改变和免疫抑制细胞募集引起的代谢竞争可抑制有效的免疫应答[39]。TME内的直接细胞间相互作用、肠道微生物组对肿瘤免疫的影响、治疗干预以及慢性炎症的存在进一步调节了促肿瘤和抗肿瘤作用之间的平衡[40,41]。这些因素的相互作用决定了TME内免疫反应的复杂性和动态性。
关于免疫细胞,如TAM和T细胞,这些细胞可以双向振荡[42,43]。它们可以驱动肿瘤生长或阻止肿瘤生长。TME表型和细胞因子控制所有这些细胞和非细胞成分的表达[44]。TAM是TME中的关键细胞,具有促进或抑制肿瘤进展的功能可塑性。这在很大程度上受到TME中存在的各种细胞因子的影响,导致TAM采用两种主要表型之一:具有抗肿瘤活性的经典活化(M1)巨噬细胞和通常为前肿瘤的替代活化(M2)巨噬细胞[45]。某些细胞因子,如干扰素-γ(IFN-γ),主要由T细胞和NK细胞产生,可以驱动TAM向M1表型发展。这些M1巨噬细胞的特点是能够呈递抗原、杀死肿瘤细胞并产生促炎细胞因子(如IL-12和TNF-α),从而增强抗肿瘤免疫力[45]。TNF-α也在诱导巨噬细胞M1极化中发挥作用,从而增加其杀瘤活性[46]。然而,其他细胞因子,如IL-4和IL-13,两者都主要由Th2细胞产生,可以诱导巨噬细胞向M2表型极化,这通常与组织修复、免疫调节和肿瘤促进有关[47]。同样,IL-10(一种抗炎细胞因子)也可驱动TAM向M2表型移动,导致巨噬细胞通常具有免疫抑制作用,并产生VEGF和EGF等因子,促进肿瘤生长和血管生成[45]。最后,已知TGF-β可诱导M2极化,并且还具有多种促肿瘤作用,包括促进免疫逃避、组织重塑和血管生成[48]。
T细胞,尤其是细胞毒性T细胞(CTL)和辅助性T细胞,在抗肿瘤免疫中起着关键作用,它们的激活、增殖和功能受到TME中各种细胞因子的影响。几种细胞因子推动T细胞对抗肿瘤。干扰素-γ(IFN-γ)主要由Th1细胞和CTL产生,可增强CTL的细胞毒性活性,促进其肿瘤杀伤能力,并增加MHC I类分子在肿瘤细胞上的表达,使其更容易受到CTL介导的杀伤[49]。IL-2在T细胞的生长和分化中起着至关重要的作用,它常用于癌症免疫治疗,主要通过促进CTL和NK细胞的增殖和活化来增强免疫系统抗癌的能力[50]。IL-12引导幼稚T细胞分化为Th1细胞,从而产生IFN-γ并促进CTL介导的肿瘤破坏[51]。另一方面,一些细胞因子诱导肿瘤发生。IL-10是一种免疫抑制细胞因子,可阻碍效应T细胞和抗原呈递细胞的功能,可能通过抑制抗肿瘤免疫来促进肿瘤生长[52]。TGF-β可以抑制T细胞和其他免疫细胞的功能,有助于肿瘤免疫逃逸。它还在癌症后期表现出促肿瘤作用,促进肿瘤细胞侵袭和转移,并启动血管生成[53]。最后,IL-6可以通过促进慢性炎症、细胞存活和血管生成来帮助肿瘤发生,并且它参与将T细胞分化为Th17细胞,在某些情况下,Th54细胞与促进炎症和肿瘤生长有关[<>]。
TME为癌症治疗提供了有前途的治疗途径。免疫抑制细胞因子如TGF-β当受到抑制时,可能会恢复抗肿瘤免疫应答并减少转移[55]。虽然IL-10通常抑制TME免疫,但其细微的作用表明调节其水平有潜在的益处,而抑制血管生成和免疫抑制性VEGF催生了FDA批准的贝伐珠单抗等疗法[56,57]。在免疫刺激方面,已知可促进T细胞生长的IL-2已经看到了治疗应用,尽管有副作用,IL-12对免疫细胞的有效激活暗示了其组合治疗潜力[58,59]。此外,靶向PD-1/PD-L1轴的检查点抑制剂(如帕博利珠单抗)可使耗尽的T细胞恢复活力以抵消肿瘤[60]。
TME共同对癌症疫苗的成功产生了重大影响。虽然疫苗旨在激活针对肿瘤抗原的免疫细胞,但TME的免疫抑制性质会阻碍这些活化的T细胞。肿瘤抗原呈递改变、MHC分子表达降低和免疫检查点表达等因素会进一步阻碍疫苗诱导的反应[61,62]。为了提高癌症疫苗的疗效,研究人员正在探索与检查点抑制剂的联合疗法、减少TME中免疫抑制细胞数量的方法、突破TME物理屏障的策略、强效佐剂的结合、细胞因子调节以及开发针对个体肿瘤抗原谱的个性化疫苗[63,64,65].这些针对疫苗机制和TME的多管齐下的战略正在指导下一代癌症治疗的方向。
3. 肿瘤疫苗
3.1. 癌症免疫学
适应性免疫或获得性免疫是对病原体和异常细胞的高度特异性和持久的防御,主要由T和B淋巴细胞管理[66]。这种类型的免疫以其免疫记忆而闻名,可以持久地抵御以前遇到过的东西,如病原体或抗原[67]。研究适应性免疫的来龙去脉对于制造疫苗和靶向免疫疗法至关重要。这些突破为预防和治疗从感染和自身免疫性疾病到癌症等一系列疾病提供了巨大的潜力[68,69,70]。
在癌症的早期阶段,免疫系统参与一个称为免疫监视的过程,在那里它追捕并消灭异常细胞,阻止肿瘤形成[71,72]。但狡猾的癌细胞使用不同的技巧来躲避免疫系统,帮助肿瘤生长和进展[73]。通过研究这些免疫逃避策略,科学家们提出了新的免疫疗法,如免疫检查点抑制剂(ICIs)和过继细胞转移。这些创新疗法旨在提高身体对抗肿瘤的能力,并克服癌症躲避免疫系统的技能[74]。
免疫疗法,如ICI和嵌合抗原受体(CAR)T细胞疗法,通过使用免疫系统对抗癌细胞,完全改变了癌症的治疗[75]。但即使有这些惊人的进展,一些患者对免疫疗法的反应不佳,甚至可能对免疫疗法产生耐药性,这意味着需要更多地了解免疫系统和癌症如何相互作用才能学习[76]。获得这些知识将有助于创建新的免疫治疗策略,并找到预测性生物标志物,使患者结局更好[77,78]。
3.2. 作用机制
癌症疫苗的基本功能在于它们动员先天性和适应性免疫应答的能力,以识别、对抗和根除肿瘤细胞[20]。以下论述将全面分析其错综复杂的作用机制(图3)。
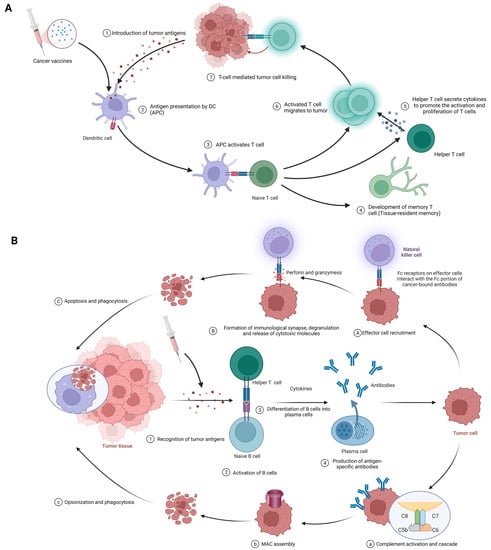
Figure 3. (A) Cellular immune response against cancer (step 1 to step 7); APCs, antigen-presenting cells; APC, antigen-presenting cell. (B) Humoral immunity response against cancer (step 1 to step 4). A–C; antibody-dependent cell-mediated cytotoxicity; a–c: complement-dependent cytotoxicity; MAC, membrane attack complex. Created with BioRender.com.
3.2.1. Cellular Immunity
The procedure of eliciting a cellular immune response against cancer, exemplified by the use of cancer vaccines, is complex and sequential. It commences with the delivery of tumor antigens and concludes with the activation of humoral immunity. The following is a detailed breakdown of each phase in this process (Figure 3A).
Introduction of tumor antigens: With cancer vaccines, referring to tumor antigens being introduced to antigen-presenting cells (APCs) like dendritic cells. Options include whole tumor cells, peptides, proteins, DNA, mRNA, or even dendritic cells loaded with tumor antigens or packing tumor-derived genetic material. Tumor vaccines play a role in this step. This crucial first step gets the ball rolling in the immune response against cancer [18,79].
Antigen processing and presentation: APCs capture, process, and present tumor-derived peptides on their surface. Dendritic cells efficiently cross-present exogenous antigens to both MHC class I and II molecules, activating both CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ helper T cells [18,80].
Activation of T cells: Presentation of tumor antigens by APCs activates and expands antigen-specific T cells. T-cell activation requires antigen recognition via the T-cell receptor (TCR) and costimulatory signals provided by the interaction between costimulatory molecules on APCs and their receptors on T cells [81,82].
CTLs and helper T cells in action: CTLs directly kill tumor cells by recognizing and binding to MHC class I molecules presenting tumor antigens, while helper T cells produce cytokines that support CTLs’ activation, proliferation, and differentiation. CD4+ helper T cells also provide help to B cells, facilitating antibody production and enhancing the function of APCs and CTLs [2,42].
刺激体液免疫:癌症疫苗诱导B细胞产生抗原特异性抗体。这些抗体靶向并消除肿瘤细胞[15,83]。
3.2.2. 体液免疫
该程序从识别肿瘤抗原开始,最终实现由抗体介导的效应功能。以下是此过程中每个阶段的详细信息。(图3B)。
肿瘤抗原的识别:与细胞免疫一样,肿瘤细胞具有免疫系统可以识别的肿瘤相关抗原(TAAs)和肿瘤特异性抗原(TSA)[84]。
B细胞的活化:B细胞通过其B细胞受体(BCR)检测和识别肿瘤抗原。加入一些来自辅助性T细胞的共刺激信号,B细胞被激活以增殖和分化[72]。
B细胞分化为浆细胞:激活后,B细胞转变为浆细胞,专门用于产生大量针对已识别肿瘤抗原的特异性抗体[84]。
抗原特异性抗体的产生:浆细胞产生抗原特异性抗体,这些抗体通过血流并附着在癌细胞上的肿瘤抗原上[85]。
抗体的效应功能:一旦结合,抗体就会对肿瘤细胞采取各种策略,例如:
-
生长因子的中和和信号通路的抑制:抗体阻碍肿瘤细胞增殖促进生长因子,并阻碍对癌细胞存活和侵袭至关重要的信号通路[15]。
第4章 癌症疫苗的种类和特点
4.1. 肽疫苗
肽疫苗代表了一种潜在的癌症免疫治疗方法,该方法采用源自肿瘤特异性或肿瘤相关抗原(TAAs)的短氨基酸序列来引起针对恶性细胞的靶向免疫反应[94]。TAAs(包括分化抗原、过表达抗原、癌/睾丸抗原和突变抗原)是多种免疫治疗技术(如癌症疫苗、过继性T细胞疗法和免疫检查点抑制剂)的可行靶点[95,96]。与全蛋白或减毒活疫苗相比,肽疫苗具有多种优点,包括易于合成、特异性和良好的安全性,因为肽疫苗引发自身免疫应答的可能性较低[97,98]。然而,它们遇到了免疫原性欠佳、体内降解迅速、CD4+T细胞反应弱以及与免疫逃避和肿瘤诱导的免疫抑制相关的挑战等局限性[97]。为了克服这些局限性,策略包括加入佐剂、优化肽序列、利用载体增强稳定性和免疫原性,以及将肽疫苗与其他免疫疗法联合使用[94]。
目前,相对成熟的多肽疫苗包括Nelipepimut-S(NeuVax)、CIMAvax-EGF和基于MUC1的多肽疫苗。Nelipepimut-S,也称为NeuVax,是一种靶向表达HER2/neu的癌细胞的肽疫苗,主要针对不适合标准HER2治疗的早期HER1 2+和2+乳腺癌患者[99]。它将HER75/neu的E2肽与GM-CSF结合,作为增强免疫应答的佐剂[100]。虽然其范围可能涵盖其他HER2/neu癌症,如卵巢癌和胃癌,但其临床开发遇到了障碍[101]。最近的III.期试验结果表明,Nelipepimut-S在乳腺癌患者中仍表现出良好的疗效和耐受性[102]。同样,靶向非小细胞肺癌(NSCLC)表皮生长因子的CIMAvax-EGF将重组EGF与蛋白质载体合并。它已显示出延长晚期肺癌患者生命的希望,已完成III.期试验[103,104]。此外,基于MUC1的肽疫苗侧重于乳腺癌和胰腺癌等癌症中异常表达的糖蛋白[105]。尽管一些药物已进入I.期和II.期试验,且安全性和免疫指标良好,但引发强有力的临床反应仍然很复杂,因此需要探索联合治疗[106,107]。总的来说,这些疫苗代表了尖端的癌症治疗方法,每种方法都有其独特的靶点和开发阶段。
4.2. 基于 DNA/RNA 的疫苗
基于DNA/RNA的肿瘤疫苗的工作原理是将编码肿瘤抗原的遗传物质输送到宿主细胞。这种方法利用病毒载体、脂质纳米颗粒或裸核酸等各种载体,刺激免疫系统识别和破坏癌细胞,从而产生适应性免疫反应[108]。与传统方法相比,这些疫苗具有多种优势,包括安全性、易于制造、强大的免疫反应诱导和对修饰的适应性。它们还提供了个性化的可能性,以满足每个患者独特的肿瘤特征[109,110]。尽管它们很有希望,但诸如将DNA/RNA有效递送和摄取到细胞中、自身免疫反应的风险以及个性化成本和时间的限制等挑战构成了重大障碍[111,112,113]。
然而,最近的进展令人鼓舞,包括临床试验中的几种疫苗和技术进步提高了疫苗递送的有效性[114]。该领域期待利用基因组测序、生物信息学和纳米技术的进步来克服当前的局限性,渴望将这些强效疫苗与其他免疫治疗策略相结合,以全面根除癌症[115,116]。其中,脂质纳米颗粒(LNP)等技术在mRNA COVID-19疫苗中具有开创性意义,正在被应用于癌症疫苗开发,以促进肿瘤特异性抗原的递送[117,118]。电穿孔和病毒载体(如腺病毒)可增强DNA/RNA的摄取,而非病毒纳米载体和微针贴片旨在增强这种递送,而不会诱导强烈的抗载体反应[119,120]。为了降低自身免疫风险,研究人员强调肿瘤特异性抗原选择、降低交叉反应性的序列优化以及瞬时表达技术,例如mRNA疫苗固有的技术[121,122]。此外,正在利用为靶向递送量身定制的破耐受性佐剂和纳米颗粒来微调免疫应答,最大限度地提高抗肿瘤功效,同时最大限度地减少对健康组织的附带损害[121,123]。总的来说,这些创新强调了癌症疫苗设计不断发展的格局,平衡了有效的肿瘤靶向与患者安全。CV9104是一种靶向前列腺癌的基于mRNA的癌症疫苗[124]。其进展达到了转移性去势抵抗性前列腺癌(mCRPC)的II.期试验[124]。这种疫苗代表了mRNA在肿瘤学中的创新应用。
4.3. 基于病毒载体的疫苗
基于病毒载体的肿瘤疫苗是癌症免疫治疗中一个有希望的发展[11]。这些疫苗利用病毒的先天能力浸润宿主细胞并有效递送肿瘤抗原,引发强烈的靶向免疫应答[125,126,127]。这种方法促使宿主细胞在感染后产生肿瘤特异性或相关抗原,导致这些抗原被显示给T细胞,随后启动对肿瘤细胞的强力防御[128]。这些疫苗的显着优势之一是它们能够触发有效的细胞和体液免疫反应。它们还可以被设计成表达多种肿瘤抗原,扩大其范围并增强免疫应答的效力[129,130]。尽管有这些好处,但仍需要解决某些挑战,例如先前存在的病毒载体免疫力的影响以及与大规模生产相关的后勤问题[131,132]。
在这些挑战中,预先存在的对病毒载体的免疫对其在肿瘤疫苗中的使用构成了重大挑战,因为免疫系统可能会在载体产生治疗作用之前中和载体[133]。为了解决这个问题,研究人员正在探索一系列策略:使用人类暴露有限的稀有或新型病毒载体,假分型改变病毒包膜蛋白,采用不同载体的异源初免-增强策略,对病毒衣壳进行遗传修饰以降低可识别性,与免疫调节剂共同给药以暂时抑制某些免疫反应,选择非静脉内递送途径,如肿瘤内给药以避免高抗体浓度,并调整剂量,要么使用高载体剂量来克服中和作用,要么重复给予低剂量以逃避免疫检测[134,135,136,137]。此外,正在探索佐剂,以将免疫应答的焦点从载体转移到递送的肿瘤抗原[138,139]。这些多方面的方法旨在优化基于病毒载体的肿瘤疫苗在面对预先存在的免疫力时的功效。
OncoVEXGM-CSF或T-VEC(T-VEC)是一种溶瘤性HSV-1疫苗,针对肿瘤选择性和GM-CSF产生进行了修饰,主要针对黑色素瘤[140]。在一项成功的III.期试验后,该药获得了FDA对不可切除的复发性黑色素瘤的批准[140]。CG0070是另一种基于腺病毒的疫苗,用于在Rb通路缺陷的癌细胞中选择性复制,并靶向膀胱癌[141]。LV305是一种基于慢病毒的疫苗,将NY-ESO-1抗原基因递送至树突状细胞,靶向表达NY-ESO-1的癌症,如黑色素瘤和肉瘤[142]。JX-594或Pexa-Vec是一种基于牛痘病毒的疫苗,经过修饰以表达GM-CSF并选择性靶向具有高胸苷激酶活性的癌细胞,并且已经进行了多项试验,包括一项针对肝细胞癌的III.期试验[143,144,145]。这些代表了肿瘤学中病毒疗法和免疫疗法的创新交叉点。
4.4. 基于树突状细胞的疫苗
树突状细胞(DC)通过连接先天免疫系统和适应性免疫系统来介导免疫应答,并且在抗原呈递和随后的T细胞活化中至关重要,因此发现它们与癌症免疫治疗策略具有实质性相关性[18,146]。这源于基于DC的癌症疫苗,该疫苗利用装有肿瘤相关抗原(TAA)的DC来促使针对癌细胞产生强大的免疫应答[147]。用TAA加载这些DC的方法多种多样,从使用肿瘤裂解物和合成肽到编码肿瘤抗原的mRNA[148]。临床试验强调了这些基于DC的疫苗在各种癌症中的前景。尽管存在这种令人兴奋的潜力,但挑战仍然存在,包括DC疫苗生产中的技术困难、疫苗效力变化、免疫抑制肿瘤微环境以及缺乏用于患者选择的可靠生物标志物[149]。然而,基于新抗原的疫苗等个体化癌症免疫疗法的最新进展为基于DC的疫苗提供了有希望的机会,将这些疫苗与其他治疗方法相结合可能会增强其疗效[8]。在最近的一项研究中,研究人员引入了一种使用叠氮糖的代谢聚糖标记技术来增强DC疫苗[150]。该方法不仅可以促进DC活化和抗原呈递,还可以促进细胞因子的有效偶联[150]。此外,它有望在各种肿瘤中广泛应用,为调节DC与其他免疫细胞之间的相互作用提供平台,并增强树突状细胞疫苗的抗肿瘤功效。
杰出的代表包括Provenge和DCVax-L。Provenge(sipuleucel-T)是FDA批准的用于晚期前列腺癌的自体细胞免疫疗法[5]。它使用暴露于融合蛋白PA2024的患者外周血单核细胞(PBMC),该融合蛋白PA151将来自前列腺癌细胞的抗原与免疫激活剂GM-CSF结合,引发针对表达抗原的前列腺癌细胞的免疫反应[152,153,154,155,156]。另一方面,DCVax-L是一种针对多形性胶质母细胞瘤(GBM)的自体树突状细胞疫苗[156]。该疫苗是通过从患者自身的肿瘤组织中加载肿瘤裂解物来制备患者的树突状细胞,使免疫系统能够识别和攻击相应的癌细胞[1]。两种疫苗都利用树突状细胞靶向癌症,但它们的临床历程和疾病靶点不同(表<>)。
4.5. 全细胞疫苗
基于全细胞的疫苗通过结合大量肿瘤相关抗原来刺激有效的免疫反应,为癌症免疫治疗提供了一种全面的方法。从机制上讲,这些疫苗利用辐照的肿瘤细胞(自体或同种异体)使免疫系统暴露于肿瘤的全部抗原库[157],从而诱导针对一系列肿瘤抗原的特异性和多价免疫应答[158]。该策略提供了广谱的已知和未知肿瘤抗原,避免了抗原丢失或下调(肿瘤采用的典型逃逸机制[157]),并且无需为每位患者识别特定抗原,这可能既耗时又昂贵[159]。但是,存在局限性。自体全细胞疫苗的生产可能是劳动密集型和个性化的,因此需要从每个患者身上分离和培养肿瘤细胞[159]。鉴于免疫抑制肿瘤微环境会限制疫苗效力,这些疫苗通常需要与佐剂或免疫调节剂共同给药以提高其免疫原性[8,159]。疫苗制剂中自身抗原诱导的潜在自身免疫也令人担忧[158]。因此,虽然基于全细胞的疫苗为癌症免疫治疗提供了一种有前途的方法,但需要进一步优化和完善这些策略以应对这些挑战和局限性。
Representatives include GVAX, Canvaxin, and Oncophage. GVAX is a whole-cell tumor vaccine, utilizing tumor cells genetically modified to secrete GM-CSF (an immune stimulant), and has been explored for cancers like pancreatic and prostate cancers, with mixed outcomes in later-phase trials [160,161]. Canvaxin, aimed at melanoma, combines irradiated autologous and allogeneic melanoma cells with the BCG adjuvant, but it failed to show significant survival benefits in a phase III trial for advanced melanoma [115,162]. Oncophage (Vitespen) is derived from patient-specific tumor heat shock proteins (HSPs) and primarily targets renal-cell carcinoma and melanoma [163,164]. It completed phase III trials with mixed results but secured approval in Russia for the treatment of kidney cancer [165]. While these vaccines showcase varied cancer immunotherapy strategies, each has faced challenges in late-stage clinical evaluations. (Table 1)
Table 1. Below is a tabular list of various tumor vaccines in the last decade.
| Types of Tumor Vaccines | Strengths | Weaknesses | Examples | Mechanisms of Action | Effects | Limitations | References |
|---|---|---|---|---|---|---|---|
| Peptide vaccines |
|
|
Nelipepimut-S (NeuVax) | HER2-derived peptide vaccine | Activation of T-cell response | Limited overall survival improvement | [166] |
| CIMAvax-EGF | EGF-based peptide vaccine | Inhibition of EGF signaling | No direct tumor targeting | [103] | |||
| MUC1-based peptide vaccine | Targeting MUC1 tumor-associated antigens | Enhanced immune response | Heterogeneous patient response | [167] | |||
| DNA/RNA-based vaccines |
|
|
CV9104 (CureVac) | Uses mRNA to encode six antigens overexpressed in prostate cancer | Induced antigen-specific immune responses in early clinical trials | Efficacy in late-stage trials yet to be established; possibility of inducing autoimmune responses | [168] |
| Viral-vector-based vaccines |
|
|
Adenovirus-based vaccines (OncoVEXGM-CSF, CG0070) | Adenoviruses are modified to express a tumor-specific antigen or an immunomodulatory molecule; these stimulate an immune response against the tumor | Effective in stimulating an immune response against the tumor | Immune response to the viral vector can limit repeat dosing | [169] |
| Lentivirus-based vaccines (LV305) | Lentiviruses are engineered to deliver tumor-specific antigens to dendritic cells to stimulate a T-cell response | Successful in initiating T-cell responses | Safety concerns over integration into the host genome | [23] | |||
| Vaccinia-virus-based vaccines (JX-594) |
Vaccinia viruses are genetically engineered to express a tumor antigen and/or immunostimulatory molecule; they can directly lyse cancer cells | Showed antitumor activity and were well tolerated in clinical trials | Immune response to the viral vector can limit its effectiveness | [170] | |||
| Dendritic-cell-based vaccines |
|
|
Provenge (Sipuleucel-T) | The patient’s own dendritic cells are exposed to a fusion protein (prostatic acid phosphatase linked to an immune cell stimulating factor) | Extended overall survival in metastatic castration-resistant prostate cancer | Limited clinical benefits, high cost, and complex manufacturing process | [5] |
| DCVax-L | Autologous dendritic cells are pulsed with tumor lysate | Prolonged progression-free survival in glioblastoma multiforme (GBM) patients | Not FDA-approved; requires personalized manufacturing | [171] | |||
| Whole-cell-based vaccines |
|
|
GVAX | Utilizes autologous/allogeneic tumor cells that have been genetically modified to secrete the immune-stimulating cytokine GM-CSF | Demonstrated a significant immune response against cancer, studied in various types of cancer, including pancreatic and prostate cancers | Production can be labor-intensive and personalized; often requires co-administration with adjuvants or other immunomodulatory agents to enhance their immunogenicity | [159,172] |
| Canvaxin | Allogeneic melanoma cells mixed with Bacillus Calmette–Guérin (BCG) to stimulate immune response | Intended for melanoma treatment, but development discontinued due to insufficient effectiveness | Limited efficacy; potential for BCG-related side effects | [173] | |||
| Oncophage (Vitespen) | Uses heat shock proteins (gp96) derived from the patient’s tumor as an autologous vaccine | Showed efficacy in extending disease-free survival in certain patients with kidney cancer and melanoma | Not universally effective; personalized manufacturing can be labor-intensive | [174,175] |
The preceding table encapsulates seminal instances of assorted classifications of cancer vaccines, encompassing peptide-based, DNA/RNA-based, viral-vector-based, dendritic-cell-based, and whole-cell-based vaccines. Each paradigm is delineated in exhaustive detail, supplemented by pertinent bibliographical citations for subsequent scholarly inquiry.
4.6. Another Cancer Vaccine Therapy: In Situ Cancer Vaccines
In situ cancer vaccines represent a therapeutic approach where the tumor inside a patient’s body is directly targeted to serve as its own vaccine [63]. Rather than extracting tumor cells for external processing and reintroduction, in situ vaccines stimulate the immune system by damaging the tumor in its native environment [63]. As the tumor cells die, they release antigens, which are then recognized by the immune system. Often, this is achieved by injecting immune-stimulating agents or oncolytic viruses into the tumor [176,177]. This not only aims to destroy the immediate tumor but also primes the immune system to recognize and combat tumor cells elsewhere in the body [176]. In situ cancer vaccines have shown promise in preliminary studies.
4.7. Influencing Factors of Tumor Vaccines
Boosting the power of cancer vaccines is a top priority for researchers, who are diving deep into adjuvants and combination therapies to ramp up immune responses, outsmart tumor immune evasion, and prevent cancers from coming back [178,179]. The efficacy of cancer vaccines hinges on several factors, including picking the right antigens, choosing the adjuvants wisely, and using the best delivery systems [2]. Antigen selection is of paramount significance. The antigens should be specific to the tumor, or associated with it, so that the immune response zeroes in on cancer cells without harming healthy tissues [180]. Plus, the chosen antigens need to be highly immunogenic and able to stimulate both CD8+ cytotoxic T cells and CD4+ helper T cells for a strong, long-lasting attack against tumors [15]. Adjuvants help by making cancer vaccines more immunogenic. They stimulate the innate immune system, encourage antigen uptake by APCs, and help activate and expand antigen-specific T cells [181,182]. There are different types of adjuvants, like alum, toll-like receptor (TLR) agonists, and cytokines, each with unique mechanisms of action and varying effectiveness [183,184]. The latest research shows that TLR agonists, such as TLR9 and TLR7/8 agonists, have shown promise by bolstering antigen-presenting cells and intensifying immune responses [185]. Researchers have developed a nanosystem that can inhibit a process called MerTK-mediated efferocytosis. This inhibition leads to the release of immunogenic contents into the tumor microenvironment, potentially boosting the body’s natural defenses against the tumor [186]. Similarly, STING agonists enhance dendritic cell activity, boosting T-cell responses against tumors [187,188]. Oncolytic viruses, while serving as direct antitumor agents, also act as adjuvants by releasing tumor antigens within an inflammatory milieu [132]. These recent breakthroughs encapsulate the dynamic progression in adjuvant research, aiming to optimize the immune system’s potency against tumors.
5. Combination Therapies
Combining cancer vaccines with other therapies has emerged as a promising strategy to enhance the overall therapeutic efficacy and overcome the limitations of single-agent treatments [189]. Cancer vaccines, which aim to stimulate a patient’s immune system to recognize and attack tumor cells, may benefit from being combined with other immunotherapies, such as immune checkpoint inhibitors, to boost immune responses and counteract immunosuppressive mechanisms within the tumor microenvironment (TME) [190].
Several types of combination therapies involving cancer vaccines and other treatment modalities have been explored in recent years, such as combining cancer vaccines with chemotherapy, targeted therapies, and radiation therapy [189]. These combination approaches hold significant promise for optimizing cancer treatment outcomes and providing more effective, personalized therapy options for patients [74].
5.1. Cancer Vaccine + Immune Checkpoint Inhibitors
These vaccines can be combined with other treatments to enhance their effectiveness. Immune checkpoint inhibitors like pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy) disable immune checkpoints, thereby unleashing a more potent attack on cancer cells [191,192,193,194]. This combination hopes to enhance recognition of cancer cells (via the vaccine) and amplify the immune response (via the checkpoint inhibitors) [5,178].
This combined approach has shown promise in preclinical models and early clinical trials by generating tumor-specific T cells and preventing their exhaustion [195]. The mechanism behind these effects is that cancer vaccines aim to boost T cells’ recognition of tumor antigens, but this immune response can be dampened by the tumor’s evasion mechanisms [196]. Enter immune checkpoint inhibitors, which block inhibitory checkpoints (PD-1 and CTLA-4) on T cells, essentially “releasing the brakes” and amplifying their antitumor activity [197]. By combining cancer vaccines, which enhance the number of tumor-recognizing T cells, with checkpoint inhibitors that ensure that these T cells are not suppressed, there is a synergistic boost in the antitumor immune response. Preliminary studies suggest that this combination augments tumor attack, potentially leading to improved patient outcomes [198,199,200,201].
5.2. Cancer Vaccine + Chemotherapy
Chemotherapy is a destructive force against cancer cells, hindering their growth and division, but may also inadvertently harm rapidly dividing normal cells such as those in bone marrow, the digestive tract, and skin [202]. The potential synergy between cancer vaccines and chemotherapy arises from some chemotherapeutic agents inducing immunogenic cell death, increasing the visibility of dying cancer cells to the immune system and potentially enhancing the efficacy of cancer vaccines [203]. Several chemotherapeutic agents have been identified to potentially enhance the efficacy of cancer vaccines due to their immunomodulatory effects. For instance, cyclophosphamide and temozolomide can deplete immune-suppressing regulatory T cells (Tregs), creating a more receptive tumor environment for vaccine action [204]. Docetaxel, used for cancers like breast and prostate cancers, can bolster antigen presentation, thereby enhancing immune recognition of tumor cells [205]. Gemcitabine targets and reduces myeloid-derived suppressor cells (MDSCs) [206]. When combined with cancer vaccines, these agents can modify the tumor environment, diminish immune suppression, or amplify the immune response against tumors, although the choice of combination depends on multiple factors, including cancer type and patient health [203].
However, there are substantial challenges to this approach. Determining the optimal timing and dosage of chemotherapy in relation to cancer vaccines remains a complex task [159]. The side effects of both chemotherapy and cancer vaccines, including chemotherapy’s often severe systemic side effects such as fatigue, infection, hair loss, and nausea, are a significant concern [207]. Furthermore, the treatment’s responsiveness is limited, as not all cancer types respond well to chemotherapy or cancer vaccines, with variability in individual patient responses adding to the complexity of treatment plans [208,209]. Additionally, the complexity of the tumor microenvironment, which can evolve various mechanisms to resist or evade treatment, may limit the effectiveness of these combined therapies [210].
5.3. Cancer Vaccine + Radiotherapy
Radiotherapy employs high-energy particles or waves, such as X-rays, gamma rays, electron beams, or protons, to annihilate or damage cancer cells. This radiation induces small breaks in the DNA inside cells, inhibiting their growth and division, and eventually leading to their death [211]. When combined with tumor vaccines, these treatments might produce a synergistic effect, with radiotherapy potentially leading to the release of cancer cell antigens and stimulating the immune system, thereby enhancing the effectiveness of cancer vaccines [212,213,214]. Research indicates that radiotherapy exerts both cytotoxic and immunomodulatory effects on the tumor microenvironment. Beyond directly damaging tumor cells, RT induces immunogenic cell death, leading to the release of damage-associated molecular patterns (DAMPs) [215]. These DAMPs serve as “danger signals”, enhancing dendritic cell function and fostering antitumor immune responses. Concurrently, radiotherapy damages the tumor vasculature, increasing its permeability due to direct effects on endothelial cells and the upregulated release of VEGF from irradiated tumor cells [215,216]. This can lead to both transient improvements in oxygen and nutrient delivery and enhanced immune cell infiltration into the tumor.
However, this combination approach has its limitations. Not all patients or cancer types respond well to either radiotherapy or cancer vaccines, making the efficacy of this approach unclear in a broad population [217]. The optimal timing and dosage of radiotherapy relative to cancer vaccines are not well understood, posing a risk of radiotherapy killing immune cells stimulated by the vaccine, and thereby reducing the effectiveness of the treatment [218]. Both treatments can cause side effects, such as skin changes, fatigue, and other symptoms for radiotherapy, and usually mild but possibly flu-like symptoms for cancer vaccines [63], and some tumors may develop resistance to radiotherapy, which could limit the effectiveness of this combined approach [219].
5.4. Cancer Vaccine + Targeted Therapy
Compared to cancer vaccines, targeted therapies obstruct specific proteins or processes that aid in cancer growth and progression, offering a more cancer-cell-selective approach compared to traditional chemotherapy and resulting in fewer side effects. Notable targeted therapies include small-molecule inhibitors, like Gleevec (imatinib), and monoclonal antibodies, like Herceptin (trastuzumab) [220,221,222]. When utilized in combination, targeted therapies aim to inhibit cancer cells’ proliferation and survival, rendering the cancer cells more susceptible to the immune response provoked by the cancer vaccine. Studies have revealed the potential of this combination, with targeted therapies able to modulate the tumor microenvironment, thereby possibly enhancing the effectiveness of the vaccine-stimulated immune response [223] and helping to prevent or delay resistance to targeted therapies [2]. However, challenges and limitations remain, including the development of resistance to targeted therapies over time [224], potential side effects ranging from mild skin rashes or diarrhea to severe liver toxicity or heart problems [225], limited responsiveness in certain cancer types or patients [208], and the complex and not fully understood interaction effects between cancer vaccines and targeted therapies, which could potentially interfere with the vaccine-stimulated immune response [16].
5.5. Cancer Vaccine + Oncolytic Virotherapy
Oncolytic virotherapy constitutes a novel paradigm in the therapeutic approach towards malignant neoplasms, exhibiting a mechanism of action that distinguishes it from traditional tumor vaccines. It capitalizes on the unique capabilities of selected or genetically engineered viruses, which are orchestrated to specifically target and eradicate neoplastic cells [19,226]. Upon administration, these oncolytic viruses infiltrate the patient’s system, subjugating cancerous cells and commandeering their biological machinery for viral replication, consequently leading to cell lysis [227,228]. This lysogenic cycle not only facilitates direct oncolysis but also liberates tumor-specific antigens, providing a catalyst for the patient’s immune system to mount an anticancer response—an underpinning that is shared with the concept of tumor vaccines [132]. This dual-action mechanism that harmonizes direct cellular destruction with immune activation embodies a promising pathway in the realm of cancer therapy. The dual-action mechanism encompasses direct tumor cell lysis, releasing tumor-associated antigens, and the unveiling of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). These elements activate both the innate and adaptive arms of the immune system, enhancing antitumor responses. As newly assembled viral entities continue their onslaught against other malignant cells, a self-propagating cycle is established.
Recent advancements in this burgeoning field have entailed the exploration of diverse oncolytic virus models, such as the Parapoxvirus ovis model, known to induce an immunogenic form of cell death termed pyroptosis [229]. Scientific investigations have also scrutinized the immunostimulatory effects of these bioengineered viruses and combinational therapeutic strategies that kindle pyroptosis, consequently fostering potent antitumor activity [230,231,232]. These engineered viruses have some potential in the delivery of antitumor drugs [233]. Pioneering therapeutic strategies, such as the KISIMA/VSV-GP heterologous prime–boost methodology and the development of adenovirus-based tumor vaccines, have further emphasized the potential of oncolytic virotherapy as a formidable armament in the arsenal of cancer immunotherapy [234].
Cancer vaccines and oncolytic virotherapy offer a potential synergistic approach for cancer treatment. Cancer vaccines introduce cancer-specific antigens into the body, training the immune system to recognize and attack cells displaying these antigens [5]. Concurrently, oncolytic virotherapy uses engineered viruses that selectively infect and eliminate cancer cells, subsequently releasing tumor antigens and new viral particles that can infect nearby cancer cells, thereby stimulating an immune response [19]. In combination, the cancer vaccine’s potential enhancement of the immune response, coupled with the direct cellular damage from the oncolytic virus, could increase therapeutic effectiveness. Initial studies suggest that this combination can result in a more robust and long-lasting immune response against tumors, even potentially overcoming some immune evasion tactics employed by cancer cells [235]. Nevertheless, this approach is not without limitations. The immune system could respond to the oncolytic virus, reducing its cancer-killing effectiveness [236]. Additionally, delivering both oncolytic viruses and cancer vaccines to the tumor site, particularly in solid tumors, is challenging [237]. The diverse nature of cancers and variability in patient responses can limit the overall responsiveness of this combined therapy [238]. Finally, safety is a significant concern, as both oncolytic virotherapy and cancer vaccines can cause side effects, with the former potentially leading to severe or life-threatening reactions in rare instances [239]. Recent advancements will change this domain. Viruses are now engineered for heightened tumor specificity, some are armed with therapeutic genes to turn tumors into producers of anticancer agents, and combinations with treatments like immune checkpoint inhibitors are showing synergistic effects [240,241]. Moreover, refined genetic engineering techniques have improved the safety profiles of these oncolytic viruses, making them more amenable for therapeutic applications [242,243]. This means that they have reduced virulence in non-target tissues and minimized side effects.
6. Personalized Cancer Vaccines
Personalized cancer vaccine therapy is an innovative approach that tailors cancer treatment to a patient’s unique tumor profile. The process can be outlined as follows (Figure 4).
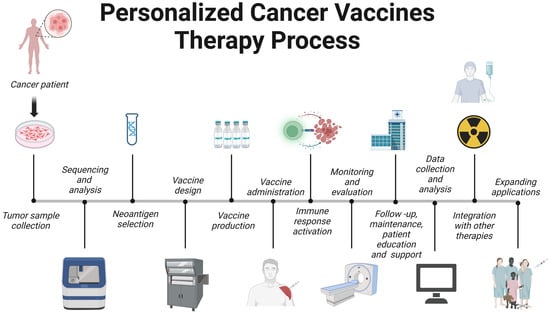
Figure 4. Therapeutic pipeline for personalized vaccines. Created with BioRender.com.
6.1. Tumor Sample Collection
A critical step in this process is the collection of tumor samples, which provide the essential genetic material needed to tailor the vaccine to the individual’s specific cancer [244]. The latest research advances have highlighted the importance of obtaining high-quality, well-preserved samples through minimally invasive techniques such as fine-needle aspiration or core-needle biopsy [245]. Additionally, there is a growing emphasis on collecting tumor samples at multiple timepoints throughout the course of treatment to account for the inherent heterogeneity of tumors and the potential for evolving cancer mutations [246]. The integration of these cutting-edge technologies and best practices in tumor sample collection is essential for maximizing the success of personalized cancer vaccine therapy [20].
6.2. Sequencing and Analysis
Sequencing and analysis empower researchers and clinicians to identify tumor-specific mutations and neoantigens that may function as potential therapeutic targets [20]. The development of next-generation sequencing (NGS) technologies, such as whole-exome and whole-genome sequencing, has greatly sped up the process and increased the precision of pinpointing tumor-specific mutations [177,247]. Concurrently, innovative computational approaches like machine learning algorithms and in silico prediction tools have emerged to forecast neoantigens with high immunogenicity, thereby expediting the selection of optimal vaccine candidates [244,248,249]. Moreover, incorporating multi-omics data, which include transcriptomics, proteomics, and epigenomics, offers a more comprehensive understanding of the tumor microenvironment and its influence on the effectiveness of personalized cancer vaccine therapy [250]. These advancements in sequencing and analysis have markedly improved the capacity to develop customized cancer vaccines, and they further emphasize the significance of multidisciplinary collaboration within the cancer immunotherapy field.
6.3. Neoantigen Selection
Neoantigen selection is vital for eliciting a robust and effective immune response [20,21]. Factors considered in the selection process include the binding affinity of the neoantigen to major histocompatibility complex (MHC) molecules, the immunogenicity of the epitope, and the likelihood of generating T-cell receptor (TCR) recognition [251,252]. Recent studies have also highlighted the importance of incorporating multi-omics data to ensure that the chosen neoantigens are effectively processed and presented on the cell surface [250]. By combining these innovative approaches, researchers have substantially improved the process of neoantigen selection, bolstering the potential for successful personalized cancer vaccine therapy.
6.4. Vaccine Design
Vaccine design directly influences the efficacy of the immune response against tumor cells [8]. Recent research advances have led to the development of various vaccine platforms, including peptide-based, nucleic-acid-based (DNA or RNA), viral-vector-based, and dendritic-cell-based vaccines, each with their own set of advantages and challenges [8,21,22]. The selection of appropriate adjuvants and delivery systems is essential for enhancing the immunogenicity of the vaccine and promoting the activation and expansion of tumor-specific T cells [253]. Innovative techniques such as liposomal and nanoparticle-based delivery systems have shown promise in improving vaccine stability, cellular uptake, and antigen presentation [254]. The swift pace of advancements in vaccine design methodologies, in conjunction with a burgeoning understanding of the tumor microenvironment and immune system, has notably augmented the potential of personalized cancer vaccine therapy, laying the groundwork for more effective and targeted cancer treatments.
6.5. Vaccine Production
The high quality of production in personalized cancer vaccine therapy is indispensable for ensuring the delivery of efficacious and safe treatment options to patients [22]. Noteworthy innovations in the production process encompass the incorporation of automation and process optimization to curtail the manufacturing duration and boost scalability [255]. Furthermore, the utilization of continuous manufacturing processes and the establishment of modular facilities have amplified flexibility and adaptability in vaccine production, thereby streamlining the supply chain for personalized therapies [256]. To ensure product quality, regulatory agencies have enforced good manufacturing practices (GMPs) and stringent quality control measures. The integration of advanced bioinformatics tools has also contributed to the acceleration of vaccine development and production, enabling more rapid clinical translation and patient access [110]. As the field of personalized cancer vaccine therapy continues to expand, further innovations in vaccine production technologies and processes will be vital to meeting the growing demand and ensuring the timely delivery of these tailored treatments.
6.6. Vaccine Administration
Vaccine administration directly impacts the induction of a robust immune response against tumor cells. The latest research advances have led to the exploration of various routes of administration, including subcutaneous, intradermal, intramuscular, and intranodal, with each route presenting unique benefits and challenges for different vaccine platforms [257,258]. The choice of administration route can influence the vaccine’s biodistribution, antigen presentation, and subsequent immune response [23]. Researchers are also investigating the optimal dosing and scheduling of these personalized vaccines to maximize their efficacy while minimizing potential adverse effects [21,259]. Recent studies have delved into the synergistic effects of merging personalized cancer vaccines with other immunotherapies to amplify therapeutic outcomes and surmount immune resistance [260].
As the realm of personalized cancer vaccine therapy continues to expand, a nuanced understanding of the optimal administration strategies, including route, dosage, and scheduling, will be pivotal for maximizing the therapeutic potential of these individualized treatments.
6.7. Immune Response Activation
The activation of the immune response is a central objective in personalized cancer vaccine therapy, aiming to instigate a robust and specific immune response against tumor cells expressing neoantigens. Recent research strides have provided a more comprehensive understanding of the mechanisms underpinning the activation of both innate and adaptive immune responses [20]. Personalized cancer vaccines endeavor to prime the immune system by presenting tumor-specific neoantigens to antigen-presenting cells (APCs) such as dendritic cells, which subsequently activate cytotoxic T lymphocytes (CTLs) to target and eliminate tumor cells [22,178,261]. A key facet of immune response activation lies in the optimization of vaccine design, thereby amplifying the immunogenicity of the vaccine and fostering the expansion of tumor-specific T cells [262].
Recent investigations have probed into combining personalized cancer vaccines with other immunotherapies to augment the antitumor immune response and counter the immune evasion strategies utilized by cancer cells [260]. As the realm of personalized cancer vaccine therapy progresses, a deeper comprehension of immune response activation and its modulation will be indispensable for maximizing the therapeutic potential of these tailored treatments.
6.8. Monitoring and Evaluation
Monitoring and evaluation are integral to ensuring the safety, efficacy, and optimization of these individualized treatments. Recent research breakthroughs have resulted in the development of comprehensive methodologies to assess both immune response and clinical outcomes in patients receiving personalized cancer vaccines [21,22]. Essential parameters for evaluating the immunological response include monitoring the expansion of vaccine-specific T cells, the production of cytokines, and the infiltration of immune cells into the tumor microenvironment [258]. These evaluations furnish invaluable insights into the vaccine’s capacity to activate and modulate the immune system.
Clinical evaluation entails tracking objective responses, such as reduction in tumor size, progression-free survival, and overall survival, while also considering the patients’ quality of life [23,263]. As personalized cancer vaccines are often administered in combination with other immunotherapies, it is crucial to identify synergistic effects and ascertain the optimal treatment regimen [260,264].
Moreover, monitoring and evaluating safety profiles is imperative for identifying and managing potential adverse effects associated with personalized cancer vaccines, such as autoimmune reactions or systemic inflammation [109]. The ongoing refinement of monitoring and evaluation strategies will contribute to maximizing the therapeutic potential and safety of personalized cancer vaccine therapies, thereby enhancing patient outcomes.
6.9. Follow-Up, Maintenance, and Patient Education and Support
Continuous follow-up empowers healthcare professionals to monitor patients’ responses to therapy, assess potential adverse effects, and make necessary adjustments to treatment plans [21,265]. Maintenance therapy, including administering booster vaccinations or adjusting combination therapies, is pivotal for maintaining the antitumor immune response and preventing cancer recurrence [22,74].
Patient education forms a key aspect of personalized cancer vaccine therapy, as it enables patients to make informed decisions about their treatment and manage potential side effects [257]. Patients should be well informed about potential adverse effects of the treatment and the importance of promptly reporting these to their healthcare providers [109,261]. Moreover, the emotional and psychological wellbeing of patients can significantly influence their response to treatment, underscoring the need for comprehensive support systems. This may include mental health counseling, peer support groups, and resources for family members, creating an environment that nurtures the patient’s resilience and determination [266]. As personalized cancer vaccine therapy continues to progress, it is crucial to maintain an integrated approach that addresses all facets of patient care, including follow-up, maintenance, education, and support. This holistic approach can maximize the therapeutic potential of personalized cancer vaccines and lead to improved patient outcomes [267].
6.10. Data Collection and Analysis
Data collection and analysis stand as the cornerstones of personalized cancer vaccine development and evaluation. Recent strides in high-throughput sequencing technologies, computational methodologies, and bioinformatics have ushered in a new era, enabling researchers to more precisely identify and prioritize neoantigens, craft personalized vaccines, and keep track of immune responses [20,22,258]. Furthermore, the application of machine learning algorithms and artificial intelligence has emerged as a powerful tool to navigate the complexity of these data landscapes. This technology bolsters the capacity to predict the immunogenicity of neoantigens and assess the effectiveness of personalized cancer vaccines [252]. Standardized data collection and analysis protocols are paramount to ensure reproducibility, facilitate comparisons across studies, and promote the development of robust and reliable personalized cancer vaccine therapies [23].
6.11. Integration with Other Therapies
The integration of personalized cancer vaccines with other therapeutic modalities offers an intriguing avenue in the realm of cancer treatment. Recent scientific findings highlight the potential synergistic effects of marrying personalized cancer vaccines with other forms of immunotherapies. Notably, ICIs, which target immunosuppressive pathways like PD-1 (programmed cell death protein 1), PD-L1 (programmed cell death ligand 1), and CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), have shown promising results in combination with personalized cancer vaccines [109]. The rationale behind this combination is that the vaccine can stimulate a specific antitumor immune response, while ICIs can further enhance the function and persistence of tumor-specific T cells [191].
Additionally, personalized cancer vaccines can be combined with conventional therapies, such as chemotherapy and radiotherapy, to induce immunogenic cell death and release tumor-associated antigens, creating a more conducive environment for the activation of vaccine-induced immune responses [139]. Furthermore, combining personalized cancer vaccines with targeted therapies, such as kinase inhibitors or monoclonal antibodies, has shown promise in preclinical models by modulating the tumor microenvironment and improving immune cell infiltration [265,268].
6.12. Expanding Applications
The exploration of personalized cancer vaccine therapy applications continues to surge, unlocking new possibilities for cancer treatment across a diverse range of tumor types and stages. Groundbreaking studies have revealed the potential and effectiveness of tailored cancer vaccines against melanoma, glioblastoma, and non-small-cell lung cancer [22,263]. Aside from solid tumors, there is growing evidence of personalized cancer vaccines proving promising against hematological malignancies such as acute myeloid leukemia (AML) and multiple myeloma (MM) [269,270]. Furthermore, researchers are delving into the possibility of implementing personalized cancer vaccines during earlier disease stages, or as supplementary therapy after surgery or radiotherapy, with the ultimate goal of preventing relapse or disease progression [126].
As the development of personalized cancer vaccine therapy progresses, gaining a comprehensive understanding of the factors impacting vaccine effectiveness—including the tumor microenvironment, individual immune responses, and the dynamic interplay among various treatment approaches—becomes essential in extending its application scope. Unrelenting research and innovation are indispensable for harnessing the full therapeutic capacity of personalized cancer vaccines and enhancing outcomes for a wide spectrum of cancer patients. The world of clinical trials is abuzz with personalized cancer vaccines and neoantigen-targeting therapies [271,272]. Challenges exist, such as optimizing vaccine design and manufacturing, plus pinpointing the patients most likely to benefit from personalized immunotherapies [195,273]. Add in resistance mechanisms, sky-high costs, and complex production, and suddenly these groundbreaking treatments seem less accessible and affordable. So, the cancer immunotherapy research needs to zero in on resistance, refine neoantigen identification tech, and make personalized therapies more cost-effective and scalable [274]. This is where multi-omics approaches and AI come into play, helping to precisely stratify patients and craft combination therapies that boost efficacy while minimizing side effects [75]. Tackling these challenges and embracing emerging technologies will be the key to revolutionizing cancer treatment and offering new hope to countless patients.
This entry is adapted from the peer-reviewed paper 10.3390/ph16101384
This entry is offline, you can click here to edit this entry!
