Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Pancreatic ductal adenocarcinoma (PDAC) represents one of the most aggressive solid tumors with a dismal prognosis and an increasing incidence. At the time of diagnosis, more than 85% of patients are in an unresectable stage.
- pancreatic ductal adenocarcinoma
- microenvironment
- neuron
- crosstalk
1. Clinical Relevance of Cancer–Neuronal Crosstalk
Besides its aggressive behavior and poor response to treatment, another major feature of pancreatic ductal adenocarcinoma (PDAC) is perineural invasion (PNI), which is defined as cancer cells surrounding at least 33% of the epineurial, perineural, and endoneurial space of the nerve sheath. PNI is present in virtually all patients [1][2]. This is a significant difference from other solid tumors in which PNI is less common [3]. PNI can be quantified using scoring systems and is primarily based on its extent and frequency (Figure 1). Interestingly, in PDAC, a significant positive correlation was found between the extent of PNI and patient survival [3][4][5]. PNI is an independent risk factor for the development of R1 resection and tumor recurrence [1][4] and an important predictor of metastatic spread along the neuronal compartment [2][6]. For instance, in a retrospective study, Takahashi et al. showed how micrometastases can be frequently found in the neuronal compartment of healthy pancreatic sections of PDAC-bearing patients [2]. This event, termed ‘intrapancreatic extratumoral perineural invasion (NEX) phenomenon’, was found in more than 50% of patients undergoing curative surgery. The occurrence of the NEX phenomenon was positively correlated with NI, whilst overall survival was negatively correlated with NEX, as NEX+ patients had a significantly worse survival compared to NEX- (350 vs. 1042 days). All patients who survived PDAC without tumor recurrence were NEX- [2].

Figure 1. Neural invasion. (A). PDAC cells (arrow) infiltrate a nerve (asterisk). More than 33% of the circumference of the nerve is affected (perineural invasion). Stained with Hematoxylin and Eosin. Image at 20× magnification. (B). Healthy pancreas with a nerve bundle (asterisk). Stained with Hematoxylin and Eosin. The entire tissue shown represents the exocrine part of the pancreas. Endocrine parts are not shown. Image at 20× magnification.
In addition, PNI also has an impact on neuronal architecture. Specifically, extensive nerve fiber hypertrophy and elongation or sprouting of nerve fibers have been usually correlated with PNI occurrence [7][8]. Nerve fiber hypertrophy appears to be a major contributor to the development of cancer-associated pain in PDAC. Approximately 80% of patients develop cancer-associated pain during the progression of the disease [7][8]. Even with modern analgesic therapies, cancer-associated pain cannot always be controlled, thus prompting the quest for novel therapeutic targets. Interestingly, two retrospective studies showed that neoadjuvant therapy significantly reduced the rate of PNI [9][10] As the role of neoadjuvant therapy is currently not well established, this is an interesting observation [11]. For instance, Barbier et al. showed that neoadjuvant chemoradiation significantly reduced the rate of PNI from 93 to 43% [9]. However, neoadjuvant chemoradiation also prevented more than half of patients from receiving resection due to cancer progression. Consequently, it is unsurprising that neoadjuvant chemoradiation did not improve overall survival compared to direct resection [11]. Whether neoadjuvant therapy has a positive impact on patient survival or on the development of cancer-associated pain, e.g., as part of an extended multimodality therapy concept, remains to be elucidated.
2. Molecular Mechanisms of Cancer–Neuronal Crosstalk and Targeted Therapies
2.1. Neurons Are Attracted to Pancreatic Ductal Adenocarcinoma Cells
Many mediators are involved in this close cancer–neuronal bidirectional crosstalk. However, our understanding of this complex interaction is still very preliminary. Currently, it is known that PDAC cells secrete mediators that promote nerve invasion into tumor tissue [12]. Cancer cells activate physiological mechanisms which in homeostasis are necessary to provide organ innervations and nerve regeneration. Among the identified mediators, neurotrophins, particularly nerve growth factor (NGF), have been best studied (Figure 2A) [12][13]. During embryonic development, tissues that need to be innervated secrete NGF. Along the NGF gradient, neurons eventually innervate the target organ. In a healthy pancreas, NGF is barely detectable. However, as early as the PanIN stage, the amount of NGF doubled in PDAC cells. In the PDAC stage, the amount of NGF in tumor cells can increase up to seven-fold. Overexpression and secretion of NGF attracts neurons leading to neurite outgrowth into tumor tissue. In human tissue, a clear correlation has been reported between NGF overexpression and an increased extent of NI. Thus, it is not surprising that NGF concentration in tumor tissue is associated with an increased metastasis rate and increased probability of R1 resection. Microdissection studies demonstrated that both PDAC cells and nerves produce NGF and both express the corresponding receptors, so that a reciprocal interaction may be established over the time course of tumor disease [13].
NGF binds to two receptors: the high-specificity trkA receptor and the low-specificity p75NTR. Upon binding of NGF to the high-specificity receptor trkA, activation of MEK and MAPK pathways occurs, which promotes proliferation and suppresses apoptosis [14]. Binding to the low-affinity p75NTR inhibits proliferation and induces apoptosis [14]. In this regard, patients with a high trkA receptor have significantly reduced survival. Patients with high p75NTR expression showed significantly prolonged survival. Interestingly, NGF receptor expression was shown to be one of the most important predictive parameters in PDAC in a multivariable model [15].
The crucial role of NGF in this bidirectional crosstalk in PDAC has also been demonstrated in preclinical models. For instance, in vitro, knockdown of NGF or its receptors trkA and p75NTR, respectively, could limit the proliferation and migration of PDAC cells (Figure 2A). Also, inhibition of the NGF—trkA axis resulted in decreased migration of Mia PaCa2 cells toward DRG neurons and decreased neurite outgrowth [1]. Similarly, Lei et al. tested the following hypothesis using a robust siRNA (gold nanocluster-associated delivery of siRNA of NGF; GNC-siRNA) for NGF knockdown, which was used both in vitro and in vivo [16]. Application of GNC-siRNA reduced proliferation of Panc1 cells and inhibited migration of these tumor cells in a migration chamber. When Panc1 cells were co-cultured with DRG neurons, neurite outgrowth was directed towards Panc1 cells. Once Panc1 cells were pretreated with GNC-siRNA, the extent of neurite outgrowth decreased compared with untreated Panc1 cells. GNC-siRNA was also examined in vivo. Three PDAC mouse models were used (subcutaneous model, orthotopic model and patient-derived xenograft). After NGF knockdown in tumor cells, the amount of neurite outgrowth into tumor tissue was significantly reduced and tumor growth was reduced by approximately 50%. Then, depending on the tumor model, reduction of the metastatic rate varied from a discrete reduction to the virtually complete absence of distant metastasis. Thus, inhibition of the NGF axis represents a promising therapeutic option [16]. Human anti-NGF antibodies are already available and used in clinical trials. As NGF also plays an important role in the development and maintenance of pain in chronic inflammation, NGF antibodies have so far been used mainly in clinical trials on painful osteoarthritis [17]. The side effects mainly observed include headache, paresthesias and hypoesthesias [17][18]. Compared to aggressive chemotherapies, these side effects are considered acceptable. To date, no clinical trial for use in pancreatic cancer has been initiated, but this may soon follow due to the promising preclinical data. Specifically, the biweekly application of anti-NGF antibodies had favorable effects in LSL-Kras+/G12D; LSL-Trp53+/R172H; Pdx1-Cre (KPC) animals [19]. The development of PDAC was suppressed, the rate of PNI was reduced by 40% and the macrometastases were not detectable (vs. 30% in sham-treated KPC animals). Inhibition of the TrkA receptor also appears to be effective, although human trk inhibitors can cross the blood–brain barrier and thus the side effects to be expected might be more critical [19]. Nevertheless, first clinical studies in solid tumors, including pancreatic cancer, have been initiated (Table 1).
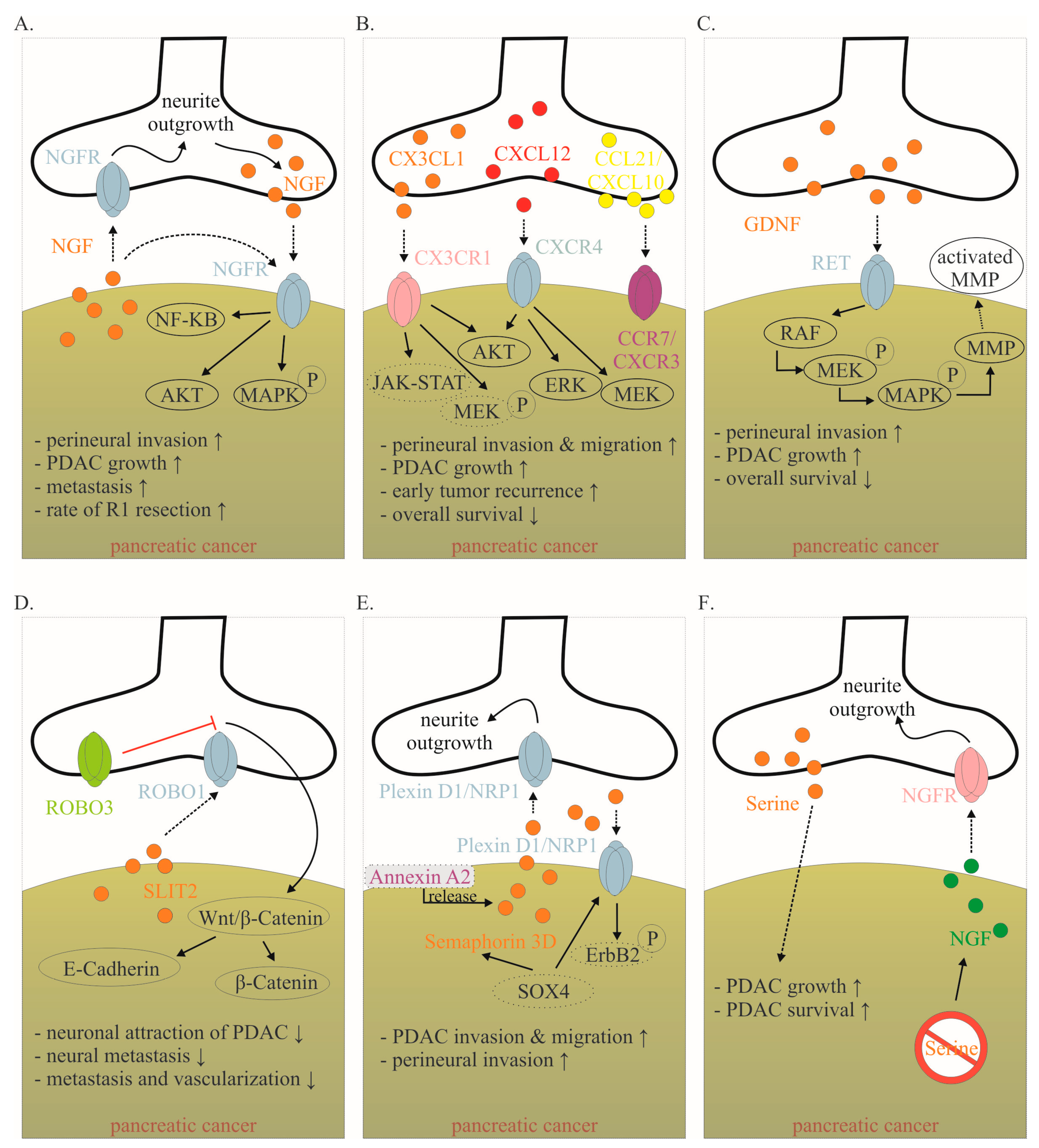
Figure 2. Mediators involved in the cancer–neuronal crosstalk. (A–F). Several mediators are involved in the close cancer–neuronal crosstalk, including NGF, chemokines (e.g., CX3CL1, CXCL10, CCL21), GDNF, SLIT2, Semaphorin 3D and Serine. Solid, black lines with an arrow symbolize activation, whereas red lines with a bar symbolize inhibition. Some signaling mechanisms are only described in non-PDAC cancers. These are labeled with a dotted line. Their role in PDAC has to be confirmed in the future. The effects of interaction (↑ increase; ↓ decrease) are listed below for each mediator. Data collected from [6][12][13][20][21][22][23][24][25][26][27]. Abbreviations: C-X3-C motif ligand 1 (CX3CL1); CX3C motif chemokine receptor 1 (CX3CR1); C-X-C motif chemokine 10 (CXCL10); C-X-C Motif Chemokine Receptor 3 (CXCR3); C-C Motif) Ligand 21 (CCL21); C-C chemokine receptor type 7 (CCR7); glial-cell-derived neurotrophic factor family of ligands (GDNF); nerve growth factor (NGF); nerve growth factor receptor (NGFR); Pancreatic ductal adenocarcinoma (PDAC); rearranged during transfection (RET); roundabout receptors (Robo); Slit glycoproteins (Slit). Red symbol crossing out Serine means Serine deprivation.
NGF also activates multiple pathways within the tumor that increase cellular invasiveness such as nuclear factor kappa–light-chain-enhancer of activated B-cells (NF-κB) [20]. In vitro data demonstrate that inhibitors of the NF-κB pathway inhibit NGF-mediated PNI and neural outgrowth. In vivo, inhibition of the NF-κB pathway leads to a reduction in neurotrophin expression, nerve density and PNI [20]. Inhibition of the NF-κB pathway was achieved on the one hand by NF-κB modulation plasmids and on the other hand by Triptolide and its water-soluble prodrug Minnelide. Triptolide is a diterpenoid triepoxide, which is extracted from the Chinese herb Tripterygium wilfordii. Among the over 300 ingredients of Tripterygium wilfordii, Triptolide is the most important bioactive component. The effect of triptolide is manifold [28][29]. On the one hand, it induces apoptosis in pancreatic cancer cells and elicits its antitumor activity through super-enhancer disruption to re-program cellular crosstalk [23]. On the other hand, it increases the cytotoxicity of various chemotherapeutic agents and inhibits the NF-κB pathway [20][29][30]. Therefore, it remains to be confirmed whether inhibition of the NF-κB pathway by triptolide leads to inhibition of cancer–neuronal crosstalk or whether other triptolide-induced pathways are responsible for the observed effects. Nevertheless, preliminary promising results on triptolide in PDAC are already available. For instance, in an orthotopic PDAC model, it was shown that the combined administration of Minnelide and paclitaxel significantly reduced tumor growth and improved survival [31]. In this model, the life expectancy in untreated animals was 13 days. After administration of paclitaxel or Minnelide as monotherapy, survival was significantly improved up to 21 days. By combined administration of Minnelide and paclitaxel, all animals were still alive after more than 6 weeks. Overall, compared to untreated animals, combined therapy reduced the rate of distant metastases by up to 90%. Based on these data, several clinical trials in PDAC (and other tumor entities) have recently been initiated (Table 1) [32].
Table 1. Selected clinical and preclinical trials targeting the cancer–neuronal crosstalk.
| Drug | Target | Current Status | Effect | Reference |
|---|---|---|---|---|
| Anti-NGF antibody or siRNA | NGF | Mouse model | Reduced neural outgrowth, reduced cancer growth, reduced metastasis | [16][19] |
| Trk-Inhibitor | Trk (unselective) or trkA (selective) | Phase I–II | NA | NCT03556228, NCT05046847, NCT02097810, NCT02568267, NCT04879121 |
| Plerixafor | CXCR4 antagonist | Phase I–II | NA | NCT03277209, NCT02179970, NCT04177810 |
| miR-383 | ROBO3 | Mouse model | Reduced cancer growth and metastasis | [33] |
| Minnelide | Several effects, including Inhibition des NF-κB | Phase I–II | NA | NCT03117920, NCT05557851, NCT03129139, NCT04896073 |
| Propranolol | Beta adrenergic receptor | Phase II | NA | NCT03838029, NCT05451043 |
| Celiac ganglion ablation | Denervation | Phase II–III | Reduction in cancer-associated pain, but no significant prolonged survival | [34] |
| Splanchnicectomy | Denervation | Phase III | Reduction in cancer-associated pain, prolonged survival only in presence of cancer-associated pain before intervention | [35] |
| Bethanechol | Muscarinic receptor M1 | Phase I–II | NA | NCT03572283, NCT05241249 |
Abbreviation: NA (not available; e.g., because the research is still ongoing or research results are not yet available).
2.2. Pancreatic Ductal Adenocarcinoma Cells Are Attracted to Neurons
Chemokines are signaling molecules that exert the attraction and directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. They are central mediators in the migration of cells, making it not surprising that several chemokines are involved in cancer–neuronal crosstalk [36].
Fractalkine (CX3CL1) is a well-studied chemokine released by neurons (Figure 2B) [37]. In the central nervous system, CX3CL1 plays an important role in neuron–glia crosstalk. Several PDAC cell lines express the corresponding receptor CX3CR1, which enables them to demonstrate PNI [37] (Figure 2B). CX3CR1 is not detectable in healthy human pancreatic tissue, whereas it is highly expressed in human PDAC tissue. Thereby, CX3CR1 expression correlates with the extent of NI, as well as local and early tumor recurrence [37]. The downstream mechanisms of CX3CR1 activation in PDAC need to be identified. However, it has been shown in prostate and breast cancer that the activation of the receptor leads to subsequent activation of several pathways, including PI3K/AKT, Raf/MEK/ERK and JAK/STAT, among others [38]. E6011 is a humanized IgG2 monoclonal antibody against human fractalkine, which has been used in phase II trials in inflammatory diseases such as rheumatoid arthritis. Clinical studies in PDAC have not yet been performed [21].
CXCL12 is also released from neurons and binds to the receptor CXCR4 (and CXCR7) on PDAC cells (Figure 2B) [21]. The activation of the CXCL12/CXCR4 axis significantly increased PDAC cells’ PNI and promoted neurite outgrowth. The expression of CXCR4 correlated highly significantly with the presence of NI in human specimens [39]. Inhibition of this pathway in vivo inhibited tumor growth and invasion of the sciatic nerve [39]. In an orthotopic PDAC mouse model, CXCR4 inhibition (using plerixafor) resulted in a significantly improved response to gemcitabine chemotherapy [40]. In human specimens, high CXCR4 expression but not high CXCR7 expression was associated with reduced overall survival [41]. While some gastric and colorectal carcinoma cells can produce CXCL12 themselves, the quantitative largest source in PDAC is derived from cancer-associated fibroblasts (CAFs) [42]. Physiologically, pancreatic stellate cells produce CXCL12 after tissue damage. In PDAC, cancer cells encourage CAFs to produce CXCL12 by secreting TNF-alpha, TGF-beta and others [42]. CXCL12 binding enhances PDAC proliferation via Akt, ERK and MEK signaling [43]. CXCL12 binding also increases treatment resistance through increased expression of pro-survival proteins Bcl-2, BclxL and Notch1 and inactivation of BAD [42]. In addition to its influence on cancer–neuronal crosstalk and tumor growth, the CXCL12/CXCR4 axis is also important for immune cell migration and possibly plays a role in modulating the response to immunotherapy [42][44]. Plerixafor is a selective CXCR4 antagonist approved to mobilize hematopoietic stem cells into the peripheral blood for collection and autologous transplantation in patients with non-Hodgkin lymphoma and multiple myeloma. Therefore, it is not surprising that CXCL12-CXCR4-axis also plays an important role in immune cell migration [42][44]. Based on the following findings, preliminary clinical studies are planned or underway. This will show the impact of the CXCL12–CXCR4 axis on cancer–neuronal crosstalk, tumor growth and response to immunotherapy.
Another prominent group of proteins secreted by nerves is the glial-cell-derived neurotrophic factor family of ligands (GDNF). The GDNF family ligands include GDNF, neurturin, artemin and persephin. GDNF family members bind to glycosylphosphatidylinositol anchor-linked GDNF family receptor alpha 1 (GFRα1), which recruits rearranged during transfection (RET) receptor tyrosine kinase for dimerization [22]. GDNF is highly expressed in the peripheral and central nervous system and stimulates the development, survival and differentiation of neuronal cells [22]. RET is expressed on several PDAC cell lines [22]. Neurons from mice deficient in GDNF had a reduced ability to attract cancer cells [12]. It has been shown that PNI is dependent on neuronal GDNF secretion and GDNF coreceptors RET and GFRα1 expressed in human PDAC cells (Figure 2C) [22]. High RET expression in human PDAC was established as a negative prognostic parameter [6]. Interestingly, PNI was blocked by treatment with PYP1, a potent RET inhibitor, showing how targeting nerves could serve as a potential mechanism to decrease PDAC growth [12]. After GDNF stimulated the RET mitogen-activated protein kinase cascade, it facilitated matrix degradation by producing more matrix metallopeptidase 2 (MMP2), MMP9 and MMP14, thereby accelerating cell invasiveness [6]. Monoclonal antibody-GDNF fusion protein was tested in parkinsonian monkeys. Focal pancreatic acinar to ductular metaplasia with transition to pancreatic intraepithelial neoplasia 1B (PanIN-1B) lesions were detected in several animals [45]. Whether this was due to the specific antibody fusion protein or to GDNF inhibition itself remains to be elucidated. Recently, protein neuroligin 1 (NLGN1) was shown to promote cancer cell invasion and migration along nerves [46]. Functionally, NLGN1 was shown to exert its effect in cooperation with GDNF (NLGN1-GDNF cooperation). Therefore, NLGN1 inhibition is a promising target for clinical trials.
Slit glycoproteins (Slit) and their roundabout receptors (Robo) are guide molecules in neuronal development, axon guidance, glial migration and angiogenesis [47][48]. Especially in PDAC, this pathway seems to play an important role in cancer–neuronal crosstalk and metastasis (Figure 2D). Thus, the expression of SLIT2 is reduced in PDAC tissue [23]. SLIT2 binds to ROBO1, which is expressed on neurons. Overexpression of SLIT2 in deficient PDAC cells reduces attraction by DRG neurons and thus PDAC migration along outgrowing neurons is inhibited [23]. Interestingly, even though the migration of PDAC cells towards neurons was reduced, their general motility was not affected. In vivo, restored SLIT2 expression was shown to reduce metastasis and vascularization [23]. Nevertheless, these findings remain to be confirmed, as the opposite effect was also observed for SLIT2-ROBO1 [49]. Inhibition of the WNT/β-catenin pathway may thus be the main mechanism by which the Slit/Robo pathway inhibits pancreatic cancer growth. Specifically, inhibition of the WNT/β-catenin pathway by Slit2/Robo signaling enhances the formation of β-catenin and E-cadherin complexes, increasing tumor cell adhesion and inhibiting tumor invasion and migration, thereby improving patient prognosis [24]. Interestingly, high expression of ROBO3, a known inhibitor of ROBO1/2 signaling, was associated with shorter survival in a cohort of 142 PDAC patients undergoing pancreatectomy with curative intent [33]. ROBO3 increases with clinical grade of PDAC and promotes cancer cell growth and metastasis in vitro and in vivo [50]. Robo3 can activate the WNT/β-catenin pathway, thus promoting pancreatic cancer growth and invasion [24]. Thus, the Slit-Robo pathway represents a very interesting target in PDAC. For example, miR-383 was identified as a suppressor of ROBO3 [50]. Besides its role in cancer–neuronal crosstalk, the SLIT-ROBO pathway is involved in other mechanisms of tumor growth and metastasis [24].
Another axon guidance molecule is Semaphorin 3D, which was found to be overexpressed in tumorigenesis [25]. Migration and PNI of PDAC is increased via secretion of Semaphorin 3D by pancreatic cells and activation of Plexin D1 on neurons (Figure 2E). Knockdown of Semaphorin 3D and loss of neural Plexin D1 reduces neurite outgrowth and metastasis in vivo [25]. Furthermore, high mRNA expression of Semaphorin 3D and Plexin A1, another molecule central to semaphorin signaling, were both associated with poor patient survival [24]. Secretion of Semaphorin 3D is increased by AnnexinA2. Annexin A2 is a metastasis-associated protein in PDAC that has been shown to be essential for the metastatic growth in genetically engineered spontaneous pancreatic tumor-producing KPC mice. By comparing tumor cells of AnnexinA2 wild type vs. knock-out KPC mice, Semaphorin 3D and PlexinD1 were among the most differentially expressed genes. Thus, disrupting the AnxA2/Sema3D/PlexinD1 signaling appears to be a promising therapeutic strategy for further clinical trials [25]. The complex signaling mechanisms of Semaphorin 3D are only partially understood. Interestingly, in non-PDAC cancer cells, the prometastatic activity of Semaphorin 3E is mediated by transactivation of PlexinD1-associated Erb2b (Figure 2E) [26].
Considering the PDAC environment is characterized by poor nutrition supply, it is becoming evident that PDAC cells are dependent on neurons to receive external growth stimuli [51]. Specifically, neurons secrete mediators stimulating cancer growth and supplying oxygen and nutrients [36][52][53]. For instance, amino acids are essential for PDAC growth. Serine is a conditionally essential amino acid that represents the second most abundant amino acid found in human proteins and can be secreted by neurons [27]. Serine is necessary for several metabolic pathways, highlighting its role in terms of tumor growth and survival in cancers [54]. For instance, human PDAC cell lines are completely dependent on exogenous serine (Figure 2F). If neurons are prevented from producing serine, tumors grow approximately 40% smaller [51]. Interestingly, after serine deprivation, PDAC cells massively overexpress NGF in order to recruit more neurons to meet its nutritional needs [51].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241914989
References
- Bapat, A.A.; Hostetter, G.; Von Hoff, D.D.; Han, H. Perineural Invasion and Associated Pain in Pancreatic Cancer. Nat. Rev. Cancer 2011, 11, 695–707.
- Takahashi, T.; Ishikura, H.; Motohara, T.; Okushiba, S.-I.; Dohke, M.; Katoh, H. Perineural Invasion by Ductal Adenocarcinoma of the Pancreas. J. Surg. Oncol. 1997, 65, 164–170.
- Liebl, F.; Demir, I.E.; Mayer, K.; Schuster, T.; D’Haese, J.G.; Becker, K.; Langer, R.; Bergmann, F.; Wang, K.; Rosenberg, R.; et al. The Impact of Neural Invasion Severity in Gastrointestinal Malignancies. Ann. Surg. 2014, 260, 900–908.
- Ozaki, H.; Hiraoka, T.; Mizumoto, R.; Matsuno, S.; Matsumoto, Y.; Nakayama, T.; Tsunoda, T.; Suzuki, T.; Monden, M.; Saitoh, Y.; et al. The Prognostic Significance of Lymph Node Metastasis and Intrapancreatic Perineural Invasion in Pancreatic Cancer after Curative Resection. Surg. Today 1999, 29, 16–22.
- Shimada, K.; Nara, S.; Esaki, M.; Sakamoto, Y.; Kosuge, T.; Hiraoka, N. Intrapancreatic Nerve Invasion as a Predictor for Recurrence After Pancreaticoduodenectomy in Patients with Invasive Ductal Carcinoma of the Pancreas. Pancreas 2011, 40, 464–468.
- Li, J.; Kang, R.; Tang, D. Cellular and Molecular Mechanisms of Perineural Invasion of Pancreatic Ductal Adenocarcinoma. Cancer Commun. 2021, 41, 642–660.
- Ceyhan, G.O.; Bergmann, F.; Kadihasanoglu, M.; Altintas, B.; Demir, I.E.; Hinz, U.; Müller, M.W.; Giese, T.; Büchler, M.W.; Giese, N.A.; et al. Pancreatic Neuropathy and Neuropathic Pain—A Comprehensive Pathomorphological Study of 546 Cases. Gastroenterology 2009, 136, 177–186.e1.
- Demir, I.E.; Friess, H.; Ceyhan, G.O. Neural Plasticity in Pancreatitis and Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 649–659.
- Barbier, L.; Turrini, O.; Grégoire, E.; Viret, F.; Le Treut, Y.-P.; Delpero, J.-R. Pancreatic Head Resectable Adenocarcinoma: Preoperative Chemoradiation Improves Local Control but Does Not Affect Survival. HPB 2011, 13, 64–69.
- Chatterjee, D.; Katz, M.H.; Rashid, A.; Wang, H.; Iuga, A.C.; Varadhachary, G.R.; Wolff, R.A.; Lee, J.E.; Pisters, P.W.; Crane, C.H.; et al. Perineural and Intraneural Invasion in Posttherapy Pancreaticoduodenectomy Specimens Predicts Poor Prognosis in Patients with Pancreatic Ductal Adenocarcinoma. Am. J. Surg. Pathol. 2012, 36, 409–417.
- Ren, L.; Mota Reyes, C.; Friess, H.; Demir, I.E. Neoadjuvant Therapy in Pancreatic Cancer: What Is the True Oncological Benefit? Langenbecks Arch. Surg. 2020, 405, 879–887.
- Gil, Z.; Cavel, O.; Kelly, K.; Brader, P.; Rein, A.; Gao, S.P.; Carlson, D.L.; Shah, J.P.; Fong, Y.; Wong, R.J. Paracrine Regulation of Pancreatic Cancer Cell Invasion by Peripheral Nerves. JNCI J. Natl. Cancer Inst. 2010, 102, 107–118.
- Ketterer, K.; Rao, S.; Friess, H.; Weiss, J.; Büchler, M.W.; Korc, M. Reverse Transcription-PCR Analysis of Laser-Captured Cells Points to Potential Paracrine and Autocrine Actions of Neurotrophins in Pancreatic Cancer. Clin. Cancer Res. 2003, 9, 5127–5136.
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Friess, H.; Ceyhan, G.O. Nerve Growth Factor&TrkA as Novel Therapeutic Targets in Cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2016, 1866, 37–50.
- Dang, C.; Zhang, Y.; Ma, Q.; Shimahara, Y. Expression of Nerve Growth Factor Receptors Is Correlated with Progression and Prognosis of Human Pancreatic Cancer. J. Gastroenterol. Hepatol. 2006, 21, 850–858.
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold Nanoclusters-Assisted Delivery of NGF SiRNA for Effective Treatment of Pancreatic Cancer. Nat. Commun. 2017, 8, 15130.
- Dietz, B.W.; Nakamura, M.C.; Bell, M.T.; Lane, N.E. Targeting Nerve Growth Factor for Pain Management in Osteoarthritis—Clinical Efficacy and Safety. Rheum. Dis. Clin. N. Am. 2021, 47, 181–195.
- Wise, B.L.; Seidel, M.F.; Lane, N.E. The Evolution of Nerve Growth Factor Inhibition in Clinical Medicine. Nat. Rev. Rheumatol. 2021, 17, 34–46.
- Saloman, J.L.; Singhi, A.D.; Hartman, D.J.; Normolle, D.P.; Albers, K.M.; Davis, B.M. Systemic Depletion of Nerve Growth Factor Inhibits Disease Progression in a Genetically Engineered Model of Pancreatic Ductal Adenocarcinoma. Pancreas 2018, 47, 856–863.
- Nomura, A.; Majumder, K.; Giri, B.; Dauer, P.; Dudeja, V.; Roy, S.; Banerjee, S.; Saluja, A.K. Inhibition of NF-Kappa B Pathway Leads to Deregulation of Epithelial–Mesenchymal Transition and Neural Invasion in Pancreatic Cancer. Lab. Investig. 2016, 96, 1268–1278.
- Tanaka, Y.; Takeuchi, T.; Yamanaka, H.; Nanki, T.; Umehara, H.; Yasuda, N.; Tago, F.; Kitahara, Y.; Kawakubo, M.; Torii, K.; et al. A Phase 2 Study of E6011, an Anti-Fractalkine Monoclonal Antibody, in Patients with Rheumatoid Arthritis Inadequately Responding to Biological Disease-Modifying Antirheumatic Drugs. Mod. Rheumatol. 2021, 31, 783–789.
- Liu, H.; Li, X.; Xu, Q.; Lv, S.; Li, J.; Ma, Q. Role of Glial Cell Line-Derived Neurotrophic Factor in Perineural Invasion of Pancreatic Cancer. Biochim. Biophys. Acta BBA Rev. Cancer 2012, 1826, 112–120.
- Göhrig, A.; Detjen, K.M.; Hilfenhaus, G.; Körner, J.L.; Welzel, M.; Arsenic, R.; Schmuck, R.; Bahra, M.; Wu, J.Y.; Wiedenmann, B.; et al. Axon Guidance Factor SLIT2 Inhibits Neural Invasion and Metastasis in Pancreatic Cancer. Cancer Res. 2014, 74, 1529–1540.
- Ding, C.; Li, Y.; Xing, C.; Zhang, H.; Wang, S.; Dai, M. Research Progress on Slit/Robo Pathway in Pancreatic Cancer: Emerging and Promising. J. Oncol. 2020, 2020, 2845906.
- Jurcak, N.R.; Rucki, A.A.; Muth, S.; Thompson, E.; Sharma, R.; Ding, D.; Zhu, Q.; Eshleman, J.R.; Anders, R.A.; Jaffee, E.M.; et al. Axon Guidance Molecules Promote Perineural Invasion and Metastasis of Orthotopic Pancreatic Tumors in Mice. Gastroenterology 2019, 157, 838–850.e6.
- Casazza, A.; Finisguerra, V.; Capparuccia, L.; Camperi, A.; Swiercz, J.M.; Rizzolio, S.; Rolny, C.; Christensen, C.; Bertotti, A.; Sarotto, I.; et al. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J. Clin. Investig. 2010, 120, 2684–2698.
- Mauro, V.P.; Chappell, S.A. A Critical Analysis of Codon Optimization in Human Therapeutics. Trends Mol. Med. 2014, 20, 604–613.
- Noel, P.; Hussein, S.; Ng, S.; Antal, C.E.; Lin, W.; Rodela, E.; Delgado, P.; Naveed, S.; Downes, M.; Lin, Y.; et al. Triptolide Targets Super-Enhancer Networks in Pancreatic Cancer Cells and Cancer-Associated Fibroblasts. Oncogenesis 2020, 9, 100.
- Kim, S.T.; Kim, S.Y.; Lee, J.; Kim, K.; Park, S.H.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Park, J.O. Triptolide as a Novel Agent in Pancreatic Cancer: The Validation Using Patient Derived Pancreatic Tumor Cell Line. BMC Cancer 2018, 18, 1103.
- Chugh, R.; Sangwan, V.; Patil, S.P.; Dudeja, V.; Dawra, R.K.; Banerjee, S.; Schumacher, R.J.; Blazar, B.R.; Georg, G.I.; Vickers, S.M.; et al. A Preclinical Evaluation of Minnelide as a Therapeutic Agent Against Pancreatic Cancer. Sci. Transl. Med. 2012, 4, 156ra139.
- Modi, S.; Giri, B.; Gupta, V.K.; Lavania, S.; Sethi, V.; Sharma, N.S.; Pandey, S.; Vickers, S.; Dudeja, V.; Saluja, A.K. Minnelide Synergizes with Conventional Chemotherapy by Targeting Both Cancer and Associated Stroma Components in Pancreatic Cancer. Cancer Lett. 2022, 537, 215591.
- Skorupan, N.; Ahmad, M.I.; Steinberg, S.M.; Trepel, J.B.; Cridebring, D.; Han, H.; Von Hoff, D.D.; Alewine, C. A Phase II Trial of the Super-Enhancer Inhibitor MinnelideTM in Advanced Refractory Adenosquamous Carcinoma of the Pancreas. Future Oncol. 2022, 18, 2475–2481.
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.-C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.-M.; Wu, J.; et al. Pancreatic Cancer Genomes Reveal Aberrations in Axon Guidance Pathway Genes. Nature 2012, 491, 399–405.
- Yan, B.M.; Myers, R.P. Neurolytic Celiac Plexus Block for Pain Control in Unresectable Pancreatic Cancer. Am. J. Gastroenterol. 2007, 102, 430–438.
- Lillemoe, K.D.; Cameron, J.L.; Kaufman, H.S.; Yeo, C.J.; Pitt, H.A.; Sauter, P.K. Chemical Splanchnicectomy in Patients with Unresectable Pancreatic Cancer A Prospective Randomized Trial. Ann. Surg. 1993, 217, 447–457.
- Gola, M.; Sejda, A.; Godlewski, J.; Cieślak, M.; Starzyńska, A. Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 5246.
- Marchesi, F.; Piemonti, L.; Fedele, G.; Destro, A.; Roncalli, M.; Albarello, L.; Doglioni, C.; Anselmo, A.; Doni, A.; Bianchi, P.; et al. The Chemokine Receptor CX3CR1 Is Involved in the Neural Tropism and Malignant Behavior of Pancreatic Ductal Adenocarcinoma. Cancer Res. 2008, 68, 9060–9069.
- Di Natale, A.; Kaur, R.; Qian, C.; Zhang, J.; Marchioli, M.; Ipe, D.; Castelli, M.; McNair, C.M.; Kumar, G.; Meucci, O.; et al. Subsets of cancer cells expressing CX3CR1 are endowed with metastasis-initiating properties and resistance to chemotherapy. Oncogene 2022, 41, 1337–1351.
- Xu, Q.; Wang, Z.; Chen, X.; Duan, W.; Lei, J.; Zong, L.; Li, X.; Sheng, L.; Ma, J.; Han, L.; et al. Stromal-Derived Factor-1α/CXCL12-CXCR4 Chemotactic Pathway Promotes Perineural Invasion in Pancreatic Cancer. Oncotarget 2015, 6, 4717–4732.
- Khan, M.A.; Srivastava, S.K.; Zubair, H.; Patel, G.K.; Arora, S.; Khushman, M.; Carter, J.E.; Gorman, G.S.; Singh, S.; Singh, A.P. Co-Targeting of CXCR4 and Hedgehog Pathways Disrupts Tumor-Stromal Crosstalk and Improves Chemotherapeutic Efficacy in Pancreatic Cancer. J. Biol. Chem. 2020, 295, 8413–8424.
- Guo, J.-C.; Li, J.; Zhou, L.; Yang, J.-Y.; Zhang, Z.-G.; Liang, Z.-Y.; Zhou, W.-X.; You, L.; Zhang, T.-P.; Zhao, Y.-P. CXCL12-CXCR7 Axis Contributes to the Invasive Phenotype of Pancreatic Cancer. Oncotarget 2016, 7, 62006–62018.
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 Axis as a Mechanism of Immune Resistance in Gastrointestinal Malignancies. Semin. Cancer Biol. 2020, 65, 176–188.
- Shen, X.; Artinyan, A.; Jackson, D.; Thomas, R.M.; Lowy, A.M.; Kim, J. Chemokine Receptor CXCR4 Enhances Proliferation in Pancreatic Cancer Cells Through AKT and ERK Dependent Pathways. Pancreas 2010, 39, 81–87.
- Biasci, D.; Smoragiewicz, M.; Connell, C.M.; Wang, Z.; Gao, Y.; Thaventhiran, J.E.D.; Basu, B.; Magiera, L.; Johnson, T.I.; Bax, L.; et al. CXCR4 Inhibition in Human Pancreatic and Colorectal Cancers Induces an Integrated Immune Response. Proc. Natl. Acad. Sci. USA 2020, 117, 28960–28970.
- Ohshima-Hosoyama, S.; Simmons, H.A.; Goecks, N.; Joers, V.; Swanson, C.R.; Bondarenko, V.; Velotta, R.; Brunner, K.; Wood, L.D.; Hruban, R.H.; et al. A Monoclonal Antibody-GDNF Fusion Protein Is Not Neuroprotective and Is Associated with Proliferative Pancreatic Lesions in Parkinsonian Monkeys. PLoS ONE 2012, 7, e39036.
- Bizzozero, L.; Pergolizzi, M.; Pascal, D.; Maldi, E.; Villari, G.; Erriquez, J.; Volante, M.; Serini, G.; Marchiò, C.; Bussolino, F.; et al. Tumoral Neuroligin 1 Promotes Cancer–Nerve Interactions and Synergizes with the Glial Cell Line-Derived Neurotrophic Factor. Cells 2022, 11, 280.
- Brose, K.; Bland, K.S.; Wang, K.H.; Arnott, D.; Henzel, W.; Goodman, C.S.; Tessier-Lavigne, M.; Kidd, T. Slit Proteins Bind Robo Receptors and Have an Evolutionarily Conserved Role in Repulsive Axon Guidance. Cell 1999, 96, 795–806.
- Chédotal, A. Slits and Their Receptors. In Axon Growth and Guidance; Springer: New York, NY, USA, 2007; pp. 65–80.
- Secq, V.; Leca, J.; Bressy, C.; Guillaumond, F.; Skrobuk, P.; Nigri, J.; Lac, S.; Lavaut, M.-N.; Bui, T.; Thakur, A.K.; et al. Stromal SLIT2 Impacts on Pancreatic Cancer-Associated Neural Remodeling. Cell Death Dis. 2015, 6, e1592.
- Han, S.; Cao, C.; Tang, T.; Lu, C.; Xu, J.; Wang, S.; Xue, L.; Zhang, X.; Li, M. ROBO3 Promotes Growth and Metastasis of Pancreatic Carcinoma. Cancer Lett. 2015, 366, 61–70.
- Banh, R.S.; Biancur, D.E.; Yamamoto, K.; Sohn, A.S.W.; Walters, B.; Kuljanin, M.; Gikandi, A.; Wang, H.; Mancias, J.D.; Schneider, R.J.; et al. Neurons Release Serine to Support MRNA Translation in Pancreatic Cancer. Cell 2020, 183, 1202–1218.e25.
- Kayahara, M.; Nakagawara, H.; Kitagawa, H.; Ohta, T. The Nature of Neural Invasion by Pancreatic Cancer. Pancreas 2007, 35, 218–223.
- Demir, I.E.; Ceyhan, G.O.; Liebl, F.; D’Haese, J.G.; Maak, M.; Friess, H. Neural Invasion in Pancreatic Cancer: The Past, Present and Future. Cancers 2010, 2, 1513–1527.
- Sullivan, M.R.; Mattaini, K.R.; Dennstedt, E.A.; Nguyen, A.A.; Sivanand, S.; Reilly, M.F.; Meeth, K.; Muir, A.; Darnell, A.M.; Bosenberg, M.W.; et al. Increased Serine Synthesis Provides an Advantage for Tumors Arising in Tissues Where Serine Levels Are Limiting. Cell Metab. 2019, 29, 1410–1421.e4.
This entry is offline, you can click here to edit this entry!
