During tissue turnover, the telomeres of cells undergoing differentiation can be damaged as a consequence of defective DNA repair caused by endogenous or exogenous agents. This may result in the emergence of new mechanism of telomere maintenance which is the final outcome of DNA damage and the initial signal that triggers malignant transformation. Instead, transformation of stem cells is directly induced by primary derangement of telomere maintenance mechanisms. The newly modified telomere complex may promote survival of cancer stem cells, independently of telomere maintenance. An inherent resistance of stem cells to transformation may be linked to specific, robust mechanisms that help maintain telomere integrity.
- DNA damage response (DDR)
- DNA damage
1.
Introduction
A crucial difference between stem and committed cells of every tissue is found in the permanente expresión of telomerase in the former and its silencing in the latter even if it can be occasionally triggered in post-stem cells by some stimuli. This property of stem cells appears to be essential for their capacity for continuous self-renewal and the long-term preservation of tissues
The molecular mechanism that restricts telomerase expresión to stem cells is not known but its downregulation is undoubtedly connected to the first cell`s decisission to exit the stem cell compartment which, in turn, leads to the loss of indefinite self-renewal ability. This universal property of tissues has led to the assumption that cancer cells directly derive from stem cells or, alternatively, from committed cells that must reacquire this property of stem cells.However, It is difficult to reconcile the former alternative with most studies on cancer phenotype.
Most, if not all, common adult cancers including the cancer stem cell population exhibit markers of differentiation corresponding to distinct differentiation stages along development posterior to the stem cell stage(1 Torres-Montaner A & Highes D. A hypothetical anti-neoplastic mechanism associated to reserve cells. The Journal of Theoretical Biology (2004), 231, 39-248) 2 )). Concurrently, their telomeres are stablized but are reduced in lenght compared to normal counterparts. This suggests that common adult cancers arise from cells that, even if they are not terminally differentiated, are in the process of differentiation and concomitant telomere erosion. Furthermore, the carcinoma in situ stage, a typical feature of common adult cancers, seems to be reached at a critical threshold of telomere erosion [3). On the other hand, tumors exhibiting an undifferentiated/immature phenotype and sudden onset that apparently precludes sustained gradual telomere erosion, such as early childhood cancers, acute leukemia and some sarcomas, do not appear to develop through a carcinoma in situ stage. However, in spite of the sudden onset and imature morpholgy, some degree of telomere erosion seems to precede development of cancers of immature cell origin ( 4) although an in situ stage is difficult to be observed perhaps due to the rapid evolution of these cancers. Instead, an accelerated exit from the stem compartment and high telomerase expresión (5) is usually associated with /or preceding immature type cancers This may suggest interference with the telomere-maintenance-specific mechanism/s of stem cells. For instance, it has been shown that c-Myc overexpression within the stem cell compartment results in cellular exit from the stem cell compartment and increased differentiation at the expense of self-renewal [6]. Progenitors that leave the stem cell compartment as a result of c-myc overexpression become vulnerable to carcinogenesis. This may be associated with the impact of c-Myc on exit from the stem cell pool and probably interference with telomere maintenance mechanism/s specific to stem cells due to the ability of c-Myc to activate hTERT expression [7]. Information gathered on therapy using human-induced pluripotent stem cells (hiPSCs) revealed that tumorigenicity caused by reprogramming factors Oct3/4, Sox2, Klf4, and cMyc should be mainly attributed to cMyc reactivation. In Yamanaka`s words, “chimeric mice made with iPSCs created by the induction of retrovirus-mediated transfection of the four reprogramming factors often developed tumors”. We detected reactivation of c-Myc retrovirus in these tumors. Chimeric mice with iPSCs that had not been induced with the c-Myc retrovirus did not show such tumors. Another subset of tumors and teratomas formed by transplanted iPSCs may be related to faulty differentiation–expansion of progenitors in the absence of a physiological microenvironment, as shown by the fact that efficient methods of in vitro-directed differentiation reduce the risk of teratoma and tumor formation [8].
Finally, some observations suggest that direct cancer origin from stem cells, that is, true stem cell tumors, may rarely occur. One instance taken from the human clinic in support of this contention is the Beckwith–Wiedemann syndrome, a genetic disease of the telomerase reverse transcriptase (TERT)-TGFβ-Smad pathway which is associated with an 800-fold risk of developing childhood cancer in many different organs [9]. Another is represented by the tumor model reported by Passegué et al. and Santaguida et al, in which JunB inactivation specifically expanded the number of long-term hematopoietic stem cells (LT-HDCs) leading to myeloproliferative disease [10, 11].
Since the growth-promoting effects of telomerase have been linked to TGFβ inhibition and the expression of the TGFβ effector gene JunB is decreased in mice that overexpress mTert, it is apparent that the same, or closely related, pathway as that of Beckwith–Wiedemann syndrome may be involved in this tumor model . One way to unify the cancer pathways of mature, immature and stem/early progenitors is to hypothesize that the common outcomes of the independent mutations that are prevalent in common adult cancers may converge in a final step of telomere attrition/dysfunction, which, similar to the crisis observed in in vitro culture, might trigger reactivation of telomerase or ALT which is indispensable for the indefinite survival of cancer cells. This implies that most oncogenic mutations affecting committed cells should impact on the telomere complex in order to induce malignant transformation. Instead, transformation of tumors of stem cell origin and probably also of some immature tumors appears to be driven by primary disturbance of the telomere complex or pathways that directly impact the telomere maintenance mechanism/s.
A prediction of this hypothesis is that most if not all oncogenic agents should be able to alter the telomere complex
- Review and Hypothesis
The DNA Damage Response. Telomeres and Cancer
Genome damage can be caused by endogenous reactive molecules, environmental agents, or internal errors arising during the recombination of genomic regions as in the assembly of T- or B-cell receptors or other differentiation events as well as stalled replication and mismatch repair of DNA. Repair of genomic damage relies on two main DNA repair pathways. The first, homologous recombination (HR), occurs between the broken DNA and the corresponding region on the opposite strand. The second, more error prone pathway, is nonhomologous end joining (NHEJ. NHEJ is carried out by DNA-dependent protein kinase (DNA-PK), its associated dimer Ku70–Ku80, and the DNA ligase IV/XCCR4 complex. Single (SSBs) and double-stranded breaks (DSBs) are the most lethal lesions of genomic DNA (12)
The response to DSBs is initiated by ATM and the MRN complex, whereas single-stranded breaks primarily activate ATR. ATM phosphorylates and activates downstream substrates such as Chk2 and p53; p53 upregulates CDK inhibitors which by inactivating CDKs lead to cell cycle arrest. In addition, checkpoint dependent phosphorylation of Cdc25 provokes its binding to 14-3-3 which results in subsequent nuclear export that precludes its interaction and activation of Cdc2-cyclib B complex(13) . Many of the downstream targets are multifunctional proteins that interact with various repair pathways. This makes it difficult to dissect the interconnected processes involved in repair, checkpoint control, and telomere maintenance. For instance, Rad9 is phosphorylated by ATM and, upon binding of 9-1-1 and TOBP1, activates ATR, leading to Chk1 activation. Similar to p53, it may be involved in cell cycle arrest and apoptosis. Additionally, it participates in five DNA repair pathways: HR, base excision repair (BER), nucleotide excision repair (NER), mismatch repair, and alternative-NHEJ (alt-NHEJ) [14].
The aim of the DNA damage response is to preserve DNA integrity. This goal requires the assistance of a temporary arrest at different phases of the cell cycle (G1/S, S, and G2/M checkpoints). Faulty DNA repair may lead to apoptosis, although its effect may be neutral or result in mutations that enhance cell proliferation. DNA repair is necessarily connected with cell cycle control that provides time for completion of DNA repair. However, even prolonged mitotic arrest has been found to be deleterious for telomeres (15) .
There is a different response addressed to cytotoxic and environmental stimuli closely connected to DDR which can also mediate cell cycle arrest and apoptosis called the stress kinase pathway. It has been shown that depletion of targets of this pathway JNK1 or JNK2 can suppress cell growth. Evidently, disruption of cell cycle should impinge mainly in telomere integrity. Apoptosis is also a frequent outcome of faulty telomeric DNA repair; however, telomere damage could also result in the emergence of aberrant mechanisms of telomere maintenance It seems that the essential characteristic of cancer stem cells, indefinite self-renewal, can only arise through different forms of telomere damage, leading to the emergence of aberrant forms of telomere maintenance in committed cells. It is difficult to envision how increased cell proliferation, per se, could result in indefinite self-renewal. According to this hypothesis, DNA lesions cannot induce transformation by themselves but must go through a final step involving alteration of the telomere maintenance apparatus.
Perusal of the literature shows that deregulation of most DNA damage response components is concurrently associated with telomere dysfunction/erosion and cancer predisposition (Tables 1 and 2).
Table 1. Components of the DNA damage response responsible for telomere dysfunction and cancer.
|
|
Telomere Dysfunction |
Cancer Predisposition |
|
* P53 |
Yes [16] |
Yes [17] |
|
Rad 9 |
Yes [18] |
Yes [18] |
|
Rad 51 |
Yes [19,20] |
Yes [21] |
|
Rad 52 |
Yes [22] |
Yes [22,23] |
|
Rad 54 |
Yes [24] |
Yes [25,26] |
|
c-Abl |
Yes [27,28] |
Yes [29] |
|
BRCA1-BRCA2 |
Yes [30,31] |
Yes [32] |
|
** PARP-1 |
Yes [33–35] |
Yes [36] |
|
*** DNA-PKcs |
Yes [28,37] |
Yes [38] |
|
Artemis |
Yes [28,39] |
Yes [40] |
|
Cerunnos |
Yes [41] |
Yes [41] |
|
Ku70-Ku80 |
Yes [42,43] |
Yes [43,44] |
|
Ligase IV |
Yes [45,46] |
Yes [46] |
|
Fanconi Anemia |
Yes [47] |
Yes [46] |
|
^ CtIP |
Yes [48] |
Yes [48,49] |
|
Rad17 |
Yes [50] |
Yes [51,52] |
|
9-1-1 complex |
Yes [53] |
Yes [53] |
|
14-3-3 complex |
Yes [28] |
Yes [54] |
|
CTC complex |
Yes [55,56] |
Yes [55,56] |
Notes regarding Table 1. * (p53) In children, telomere length was found to be shorter in carriers affected with cancer than in unaffected carriers and with respect to controls. The same pattern was observed in adults. Telomere attrition between children and adults was faster in the carriers than in the controls. These results support the role of Mdm2-SNP309 as a genetic modifier of LFS. The finding of accelerated telomere attrition in LFS patients suggests that telomere length could explain the earlier age of onset in successive generations of the same family with identical TP53/MDM2-SNP309genotypes. ** (PARP1) The role of PARP1 in telomeres is controverted but it is acknowledged that it is required for the maintenance of damaged telomeres and associates with R-loops to promote their resolution and genome stability. According to [35], it may not have an effect on telomere length but may affect telomere dysfunction. However, its role in cancer is not well understood. In p53, w.t. PARP overexpression correlated with high expression of stem cell markers in colorectal tumor samples as well as with sphere-forming ability but its overexpression was reduced in a mutant p53 context. PARP expression increased with advanced dedifferentiation but not in the p53 mutants. *** DNA-PKcs. Somatic mutations in DNA-PKcs have been identified in patients with breast and pancreatic cancer. Ablation of mouse Thr2605 leads to multiple stem cell defects and increased chromosomal alterations. ^ Human CtIP. CtIP is a multifunctional protein that interacts with multiple partners, including the oncogenic corepressor CtBP, tumor suppressor RB, promoter of cell proliferation Pin1, and oncogene and DNA repair protein BRCA1. It is involved in the activation of the G2/M cell cycle checkpoint and in collaboration with the MRN complex 5′-3′ resection of broken DNA ends. CtIP deficiency impairs DSB repair. However, in contrast to BRCA1, CtIP is amplified in a substantial proportion of pancreatic cancer, high-grade serous ovarian cancer, esophageal carcinoma, and gastric adenocarcinoma. Germline mutations have been identified in patients with early-onset breast cancer who are negative for BRCA1/2 mutations. However, some functions of CtIP could counteract tumorigenesis, and it was initially postulated to be a tumor suppressor based on the shortened lifespan and tumor predisposition of ctip+/− heterozygous mice. In short, CtIP plays very intricate roles in both DNA response and tumorigenesis but its involvement in telomere homeostasis has been demonstrated.
Table 2. Components of the DDR which are responsible for DNA repair syndromes that manifest early in life as clinical entities and are concurrently associated with cancer predisposition and telomere homeostasis.
|
|
Telomere Dysfunction |
Cancer Predisposition |
|
(See comment) ERCC6like2 (ERCC6L2) |
Yes [57] |
Yes [46] |
|
CMMRD-Xeroderma pigmentosum |
Yes [58] |
Yes [33,46] |
|
Bloom syndrome |
Yes [59] |
Yes [46] |
|
Werner syndrome |
Yes [59] |
Yes [46] |
|
Rothmund–Thomson syndrome |
Yes [60] |
Yes [46] |
Note regarding Table 2. Ref. [49] described three new cases of trilineage bone marrow failure, microcephaly, and learning difficulties consistent with ERCC6L2 deficiency. Exome sequencing identified homozygous variants of ERCC6L2 in two cases. Telomere shortening was present in two.
Finally, deregulated expression of chromatin remodeling factors may also drive tumorigenesis and may be mediated by telomere dysfunction.
2.2. False Exceptions to the Correlation between Cancer–Telomere Dysfunction: Seckel Syndrome, Cockayne Syndrome, Trichothiodystrophy
In humans, Seckel syndrome type 1 is caused by an autosomal recessive mutation in ATR. Patients with this condition exhibit developmental defects but no cancer predisposition. Nevertheless, an autosomal dominant germline ATR missense mutation associated with oropharyngeal cancer was found in 24 individuals from a five-generation pedigree. ATR mutation segregated with the disease and resulted in lower p53 levels upon ATR activation. Loss of heterozygosity has been observed in tumor tissue [61]. Mice heterozygous for ATR develop normally and suffer increased cancer incidence after 18 months of age, while adult mice null for ATR displayed an age-related phenotype reminiscent of human Seckel syndrome without tumor development. ATR is specifically activated by single-strand DNA lesions. Initially, ssDNA intermediates are rapidly coated with replication protein A (RPA) which is quickly bound by ATRIP, providing a binding site for ATR. The DNA repair process that follows requires recognition of the junctions of the DNA lesion by Rad17 and the 9-1-1 complex and activation of several further components of the pathway, including TOBP1 and Fanconi Anemia factors. This suggests that DNA repair in Seckel syndrome is aborted in its initial stages, which may conceivably lead to arrested proliferation or be incompatible with cell life owing to ATR loss of function [62]. Dramatic proliferation failure of ATR-deficient cells is observed in vivo as well as in in vitro cell culture, apparently due to the lack of stabilization of replication forks in the absence or deficiency of ATR. Chromosome breaks are observed in ATR knockout cells even without exogenous replication stress, suggesting that stalled DNA replication may be a common event during normal replication. The rapid exhaustion of replication ability in ATR-deficient cells may explain the rarity of tumors either in ATR null mice or in human Seckel syndrome [63] or even the small occurrence of tumors in heterozygous mice suggesting that unrepaired DNA is incompatible with cell life, reinforcing the view that sustained cell proliferation must be supported by a putative anomalous telomere-associated mechanism rather than by genomic mutation; in other words, unrepaired DNA leads to cell death rather than cancer.
Cockayne syndrome (CS) is caused by a specific failure in the mechanism of transcription-coupled repair due to defects in either of the two genes ERCC6 (CSB) or ERCC8 (CSA). Following UV irradiation, transcription is arrested through the binding of ATF3, the product of an immediate early gene (IEG) to CRE/ATF binding sites present in numerous genes. This prevents binding of RNA pol II and inhibits RNA synthesis. Surprisingly, not only are transcribed genes inhibited by this mechanism but so are inactive genes. RNA sequence analysis showed that 5895 genes were downregulated (70%) in CSA and CSB cells. Inhibition of RNA synthesis is short-lived in cells proficient in CSA and CSB but considerably delayed in CS cells. ATF3 is removed from CRE/ATF sites at promoters by a ubiquitin proteasome degradation mechanism that involves Mdm2. Mutations that interfere with this process lead to the persistence of ATF and continued repression of RNA transcription similar to that caused by CSA or CSB deficiency [64]. Functionally, the end result appears to be analogous to that of Seckel syndrome in that interference with RNA transcription can make the cell unable to proliferate and execute DNA repair. As in the case of Seckel syndrome, this can be incompatible with cancer transformation.
A similar mechanism could explain the lack of cancer predisposition in trichothiodystrophy (TTD). TTD is caused by TTDN1, a gene of unknown function or by TTDA which encodes a small subunit of TFIIH. THIIH has a vital function in the initiation of RNA transcription [65].
.2.3 Mutation of the Mre11 Component of the MRN Complex Does Not Result in Telomere Erosion nor Cancer Predisposition
The MRN complex formed by Mre11, Rad50, and Nbs1 proteins is the main sensor of double-strand breaks. It is involved in both the HR and NHEJ DDR pathways. MRN associates with chromatin and recruits inactive ATM dimers to DSBs where the active monomer initiates the ATM phosphorylation cascade. However, MRN or its components can also act downstream of ATM and, together with ATM, can promote the activation of ATR [66, 67)]. Germline alterations of MRN components give rise to hereditary cancer predisposition syndromes ataxia-telangiectasia like disease (A-TLD) and Nijmegen breakage syndrome (NBS), caused by hippomorphic mutation in the gene encoding Mre11 nuclease and NBS1 (encoding the protein NBN also known as nibrin) (null mutation of Nbs1 causes embryonic lethality). These syndromes are phenotypically similar to ataxia-telangiectasia, (AT), a hereditary cancer predisposition syndrome caused by ATM deficiency which presents with cerebellar degeneration, immunodeficiency, reduced fertility, radiosensitivity, and cancer predisposition. Similar phenotypic features are shared by Nijmegen breakage syndrome (NBS) except that it presents with microcephaly in place of cerebellar degeneration and A-TLD, although the latter may have a milder clinical expression [67,68]. Cancer predisposition of AT and NBS is conspicuous and manifests mainly in lymphomas related to chromosomal aberrations at the sites of T-cell receptor or immunoglobulin chain rearrangement where V(D)J recombination spontaneously induces DSBs. However, other cancer types may occur. Tauchi et al. [69] reported that 22 out of 55 patients in the International NBS registry in 2002 (age range 1–22 years) had developed a malignancy. Of these, 16 were lymphomas and the remaining six had developed leukemia, glioma, medulloblastoma, or rhabdomyosarcoma. Similar to AT, cells derived from patients with NBS exhibit accelerated shortening of telomeres, loss of intra-S and G2/M checkpoint control, and genomic instability [69]. Another study including 241 cases reported a median age of cancer onset of 9.1 years with a probability of 20-year survival of 44.6% [70], The association of cancer with A-TLD is more ambiguous, partly due to the rarity of this syndrome, although it is clearly much lower than that of AT and NBS. By 2020, only 23 cases had been identified, but only two patients died of cancer (lung adenocarcinoma) at 9 and 14 years of age. These were two brothers with two distinct mutant alleles, one from each parent.
Spehalski et al (71) introduced the Mre11H129N mutation in B lymphocytes through CD19-Cre expresión. This,did not affect the survival of p53 null mice and thymic lymphomas typical of p53 deficiency developed with the same frequency regardless of Mre11 deficiency.
Next, these authors examined the impact of Mre11 deficiency on the Artemis mouse model strongly predisposed to B lineage lymphomas. Artemis is required for processing the DNA ends generated during V(D)J recombination. Mutations in Artemis cause immunodeficiencies and tumors. When combined with p53 deficiency, aggressive pro-B lymphomas harboring chromosomal translocations involving IgH and c-Myc or N-Myc loci develop. The results were as follows:
Artemis null, p53 null, Mre11 null (17 weeks survival).
Artemis null, p53 null Mre11H129n/− (19 weeks survival).
Artemis null p53null Mre11+/− ® (14 weeks survival).
Thus, the B-cell-specific loss of Mre11 or Mre11 nuclease activity did not markedly alter survival. In fact, survival was slightly prolonged in Mre11 null or Mre11/−. The majority of Artemis/p53 double nulls succumbed to progenitor B lymphoma and a smaller subset of thymic lymphoma of progenitor T-cell origin. Remarkably, no pro-B-cell lymphoma arose in Artemis/p53 double null mice with either Mre11−/− (n = 11) or Mre11−/H129N. The majority of lymphomas that developed in these mice were thymic lymphomas. These results indicate that the Mre11 mutation suppresses pro-B-cell lymphomas in a context where pro-B-cell lymphomas normally arise in the majority of mice by 8–10 weeks of age in spite of the preserved ability of p53 null Mre11 null and p53 null Mre11H129N/− to generate oncogenic chromosomal translocations (including IgH: Myc translocations) and also chromosomal translocations outside of the IgH locus, not only in B cells but also in nonlymphoid cells. In summary, inhibition of Mre11 nuclease activity through gene mutation precludes or attenuates tumor promotion (71) This is supported by the work of Theunissen et al. [72] using the Mre11ATLD/ATLD mutant. They showed that Mre11ATLD/ATLD mice are not prone to lymphomagenesis in spite of having defective cell cycle checkpoints and chromosomal translocations similar to that of ATM and NSB1 deficiencies
A feature displayed by the Mre11ATLD/ATLD mutation may be significant in order to explain the different tumor-promoting activities of MRN component Mre11 versus Nbs1 and ATM. Shortened telomeres associate with defective ATM or Nbs1 function [69], but telomere shortening is absent in cells homozygous for Mre11ATLD/ATLD [73]. Attwool et al. reported that no telomere attrition was seen in immortalized Mre11ATLD/ATLD MEFs over the course of over 300 population doublings. At cPDL 300, there was no difference in telomere shortening between Mre11+/+ Tert∆/∆ and Mre11ATLD/ATLD Tert∆/∆. Also, maintenance of the G-strand overhang was unaffected.(73)
Another intriguing and significant finding in this context is that development of thymic lymphoma in p53-deficient mice does not require V(D)J recombination (74). This may suggest that Artemis mutation that impairs V(D)J recombination is rescued by the telomere preservation associated to Mre11 mutation. Mre11 mutation could not achieve the same telomere effect when brought about by p53
In summary, although DNA repair, checkpoint control, genomic stability, and telomere maintenance appear to be disrupted simultaneously by mutations of ATM or Nbs1, and are associated with their cancer predisposition, mutation of the MRN component Mre11 (Mre11ATLD/ATLD) does not impair telomere integrity despite affecting to some extent other branches of the DDR. Concomitantly, this mutation is associated with a much smaller cancer predisposition than that associated with ATM or NBS1, suggesting that telomere disruption is the main culprit for the ensuing cancer predisposition (Table 3).
Table 3. Common and differential features of ataxia-telangiectasia mutated, ataxia-telangiectasis-like disease, and Nijmegen breakage syndrome.
|
ATM-Mutated |
Mer11 (AT-LD) |
NBN (Nijmegen Breakage Syndrome) (NBS) |
|
Deficient initial DNA repair. |
Deficient initial DNA repair. |
Deficient initial DNA repair. |
|
Hypersensitivity to IR-induced chromosome breakage, increased translocations (chromosomal instability). |
Hypersensitivity to IR-induced chromosome breakage, increased translocations (chromosomal instability). |
Hypersensitivity to IR-induced chromosome breakage, increased translocations (chromosomal instability). |
|
IR-induced thymocyte apoptosis relative to wild-type: 58% versus 90%. Severe intra-S and G2/M checkpoint defects. Telomere shortening. Cancer predisposition. |
IR-induced thymocyte apoptosis relative to wild-type: Sightly reduced (81% versus 90%. Intra-S and G2/M checkpoints less severe than in ATM deficiency and comparable to Nsb1 null. No telomere shortening. No cancer predisposition (or mild). |
IR-induced thymocyte apoptosis: Extreme variations in apoptotic capacity (in neural tissue similar to wild-type). Intra-S and G2/M checkpoints less severe than in ATM deficiency and comparable to Mre11 hypomorphic. Telomere shortening. Cancer predisposition. |
Mutations in Rad50, the other subunit of the MRN complex, are very rare. To date, only two patients with germline biallelic variants of Rad50 have been described. The clinical presentation was similar to both NBS and A-TLD. The association of either homozygous or heterozygous variants of Rad50 with cancer predisposition has been debated [75]. However, Rad50s/s or BRCA1 defects giving rise to progeroid phenotypes, shortened lifespan, cancer predisposition, and hematopoietic stem cell failure has been described [76].
2.4 DNA Damage Associated with Defective Genomic Recombination
DNA damage response integrates functions of DNA repair, checkpoint control and apoptosis but is also inextricably linked to telomere maintenance as telomeres are the latest región of the genoma to be replicated and its replication is closely coordinated with the cell cycle. Therefore, checkpoint defects can easily affect telomere maintenance. Perturbed apoptosis may also impinge on checkpoint control as the same factors can perform both tasks and therefore result in telomere damage. On the course of tissue differentiation dysregulation of the interplay between differentiation events and DDR manifest mainly on disruption of apoptosis and/or cell cycle arrest that lead to tumor formation.
2.5. Oncogenesis and Altered Differentiation to Plasma Cells
An example of it, is provided by the work of Sherman et al. [77] . These authors analyzed a single differentiation event in hematopoietic development: the transition from germinal center lymphocytes to memory B cells and plasma cells. Their work showed that the impairment of normal differentiation can lead to neoplastic transformation in a process that recapitulates most of the features involved in developmental blocks. This analysis also showed that aberrant DDR is functionally equivalent to a differentiation block.
In the germinal center (GC) reaction, physiological double-strand breaks are produced during the diversification of antigen receptor genes and the formation of higher affinity antibodies associated with class switch recombination. They showed that, following a double-strand break (DSB), one of the substrates phosphorylated and activated by ataxia-telangiectasia-mutated (ATM) is LKB1 (also called STK11 (serine-threoninkinase 11), which, through adenosine-monophosphate-activated protein kinase (AMPK), induces cytoplasmic translocation and inactivation of CREB-regulated transcriptional coactivator 2 (CRTC2). This occurs over an interval of 3 to 7 days, correlating with a gradual decrease in the association between CRTC2 and the TCL1 promoter followed by concomitant gradual decrease in the expression of T-cell leukemia/lymphoma 1 (TCL1) oncogene. During the first phase of the GC reaction, B lymphocytes undergo enhanced proliferation due to TCL1 oncogene and BCL6 expression. B-cell lymphoma 6 (BCL6) imposes a pause in plasma cell differentiation through repression of the PR domain zinc finger protein 1 (PRDM1) (encoding B-lymphocyte-induced maturation protein 1 (BLIMP-1)) and suppresses some components of the DNA damage reaction (DDR) including ataxia-telangiectasis and Rad3-related (ATR) p53 and p21. This allows unrestricted B-cell proliferation, which must be inhibited in the second phase of the process. This requires BCL6 downregulation to allow for the next final differentiation step into plasma cells. The pathway in charge of this second phase, which abates the initial cell proliferation and permits differentiation, is also triggered by the physiologically generated double-strand break, in this case through activation of the ATM cascade, which antagonizes the repression of differentiation induced by BCL6. In addition to classical substrates activated by ATM, such as p53 and p21, these authors identified the tumor suppressor LKB1, which inactivates CRTC2. The timing of cytoplasmic translocation and inactivation of CRTC2 appears to be critical for the development of two opposing phases that entail delayed plasma cell differentiation in the initial GC reaction and proper differentiation and halted proliferation in the second phase, as reproduced in an in vitro B-cell differentiation system using naïve human tonsil B cells. In summary, the response to physiological DSBs is chronologically regulated and involves a first phase of repressed differentiation and enhanced proliferation, which is subsequently antagonized by mechanisms that inhibit proliferation and allow cell differentiation. The normal function of the DDR underlies the normal course of this process, and disruption of the second controlling phase (either by cell cycle arrest or apoptosis) has potentially damaging consequences that may lead to cancer development.
3. Disturbed Differentiation and Notch Signaling
The lack of coordination between physiological DNA damage and the subsequent phases in command of proliferation and/or mitosis control has been studied in an experimental model commented in a former paper (78) describing the association between hematopoietic developmental blocks and leukemogenesis:
Notch1 signaling controls the early development of progenitors into the T-cell lineage while inhibiting B-cell differentiation. Lineage commitment is followed by VβDβJβ recombination which, in combination with a surrogate light chain and other molecules such as CD3 chains, forms the pre-T-cell receptor (pre-TCR) complex. Completion of the pre-TCR divides the DN3 stage into DN3a and DN3b stages. Pre-TCR signaling is required for the differentiation of CD44- CD25+ (DN3) into DN4 and double-positive (DP) cells. Disruption of pre-TCR arrests T-cell development at the CD44- CD25+ stage. Rag null cells cannot rearrange TCR genes and lack pre-TCR. Therefore, they fail to form DP T cells and arrest at CD44- CD25+ (DN3 stage). When Rag null are transduced with Notch ligand (ICN1), they do not generate DP. If, in addition, they are transduced with a TCRβ transgene, they form DPs and rapidly expand, indicating that pre-TCR signals complement Notch to develop DPs. ICN1 (intracellular Notch 1)-induced bone marrow (BM) DPs cells from both WT and Rag null x TCRβ rapidly expand. In contrast, ICN1-induced Rag null did not, suggesting that pre-TCR signals are required for the proliferation burst that accompanies thymocyte differentiation. Mice repopulated with ICN1-transduced WT hematopoietic stem cells (HSCs) generated extrathymic DP T cells within 3 weeks. In contrast, mice repopulated with ICN1-transduced Rag-2−/− did not generate DP T cells. These findings are consistent with a model in which Notch commits lymphoid precursors to the T lineage; however, pre-TCR signaling is required for the proliferative burst that accompanies thymocyte differentiation.
Recipients of ICN1-transduced HSCs from Rag null mice remained alive for >1 year after transfer. In contrast, all mice receiving ICN1-transduced BM cells from Rag null mice expressing a TCRβ transgene developed T-cell leukemia between 9–11 weeks after transfer. Thus, ICN1-mediated transformation of T-cell progenitors required the expression of a TCRβ chain and development of CD4+ CD8+ T cells [116,79]. Under physiological conditions, thymocyte proliferation ceases shortly after the cells reach the DP stage, and Notch signaling fails to influence thymocyte development following β selection [117,80]. In these experiments, three situations may be distinguished: the physiological situation when DNA recombination is followed by cellular proliferation and differentiation, which abates shortly after cells reach the DP stage 2/arrested cell proliferation–differentiation in the absence of the necessary signals delivered by the Notch signaling, plus preTCR (in it lack of cell proliferation prevents cancer development) 3/cell proliferation–differentiation extending beyond the DP stage in the presence of transduced TCR and constitutive Notch signaling. This situation leads to leukemic transformation but is not preceded by V(D)J recombination as Rag has been deleted. Here, the parallelism with GC-derived tumors apparently seems to fail as there is no DSB at the start of the process. However, it is tempting to speculate that some minor form of DNA perturbation may be present since DP cells are formed and there are DNA modifications that must concur with Rag at this crucial differentiation step. For instance, single stretches of DNA may be induced by cofactors that participate along with Rag in the formation of the pre-TCR, such as the surrogate light chain and CD3 chains. This may well be the case since injection of anti-CD3ε mAb into Rag-deficient mice allows progression of DN thymocytes to the DP stage [118,81]. On the other hand, similar to a GC reaction cell proliferation may not cease due to constitutive Notch expression concurrent with the DP stage
4.A DNA Damage Response Is Triggered by Oncogenes
A two-phase response similar to the germinal center (GC) reaction is also elicited by c-Myc. It differs from the GC reaction in the identity of the regulators driving the proliferative phase which, in this case, is directly induced by Myc and its targets, whereas the following growth suppression phase is triggered at least in part, as in the GC reaction, by a DDR response. As a matter of fact, in addition to signaling to p53 through the ARF tumor suppressor, enhanced Myc expression was shown to induce increased levels of total and phosphorylated p53 through ATM activation as part of a DDR response. Overexpression of Myc in squamous epithelium using K5-Myc transgenic mice demonstrated that Myc induced multiple γH2AX and phospho-SMC1 foci similar to those induced by ionizing radiation (IR) as well as malignant transformation.
It has been reported that forced expression of other oncogenes, including E2F1, cyclin E, or cdc25A, induce the ATM signaling pathway [82].
5. Double-Stranded Breaks versus Chromatin Alteration
Germline mutation of ATM, the central player of the DNA damage response associated with ataxia-telangiectasia síndrome focussed the attention of researchers on the role played by double DNA strand breaks in cancer. However, the relation of DDR with cancer is more complex than initially thought.
The role of physiological DSBs and their alterations in tumorigenesis was studied in an animal model by Petiniot et al. [83]. These authors showed that mice double deficient for the recombinase-activating gene Rag 2 and Atm were still subject, in the absence of physiological V(D)J recombination, to increased tumor development both in lymphoid tissue and other tissues. It is known that lymphomas arising in ATM-deficient mice display consistent cytogenetic abnormalities at the Tcr/α/δ locus. The work of these authors demonstrated that development of malignant lymphoma in Atm−/− mice is not prevented by loss of Rag2. However, the appearance of these tumors was somewhat delayed, and lymphomas did not exhibit the Tcr/α/δ rearrangement typical of ATM deficiency. In order to evaluate the role played by developmental arrest produced as a result of lack of Tcr, these authors generated HyTcrTg+ Rag2−/− Atm−/−mice that overcome arrest by HyTcr transgene expression. These mice did develop lymphoma comparable in frequency and phenotype to those of Rag−/− Atm−/−, and cytogenetic analysis demonstrated absence of translocation within the Tcrα/δ locus.
As expected, no tumors developing in Rag2−/− Atm−/− mice express surface TCR. However, when the arrest in T-cell development caused by Rag2 deficiency is rescued by TCR transgene expression, as in HyTcrTg+ Rag2−/− Atm−/− [84], all tumors expressed the TCR transgen Vβ8+. The predominance of tumors expressing the transgene might suggest that the proliferation burst provided by TCR expression may contribute to tumor development. Nevertheless, the ability to proliferate and differentiate must not be completely abolished in the absence of TCR
5.1Chromatin Remodeling Factors Are Concurrently Associated with Telomere Dysfunction and Cancer
T-loops are RNA: DNA hybrids formed between a single RNA strand and a double-stranded DNA. Telomeric repeat containing RNA (TERRA) is transcribed from subtelomeric regions to telomeres and can invade telomeric dsDNA to form telomeric R-loop structures [85]. The SWI/SNF chromatin remodeling complex plays an important role in resolving R-loop conflicts. Consequently, malfunction of this complex may result in telomere dysfunction. Deregulation of SWI/SNF has been observed in undifferentiated/rhabdoid-type tumors [86,87]. This suggests that its normal function must occur at a very early stage of cell differentiation and its impairment must be reflected in the very immature phenotype of the tumor cell population.
BCL11B is a transcription factor that interacts with nucleosome remodeling and the histone deacetylation complex NuRD [88]. It may also play a role in telomere structure and function [89,90]. Mutations in BCL11B have been associated with lineage ambiguous leukemias [91] and undifferentiated nervous tissue tumors [92]. There are several other cases where chromatin remodeling dysfunction is associated with acute leukemias and sarcomas with an extremely undifferentiated phenotype, suggesting that these tumors arise through errors produced in very early differentiation events at the immediate post-stem cell stage.
Another member of the SWI/SNF chromatin remodeler group, ATRX, plays an important role in telomere function and cancer which is beginning to be unraveled. Loss of ATRX or its partner DAXX frequently occurs in cancers with the ALT phenotype. It has been shown that the loss of these factors induces telomere dysfunction that culminates in the inception of an ALT mechanism that supports cell immortalization [93].
.6. Replication Stress Associated with Developmental Blocks Underlies Cancer
Transformation
Examination of hematopoietic development showed that any developmental block at any step of the differentiation ladder is always associated with leukemic transformation [78]. On this basis, it was proposed that replication stress induced by blocked differentiation induced transformation by disrupting telomere integrity and/or the re-expression of telomerase [78]. Developmental blocks create favorable conditions for DNA damage by altering chromatin structure, delaying or stalling transcription, and generating recombination breaks that, when not properly handled, can be converted to single- and double-stranded DNA breaks. Moreover, it has been shown that cells unable to transition to the next differentiation step proliferate extensively, and, consequently, a local shortage of nucleotides may be generated, an important feature of replication stress [94].
Replication stress (RS) refers to disturbances in DNA dynamics that are reflected in the slowing and asymmetry of replication fork progression. It also affects deregulation of replication origins. There are multiple causes of replication stress, including oncogenes, shortage of nucleotides and replication factors, UV or ionizing radiation, and reactive oxygen species, as well as difficulties arising in the process of DNA replication due to special features of particular DNA regions, such as repetitive elements, late replication or anomalous chromatin configuration. Some of these causes have been shown to be interrelated, such as oncogenes and exhaustion of nucleotide pools that preferentially influence certain regions of DNA such as common fragile sites (CFS). Concerning oncogenes, Bester et al. showed [94] that keratinocyte proliferation induced by deregulation of the Rb-E2F pathway by HPV-16 E6/E7 or cyclin E oncogenes resulted in slower cellular DNA replication fork rate, decreased symmetry between the right and left fork progression, and increased number of active origins. They detected increased copy number variation (CNV) and loss of heterozygosity (LOH) preferentially associated with fragile sites. LOH measured after 100 days in three representative fragile sites (FRA3B, FRA16D, and FRA7G) was 4.1% significantly higher than that found in the entire genome, 1.4%. Instability increased with time. It was firmly established that both replication slowing and DNA damage were caused by an insufficient rate of nucleoside synthesis which was unable to cope with the needs of enhanced cell proliferation induced by the Rb-E2F pathway. In fact, exogenous supply of nucleosides corrected the DNA-replication-induced defects without affecting cell proliferation. The main form of DNA damage detected was activation of the ATR DDR pathway caused by the slowing of replication forks [100].
Saldivar et al. [95] demonstrated that deletion of the FHIT gene located within the FRA3B fragile site results in nucleotide exhaustion because FHIT knockdown suppresses thymidine kinase 1 (TK1) expresión (dTTP is synthesized by two pathways via thymidilate synthase (TYMS) or rhe scavenger pathway via thymidine kinase, TKI). The primary DNA defect observed was deficient replication fork progression which led to fork stalling and collapse. FIHT knockdown resulted in a threefold increase in the fraction of cells containing phosphorylated ATR and γH2AX foci but they did not detect phosphorylated Chk1, suggesting that the S phase checkpoint was not activated. In fact, S and G2 checkpoints were defective and cell proliferation continued. This suggests that it is the branch of the DDR responsible for delaying cell division that is mainly perturbed. Loss of FHIT is frequent in precancerous lesions and has been proposed as a soil for further cancer transformation. More recently, Tummala et al (96) have reported individuals with clinical features of the cancer-predisposition syndrome dyskeratosis congénita (DC) that harboured los of function mutation of TYMS gene(thymidylate synthase) combined with an epistatic effect from its antisensense regulator. This represents a new molecular pathway for DC. TYMS-ENOSF1 defects lead to decreased deoxyribonucleotide pools, ribonucleotide reductase inhibition and a DNA damage response that affects expresión of proteins involved in telomere maintenance. In budding yeast regulation of dNTP production is considere dan essential function of DNA damage signaling. In mammals, ATR stablizes RRM2 ribonucleotide reductase subunit (RNR) regulatory subunit). It has been suggested that an extracopy of RNR can extend the lifespan of ATR-deficient mice (97, 98). Interestingly, p53 may promote transcription of the RRM2B subunit of human RNR (97)
Similarly, hydroxyurea (HU) causes depletion of dNTPs through inhibition of ribonucleotide reductase (RNR), and replication fork stalling is typically observed after treatment with hydroyurea (HU) or aphidicoline (APH) 94,95).
DNA regions particularly sensitive to RS were revealed by the formation of recurrent DNA breaks after exposure of cells to APH, an inhibitor of polimerase α and were termed common fragile sites (CFS). Formation of these sites can be observed in early preneoplastic phases following oncogene activation or tumor suppressor inhibition. Other chromosome regions specially sensitive to RS due to their content in repetitive DNA are ribosome, centromere and telomere regions. All these sites often remain incompletely replicated until mitosis and may result in formation of anaphase bridges unless they are processed by mechanisms that allow mitotic rescue of RS. In cancer cells, candidate suppressors of RS such as PIGN, MEXC or ZNF516 are often inactivated. FANCD2 in cooperation with BLM can promote resolution of ultrafine bridges (UFBs), DAPI-negative DNA threads arising from replication intermediates that persist until mitosis and associate with sister chromatid entaglement. Five types of UFBs have been described: centromeric (c-UFBs), ribosomal (r-UFBs), common fragile site (CFB-UFBs), telomeric (t-UFBs), and the newly identified HR-UFBs derived from HR recombination intermediates (99). T-UFBs can be induced by RS similar to CFS-UFB and by telomeric repeat binding factor (TRF1) but also by topoisomerase II inhibition, similar to r_UFBs and r-UFBs
Tolerance to low levels of replication stress or defects in checkpoint and mitotic control resulting from deficient resolution of chromatid entanglement can easily lead to the loss of DNA sequences. In CFSs, this may underlie the frequent inactivation of tumor suppressors in these regions while in telomeres it will likely be reflected by changes in length and/or telomere dysfunction, whereas loss of centromeric or ribosomal DNA will most likely lead to impaired cell function or even cell death. We hypothesize that loss of tumor suppressors or mutations in genomic DNA can enhance cell proliferation, which cannot result in transformation unless a new telomere maintenance mechanism arises. Telomere dysfunction can also result in cell arrest or cell death, but in some cases may lead to escape of cell death through different mechanisms that maintain telomere length and continuous cell division. It is not easily envisioned how this outcome may result from signaling from other DNA regions.
In vivo, preferential damage to telomeres has been demonstrated (100). Hewitt et al have shown that telomere associated foci (TAFs) induced by x-ray irradiation and other DNA damaging agents are more numerous and remain longer than genomic DNA foci. Telomerase expression and telomere length do not influence this result. Consequently the relative proportion of TAFs increases with age. Interestingly, TAFs were frequently detected in the transit amplifying zone of intestinal crypts of old mice but not at the bottom of the crypts where intestinal stem cells are localized(100)
7. Other Roles of ATR and the Fanconi Anemia Pathway
The FA pathway is thought to comprise 22 genes that have been organized into three groups. Group I consist of 8 proteins which form the FA core plus associated proteins FANCM-FAAP24, FAP20 and FAP100. The primary function of this pathway is the protection of DNA replication forks and repair of interstrand cross-links (ICLs). Single strand and double strand breaks are subproducts of ICL processing. FANCM can recognize ICLs during S phase and recruits the FA core complex (101, 102) which leads to mono-ubiquitination of the FANCD2-FANCI (group II or ID2) and further recruitment of group III proteins that mediate different aspects of the DNA repair function such as DNA incision, ICL elimination, translesion synthesis, homologous recombination, etc. (101, 102).
Efficient activation of ATR signaling and remodeling of stalled replication forks requires the Fanconi Anemia pathway (FA). The FANCD2 component of FA has been shown to attenuate common fragile site (CFS) transcription thereby promoting CFS stability (103). ATR but not ATM is involved in the maintenance of CFS (104). As stated above, telomeres resemble fragile sites as demonstrated by aphidicolin-induced breaks that increase after ATR inhibition (105)
FANCM depletion led to ALT-specific telomere replication stress. FANCC deficiency accelerates telomere attrition in bone marrow cells (102) and downregulation of FANCM or FAAP24 compromise ATR/Chk1 signaling. (106). Clinical studies have shown that telomere length diminishes with age in FA patients. In contrast, telomerase activity is similar in heterozygous carriers and controls but its level increases in FA patients (107)
An interesting aspect of FANCM and FAPP24 proteins is their ability to bind HLCK2 independently of the FA core complex (108). The HCLK2/Tel2 gene, originally identified in C.elegans, is one of the most enigmatic players in the DNA damage response. There is no evidence that its higher eukaryote ortholog can bind DNA. Nevertheless, it has been shown to be involved in DNA damage and checkpoint control. Furthermore, HCLK2 function seems to greatly influence the balance between DNA damage/repair and checkpoint signaling. Horejsi et al showed that a partial reduction in ATR levels resulted in a severely compromised checkpoint response (108). This unbalanced situation which is associated or can precede malignant transformation might be communicated to the telomere complex either through an HCLK2/Tel2 downstream target or through HLCK2 itself. This function of HCLK2/Tel2 could be supported by its ability to differentially regulate members of the PIKK family but more probably would rely in a capacity that has been demonstrated in its yeast homologue Tel2 called telomere position effect that enables this gene to control telomere length and silencing of genes in the sub-telomeric regions (109). Disruption of the telomere position effect may influence distinct functions of the telomere complex such as hTERT expression (110) and, in turn, aberrant TERT expression may initiate transformation. The ability of HCLK2/Tel2 to transmit the initial activation of DNA damage from ATR to the telomere provides a hypothetical common mechanism that may underlie oncogenic signaling of both familial cancer syndromes such as Fanconi’s anemia and sporadic cancers. A link between a perturbed DNA damage response and the telomere oncogenic pathway could be mediated by ATR-HCLK2/Tel2-Telomere signaling
8. Telomere Complex Alterations Can Directly Induce Cancer
Cancer Induced by the Sheltering Protein TRF2
Overexpression of TRF2 under the control of Keratin5 promoter leads to increased UV-induced tumors as well as spontaneous cancer [111,112]. TRF2 overexpression results in telomere degradation even in the presence of telomerase, but telomere shortening can be rescued by deletion of XPF nuclease. As this nuclease, which is involved in the repair of UV-induced lesions, can also degrade the single-stranded G-overhang at telomeres, it was hypothesized that TRF2 would sequester XPF at telomeres, resulting in deficient XPF elsewhere at the genome, thereby diminishing protection against UV irradiation and UV-induced tumors. However, TRF2 is abundantly expressed in different cancers apart from skin carcinomas, such as breast, liver, and lung carcinomas. On the other hand, TRF2 in the K5-TRF2 model resulted in both UV-induced and spontaneous tumors. In a new approach, these authors made use of successive generations of Terc−/− mice that were crossed with K5TRF2 mice to study the presumed protective role of short telomeres on tumor development. Surprisingly, telomerase deficiency in the K5TRF2/Terc−/− mouse model led to an increased frequency of chromosome aberrations consistent with increase telomere shortening caused by TRF2, which adds to the effect of Terc deletion. However, carcinogenesis was also significantly promoted [111,112]. The findings showed that despite the role of TRF2 in protecting t-loops through ATM inhibition, there was normal activation of the ATM signaling pathway in response to IR, as attested by Chk2 phosphorylation and increased p53 level. It was uncertain whether deficient p53-induced apoptosis might have favored tumor development, as p53 levels were found to be decreased in tumor tissue in two cases as compared to noninvolved surrounding tissue and showed variable levels in other two tumors. Interestingly reversal of telomere shortening was evident in late generations of K5TRF2/Terc−/− mice together with increased intensity of telomere fluorescence, telomere elongation, and other parameters indicative of an ALT mechanism, such as elevated numbers of telomere sister chromatide exchanges (T-SCE) and colocalization of PML with telomeres [113].
The explanation offered by the authors for the promotion of tumor development in these independent models invoked the ability of telomerase in the K5TRF2 telomerase proficient model to provide for continuous cell division, whereas a “DNA damage response (DDR) induced by short telomeres” would be responsible for tumorigenesis in the telomerase-deficient K5TRF2/Terc−/− mice. In both cases, they pointed out that increased chromosomal instability may result from the combination of telomere shortening and a defective NER pathway. In my view, deficient apoptosis through a deficient checkpoint arm of the DDR, as could be the case in the tumors displaying decreased p53 levels, may have contributed to malignant transformation. This was suggested to have resulted from the cancellation of ATM by TRF2. My own suggestion in the case of telomerase-proficient mice is that telomere shortening induced by TRF2 may have led to immortalization through reactivation of telomerase, by a process similar to immortalization after crisis, whereas immortalization in the telomerase-deficient mice should have arisen through activation of an ALT mechanism, as suggested by the intense telomere fluorescence signals, associated PML bodies, and telomere elongation observed in tumoral tissue of G2K5TRF2/Terc−/− not present to the same extent in nontumor surrounding tissue.
Although the role of TRF2 is far from being understood, current knowledge is compatible with this interpretation. Confirming data gathered in the above reports, it has been found that overexpression of TRF2 may cause replication stalling at telomeres and, hence, telomere attrition. On the other hand, induction of ALT by TRF2 has been reported by other authors. SUMOylation of TRF2 has been found to occur at dysfunctional telomeres, inducing elongation via ALT [113]. TRF2 appears to have divergent function in telomeres and extratelomeric DNA. It protects telomeric t-loops by inhibiting ATM and it has been detected at DSB sites next to other DDR factors. A complex interplay between extratelomeric DNA and TRF2 has been described previously. Genomic damage can transiently increase TRF2 expression, whereas telomere damage may lead to increased TRF2degradation and accelerate telomere shortening. TRF2 expression has been found to be elevated in breast and colorectal cancers, whereas loss of TRF2 has been observed in Hodgkin’s disease [113)
9. The PI3K/AKT pathway
The DNA damage response may disrupt the telomere complex which appears to be a hub that sends signals for final transformation through molecules such as TRF2. Some downstream targets of the PI3K pathway could mediate effects on both directions
The PI3K/AKT pathway is connected to the DNA damage response and can be activated following DNA damage. Bozulic et al. [114] reported that Akt/Pkb is activated by one of the central kinases of the DNA damage response pathway, DNA-PK, following double-strand breaks. It has also been shown that pTen, the phosphatase that antagonizes AKT activation, is a target of ATM, the main central sensor of the DDR pathway [115]. The PI3K/AKT pathway is the most frequently mutated pathway in cancer. Some of its individual components, such as p110α and Pten, are among the most frequently mutated genes in cancer; however, many other lesions that activate this pathway are also involved in tumorigenesis (oncogenic Ras, AKT, loss of LKB1, etc.) [116].
The pathway is initiated by the conversion of phosphatidylinositol (3,4) bi-phosphate (PIP2) into the second messenger (PIP3). PIP3 binds PKB/AKT, provoking a conformation change which allows protein PDK1 to phosphorylate AKT at position T308. Full activation of AKT requires phosphorylation of AKT at Ser473 carried out by mTORC2. The phosphatase and tensin analog (Pten) negatively regulates AKT through its phosphatase activity, but Pten mutations outside the phosphatase domain demonstrate an additional role for Pten in the control of chromosome stability. Nuclear Pten binds centromeric protein C (CENP-C) and targets different proteins such as focal adhesion kinase (FAK). Pten can participate in DSB repair through induction of RAD51 and may regulate the cell cycle through the PI3K-p27-CDK2 axis [117,118]. In agreement with the central and many-branched role played by Pten, deletion of this gene represents one of the more effective and faster means of cancer induction. Given the complexity of signaling pathways arising from this central node, numerous studies have been devoted to dissecting the impact of Pten deletion, Akt/Pkb activation, and their downstream targets in tumor development. Phosphorylation of Akt is followed by phosphorylation of GSK3β, FOXO1, and mTORC1.
Some initial studies suggested that transformation in the PI3K pathway was mediated exclusively via mTORC1 activation [119]. Partial reduction of Akt through Akt1 deletion significantly reduced skin carcinogenesis as well as Harvey Ras-induced tumors. Deletion of both Akt1 and Akt2 resulted in almost complete resistance to oncogenic transformation. In subsequent experiments, the same authors observed that Tsc2 deletion led to high mTORC1 activity and enhanced cellular replication, as measured in vitro, while Akt phosphorylation was diminished as a result of a feedback inhibitory loop. On the contrary, increased Tsc2 expression led to downregulated mTORC1 activity, decreased number of foci, and increased Akt activity. From these results, they concluded that Akt-mediated transformation relied mainly or exclusively on mTORC1 activity. However, this conclusion was based on the presumed absolute equivalence of in vitro proliferation foci with oncogenesis in vivo. The latter was not measured in these experiments. mTORC1 controls coordination of ribosome biogenesis and cell growth through its downstream targets S6 and 4E-BP1s. mTORC1 regulation of ribosome biogenesis requires S6 kinase and is mediated by upstream binding factor (UBF), a cofactor of RNA polI [120]. Suppression of UBF in the face of unaltered levels of other proliferation-associated proteins has been shown to lead to apoptosis [121], presumably due to cell proliferation unsupported by lack of protein synthesis. In addition, the inhibition of mTORC1 function by conditional deletion of Raptor, a component of the mTORC1 complex, revealed that phosphorylation of p70S6 and 4E-BP1 was dependent on the cell developmental stage. Consequently, a faulty mTORC1 function is more disruptive for transit, amplifying cells that are actively replicating and need a higher protein supply than stem and quiescent cells. This is reflected in the response of different populations of acute leukemia cells (AML) to mTORC1deprivation. mTORC1 loss leads to apoptosis of mature cells and reduction of tumor burden but does not affect leukemia stem cells (LSCs) (122)
After Pten deletion, mice develop hematological malignancies, the most prevalent of which is T-cell acute leukemia/lymphoma. The incidence of malignancies was decreased in Pten/Raptor double knockouts but incidence was still significant. In contrast, this study did not find acute leukemia/lymphoma development in Pten, Rictor double-deleted mice. Cheng et al. [123] induced mTORC1 activation by conditional Tsc1 deletion. mTORC1 activation induces HSC proliferation followed by exhaustion. It was shown that increased ROS levels were responsible for HSC exhaustion because treating the mice with the antioxidant N-acetylcysteine prevented bone marrow hypocellularity and restored reconstitution capacity. However, in contrast to Pten deletion, which, like Tsc1 deletion, results in mTORC1 activation, leukemogenesis did not follow HSC exhaustion in Tsc1 KO HSCs. Contrary to the restoration of repopulating ability by N-acetylcysteine observed in Tsc1 null-HSCs, the loss of repopulation ability caused by Pten deletion could not be restored by antioxidant treatment [124]. These discrepancies might be due to the different experimental models used, but are more likely due to a greater impairment of HSC function of Pten null HSCs.
Similarly, in leukemias associated with Pten chromosomal translocations, it has been repeatedly observed that inhibition of mTORC1 signaling by rapamycin delays the onset of leukemia and reduces tumor burden but it does not affect the leukemia stem cell. From a clinical perspective, it is worth noting that mutations in TSC1 or TSC2 are responsible for tuberous sclerosis, which is associated with hamartomas rather than carcinomas. Nevertheless, mTORC1 contributes to malignant transformation induced by Akt activation because rapamycin decreases the incidence of T-cell lymphomas in murine models using constitutively active AKT [125]. Thus, the high efficiency of the PI3K/AKT/Pten pathway in promoting leukemia may result from the convergence of oncogenic signals mediated by several downstream targets of this pathway. This is consistent with the concept that cell proliferation may act in concert with deregulation of the mitotic cycle to elicit oncogenic transformation.
GSK3β is a downstream node of the PI3K pathway from which divergent signaling for HSC self-renewal and lineage commitment emanate. Activated Akt phosphorylates GSK3β, leading to its inhibition and subsequent activation of Wnt-βcatenin signaling which enhances HSC self-renewal. Concomitantly, GSKβ inhibition also activates mTORC1, promoting lineage commitment. By simultaneously stimulating the Wnt-βcatenin pathway with inhibitors of GSKβ CHIR99021 or lithium and inhibiting the mTOR pathway with rapamycin, Huang et al. [126] were able to maintain long-term multilineage hematopoiesis in cytokine free cultures treated with both inhibitors. These culture conditions allowed tri-lineage hematopoietic reconstitution and gave rise to mature myeloid cells, contrary to control cells cultured in a standard cytokine cocktail. A higher percentage of c-Kit+ quiescent cells in G0 as well as a small increase in S/G2/M, indicating some increase in cell growth, were seen in the treated cultures. Transplantation to secondary recipients confirmed the improved preservation of HSC function as assessed by a higher chimerism detected in recipients of cells treated with inhibitors [126]. Although HSC proliferation and subsequent exhaustion mimic similar outcomes induced by Pten deletion, there are important differences that have been revealed by these experiments. First, HSC exhaustion induced by GSKβ inhibition is reached more slowly and becomes evident only through serial transplants and competitive repopulation assessment. More importantly, Pten loss or constitutive Akt activation leads to the rapid development of leukemia which has not been observed so far in GSK3β-rnai transplanted mice or lithium, whose use in the treatment of bipolar disorders has never been associated with increasing risk of malignancies [127]. In addition, the preservation of physiological tri-lineage hematopoietic differentiation induced by Wnt-β-catenin signaling contrasts with the biased expansion of immature myeloid progenitors subsequent to HSC mobilization observed after Pten deletion or Akt constitutive activation [125]. Again, exit from the stem cell pool appears as a step to malignant transformation. These observations suggest that cell proliferation induced by mTORC1 is not sufficient to induce transformation, and point to an essential oncogenic contribution from FOXO, another downstream target of activated AKT.
The FOXO group of transcription factors (FOXO1 (FXHR), FOXO3a (FKHRL1), and FOXO4 (AFX)) act downstream of the PI3K/AKT pathway [128] Interestingly, sites of Akt phosphorylation at threonine 308 (pAKTThr308) and Serine 473 (pAKTSer473) are essential for full Akt activation, but pAKTSer473 is dispensable for AKT-mediated phosphorylation of TSC2 and GSK-3β and is required for phosphorylation and inactivation of FOXOs [129).
FOXO proteins are normally present in an active state in the cell nucleus where they are involved in cell cycle arrest or apoptosis (depending on the physiological and cell context), control of cell cycle progression (e.g., induction of cdk inhibitor p27kip1 and p57 as well as long-term survival and control of factors involved in stemness such as OCT4 and SOX2 [128,130,]. Nuclear exclusion (inactivation) of Foxo3a is associated with the first step in hematopoietic differentiation, as Foxo3a is present in the nucleus of freshly isolated Foxo3a+/+ CD34− KSL cells (HSCs) but appears in the cytoplasm of freshly isolated Foxo3a+/+CD34+ KSL cells (progenitors) 130]. Clonogenic assays show that Foxo3a deficiency impairs the long-term (16 weeks) reconstitution ability of CD34− KSL (HSCs) but not the short-term reconstitution capacity of CD34+ KSL cells (progenitors). The decline of repopulation efficiency of HSCs is associated with loss of quiescence and decreased levels of cell cycle inhibitors p27 and p57 in Foxo3a−/− CD34− KSL cells [138, 129]. Active FOXOs play an essential role in the maintenance of stemness; consequently, loss of stemness and exit from quiescence can result from Foxo nuclear exclusion and inactivation.
Finlay et al. [131] showed that “PDK1 has an obligatory function in controlling the phosphorylation and transcriptional inactivation of Foxo1, 3a and 4 in Pten-null cell” and that “Pten null T cell progenitors cannot transform or develop into invasive and fatal T lymphoma without PDK1”. Other findings lend further weight to the role of this oncogenic mechanism, such as the requirement of the mTOR complex 2 for development of prostate cancer in Pten null mice [132] or the suppression of leukemogenesis in Pten null mice by concomitant deletion of Rictor (an essential component of mTORC 2) [133] (there has been a hot debate on the role of PDK1 in Akt phosphorylation at Ser473). Other kinases referred to as PDK2 activity, and more recently mTORC2, have shown to be responsible for AktSer473 phosphorylation [134]. Nevertheless, this does not invalidate the conclusions of Finlay or the ideas defended here that boil down to the paramount role of Foxo inactivation induced by pAktSer473 on T-cell lymphomagenesis [133]. Magee et al. showed that Rictor deletion with the subsequent suppression of mTORC2 prevented leukemogenesis and HSC depletion in Pten-deleted adult mice [133). The expression of myr-AKT in HSCs that is associated with HSC mobilization and exit from the stem cell compartment must correlate with inactivation and cytoplasmic translocation of Foxo 3a (the main form of Foxo in hematopoietic cells) as it has been observed that transition of HSCs to progenitors is associated with inactivation and cytoplasmic translocation of Foxo [130]. In normal HSCs, the activity of Akt is attenuated as required for quiescence, whereas Akt activation rises in normal granulocyte-macrophage progenitors (GMPs). Paradoxically, Sykes et al. observed growth suppression in the AML-AF9 leukemia model [135] after enforced activation of AKT in HSCs by means of the MSCV-IRES-GFP-myr-Akt construct. The leukemia stem cell (LSC) shared the immunophenotype of GMPs (lineage low, c-Kit high, FcγRII/III+, CD34+) but had the attenuated Akt pattern of a normal stem cell associated with active nuclear Foxo. Since this expression pattern seems to be related to maintenance of stemness, it is probably a general feature of the transformation process rather than a specific feature of the AML-AF9 leukemia. This suggests that the differentiation of HSCs into committed progenitors is an obligatory step in the process of transformation after which the committed progenitor must undergo a partial reversion in order to acquire the attenuated pattern of Akt expression that supports quiescence. A possibility was that activation of mTORC1 induced by pAKT with subsequent differentiation of tumor cells was responsible for this paradoxical beneficial role of pAKT in leukemic growth. Akt activates mTORC1 by relieving inhibition of mTORC1 by TSC2. As this explanation was ruled out under rapamycin treatment, other actions of pAkt, independent of mTORC1 activation, must be responsible for myeloid maturation and growth inhibition. Given the substrate selectivity of pAKTSer473, the involvement of Foxo inactivation was an obvious choice. This was confirmed by inhibiting Foxo3a with shRNA in MLL-AF9 as well as in AML cell lines that do not carry MLL translocations [135). Foxo inhibition lowered tumor growth and induced myeloid-maturation-related death, but Foxo inhibition does not affect the generation or survival of leukemic stem cells. Thus, pAKTSer473 induced Foxo3a cytoplasmic translocation and exit from the HSC compartment (commitment) concomitantly, but generation of the leukemia stem cell involves reversion of a committed cell to the attenuated AKT pattern of the normal stem cell. In other words, HSC differentiation and subsequent leukemic transformation are two outcomes of Foxo3a signaling that affect tumor biology in opposing ways, but once leukemic transformation is established only Foxo’s deactivation role on cell differentiation is observed. Obviously, deletion of Foxo in AML-F9 transplanted Cre+ recipient mice extended latency and survival due to differentiation-related cell death, but the majority of mice eventually succumbed to leukemia, suggesting that, independently of its effect on myeloid maturation and apoptosis, Foxo3 depletion cannot eradicate LSCs after transformation. Collectively, these results imply that putative transformation of a stem cell requires a previous step of differentiation (Figure 1).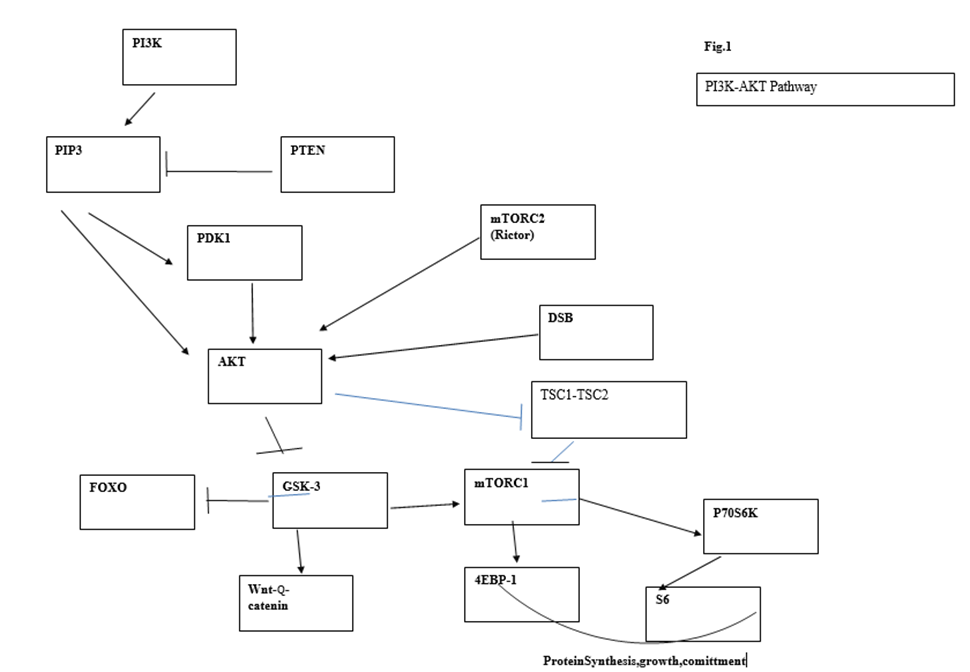
Figure 1. Main circuits of the PI3K/AKT pathway and its connection to DSBs
Hu et al. [136] revealed an Akt-independent mechanism of Foxo3a inactivation dependent on IκB kinase (IKK). Constitutive expression of IκB kinase leads to Foxo3a inactivation and nuclear exclusion, cell proliferation, and tumorigenesis, a clear indication that the proleukemogenic role of Foxo3a predominates over its antileukemic effect. Degradation of IκBα by IκB kinase is accompanied by the activation and nuclear translocation of NF-κB, which is known to be associated with the upregulation of cyclin D1 and cell proliferation. However, tumorigenesis was attributed to Foxo inactivation because cell clones transfected with, and expressing, IκB kinase induced mammary tumors in nude mice which could be suppressed by re-expression of Foxo3a [144, 135]. The interplay between Foxo and NF-κB may be crucial for the integrity of the cancer stem cell and this interplay may be influenced by telomere alterations through the demonstrated ability of telomere protein Rap1 to bind to IKK, thereby promoting degradation of IκB and subsequent translocation of NF-κB to the nucleus [137]. The same outcome (IKK activation and NF-κB nuclear translocation) can be elicited by DNA double-strand breaks (DSBs) but not by proinflammatory stimuli [138). Following DSBs, ATM can activate IKK, leading to the degradation of IκB and release of bound NF-κB which translocates to the nucleus. In contrast to upregulation of cyclin D1 expression, antiapoptosis, and cell proliferation associated with nuclear NF-κB, nuclear FOXO drives cell cycle arrest or apoptosis, whereas cytoplasmic Foxo drives cell proliferation. Under physiological conditions, AKT activity is low in quiescent cells and it is associated with retention of FOXO factors in the nucleus, where they upregulate expression of target genes that control cell cycle, i.e., p27kip1, Rb2(p130), mitosis (cyclin B and polo-like kinase, metabolism, or apoptosis (Fas ligand and Bim)) [128,130,134,139). Some of these phenotypic traits, like upregulation of p27 and cell cycle arrest, can also be induced by re-expression of Pten. In contrast, Pten deletion and subsequent Akt activation led to FOXO inactivation and nuclear exclusion, and presumably cycle control deregulation. The proapoptotic function of Foxo proteins is in great part dependent on their collaboration with E2F1 protein (139) This collaboration is required for E2F1-induced apoptosis but not for E2F1-induced proliferation. A protective DNA damage reaction increases apoptosis through E2F1-Foxo because it stabilizes E2F1 through ATM and Chk2 phosphorylation, whereas the Foxo-dependent arm of E2F1 signaling in charge of apoptosis was found to be reduced in most tumor types examined relative to counterpart normal samples. These findings highlight the relevance of the cross-talk between the oncogene-induced DNA damage reaction and the PI3K pathway [139]. Pin1, a target of E2F1 essential for Neu/Ras-induced transformation of mammary epithelial cells through activation of cyclin D1 [140] (to be discussed later), has also been reported to induce nuclear accumulation of NF-κB.
The simultaneous cytoplasmic translocation–inactivation of Foxo leading to deficient apoptosis, differentiation (exit from the stem cell pool) and prosurvival effect, and induction of cell proliferation by nuclear translocation of NF-κB appear to reinforce each other in the transformation and survival of the cancer stem cell.
10. Resistance of Neonatal Cells to Leukemia Development
The Ser473 AKT mTORC2 site required to inactivate Foxo3a is also required to mobilize HSCs, as demonstrated by the decrease in the S/G2/M fraction of HSCs after Pten deletion when Rictor, a component of mTORC2, is deleted. The mobilization of HSCs has an important proleukemogenic effect, as demonstrated by the antileukemic role of Rictor deletion in Pten-deficient cells [141]. Rictor deletion, but not rapamycin treatment, reduced HSC mobilization after Pten deletion. Rapamicin treatment and Rictor deletion both reduced HSC proliferation after Pten deletion and additively reduced the severity of myeloproliferative disorder following Pten deletion.
An experiment by Xue et al. [141) revealed a special form of cancer resistance linked to cell or organ immaturity, which may underlie the resistance of stem cells to cancer development. They described a different behavior of HSC in neonatal mice compared to that in adult mice in their response to Pten deletion. When Pten deletion was performed 2 days after birth, the expected increase in HSCs number was only observed in 8-week-old mice (>40-fold) but it had no effect on the number of HSCs in 2-week-old mice. Overall proliferation of unfractionated bone marrow cells at 2, 3, 4, and 5 weeks of age was not affected by Pten deletion. On the other hand, control neonatal HSCs divided more actively than adult HSCs, with transition from neonatal to adult phenotype taking place between 4–5 weeks. In transplantation experiments, Pten-deficient neonatal (before 6 weeks of age) HSCs showed long-term multilineage reconstitution lasting from 16 to 24 weeks, and even this constraint (16–24 weeks) appears to reflect their maturation to an adult phenotype. In contrast, Pten-deficient adult HSCs showed only short-term reconstitution lasting less than 16 weeks. It was also found that Pten prevented leukemia of adult but not neonatal mice. The fact that adult mice succumbed to leukemia 12 weeks after Pten deletion made it difficult to test whether neonatal mice were resistant to leukemia, since their HSCs would mature before the onset of leukemia. To circumvent this difficulty, pIpC was administered to Mx1-Cre, Ptenfl/fl, p53−/− mice, and littermate controls lacking Mx-1-Cre at 2 days or 6 weeks after birth. All mice in the 6 weeks group died before 20 days of pI-pC treatment, whereas at 21 days after pI-pC treatment, none of the doubly deficient Pten, p53 neonatal mice showed any sign of illness and did not die until 44 to 60 days after pI-pC treatment. Additionally, comparison of PI3K kinase activation in Pten-deleted neonatal and adult mice showed that Pten deletion increased phosphorylation of most substrates of the pathway (AKT, S6, and GSK3β, but not MAPK or AMPK) in LSK from adults but not neonatal mice. Nevertheless, HSCs from neonatal mice can activate this pathway, as demonstrated in in vitro cultures with 2% bovine serum. AKT phosphorylation at Ser473 distinguished Pten-deficient HSCs from HSCs that are dividing under physiological conditions, and Rictor deletion, which suppresses mTORC2 function, substantially reduced AKTSer473 phosphorylation, consistent with mTORC2 being less active in neonatal HSCs. Rapamicin did not affect AKTSer473 but Rictor deletion modestly reduced mTORC1 (modest reduction in S6 phosphorylation). Proliferation of HSCs after Pten deletion depends on mTORC1 as it is reduced by rapamycin. In contrast, Rictor deletion in Pten null HSCs reduced the frequency of HSCs in cycle. Also, in adult mice, Rictor deletion significantly reduced MPPs but not other lineages. All these findings show that mTORC1 induces HSC proliferation–differentiation and is the main regulator of these activities under physiological conditions, whereas Pten deletion induces HSC proliferation and mobilization of HSCs through mTORC2-dependent and -independent mechanisms that do not mimic physiological HSC self-renewal. These findings are consistent with the role previously described of AktSer473 in relation with Foxo. Moreover, Rictor deficiency in Pten null cells restored long-term reconstitution ability (of myeloid and T cells, although not B lymphoid cells), which suggests that loss of long-term reconstitution is associated with HSC mobilization (and accelerated differentiation to MPPs since Rictor deletion reduced MPP number) and, in turn, prevention of leukemia development by Rictor deletion correlates with restored repopulation efficiency due to decreased HSC mobilization and early differentiation to MPPs.
Therefore, the resistance of neonatal cells to leukemia development appears to depend on a deficient mTORC2 activity in neonatal HSCs. However, the question remains whether antileukemic protection afforded by deficient mTORC2 activity may do so by preventing exit from the stem cell pool and loss of self-renewal potential associated with stem cells.
In addition, the work of Xue et al. [141) demonstrates the role of a DNA damage response in the initiation of oncogenesis through the PI3K/AKT pathway. T-cell thymomas that develop in Pten-deficient mice are CD4+ CD8− mature T-cell thymomas. These researchers observed that premalignancy only starts at 9 weeks of age in the preceding stage of double-positive (DP) thymocytes and is accompanied by classical markers of a DNA damage reaction such as phosphorylation of DDR substrates p53, chk1, and chk2 in DP thymocytes. These markers were highly phosphorylated in w.t. thymocytes consistent with the stage where V(D)J recombination occurs (it was negligible in CD4SP and naïve w.t. T cells). Pten-deficient DP thymocytes expressed higher levels of these proteins but not of γH2AX (no thymocyte populations expressed γH2AX). They also expressed higher levels of p19 and p21. AKT phosphorylation was not detected before 6 weeks of age in Pten-deficient T cells. Concomitantly, Foxo3a was heavily phosphorylated in DP T cells but not in SP or naïve T cells of Pten-deficient 9-week-old mice. GSK3β was only slightly activated but S6K was unaffected. They concluded that, although Pten is lost since birth, premalignancy does not start until mice are 6 weeks old and activation of AKT in DP cells differentially affect downstream targets of the PI3K pathway. DP thymocytes from both controls and Pten-deficient cells were Ki67 positive (a marker of non-G0 cells) but did not proliferate. The most striking changes were seen in the expression of p27 that was expressed at high level in w.t. DP thymocytes and was significantly reduced in Pten-deficient DP thymocytes [141].
The association of stem cell resistance to leukemogenesis with the need for a short period of organismal maturation before a cell becomes vulnerable to cancer transformation points to obscure mechanism/s of anticancer protection linked to the cell immaturity, which may underlie the observation that, contrary to a widespread assumption, there are few true stem cell cancers. On the other hand, the synchronous appearance of premalignancy at the DP stage suggests that the DNA strand breaks that occur at this developmental stage along with V(D)J recombination initiate a DDR that, in conjunction with Pten deficiency, is responsible for malignant transformation.
Further Comments and Conclusions
The preceding discussion is aimed at supporting the proposal that oncogenes and other cancer-inducing events, including mitotic disruption and chromosome instability, may lead to disturbances in the telomere complex, which are ultimately responsible for oncogenic growth signaling through known or still-unknown mechanisms of telomere maintenance. A faulty repaired DNA may lead to mutated DNA, leading to enhanced cell proliferation or, if unrepaired, to cell death, but it would be the subsequent checkpoint defect perhaps boosted by stimulation of cell proliferation that would directly induce cell cycle deregulation and telomere dysfunction ultimately responsible for malignant transformation. Furthermore, even in the absence of direct disturbance of the cell cycle, DNA alterations may directly impact the telomere. For instance, it has been reported that deficiency of DNA mismatch repair increases the rate of telomere shortening [58].
The above observations regarding the proleukemogenic role of loss of quiescence, the frequent initiation of cancer following a DNA damage reaction in a committed cell, and the rare description of true stem cell tumors suggest that stem cells are much less susceptible to DNA damage reactions that are followed by malignant transformation. However, from the earlier stages of lineage commitment, the cell becomes vulnerable to malignant transformation although cancer transformation may require the acquisition of stemness by a committed cell. One important characteristic that separates stem cells from the earliest committed cells is the capacity for indefinite self-renewal, which appears to be linked to a mechanism of telomere maintenance that is, probably, stem-cell-specific. This privileged situation of the stem cell could be lost rapidly through stem cell mobilization. Moreover, stem cell mobilization might be caused by a mechanism that interferes with normal telomere maintenance of the stem cell, for instance, Myc activation of TERT. On the other hand, malignant transformation implies the sudden acquisition of a mechanism for indefinite telomere maintenance that the committed cell did not possess previously. Many studies indicate that the generation of such mechanism requires sustained erosion or other form/s of telomere damage, which may be elicited through different types of DDR. Evidently, DDR occurs more frequently during tissue turnover and differentiation than in the stem cell compartment and may likely lead to disturbances of the telomere complex (erosion, ALT, gain-of-function mutations at the TERT promoter). One of the probable outcomes is telomerase re-expression (or one of its downstream targets), which has been demonstrated to induce transformation independently of its canonical enzymatic activity. All this suggests two interpretations that, far from being mutually exclusive, may be interdependent:
Different types of DNA damage reaction might converge on distinct modes of telomere alteration that could induce transformation independently of canonical telomere maintenance but be associated with it (i.e., telomerase can induce transformation independently of telomerase activity, for instance, through its downstream target TGFβ and TGFβ target JunB [142)). Distinct oncogenic pathways are initiated by different telomere lesions caused by diverse forms of DNA damage, which can result in transformation through the emergence of new mechanisms for telomere maintenance. This is consistent with the fact that radical changes in telomere maintenance are associated with cancer transformation. Thus, normal stem cells might be protected from direct cancer transformation by mechanism/s of telomere protection (or protection from DNA damage) that are more efficient in stem cells than in committed cells (telomerase expression or others not well known, such as the capacity for chromosome healing that has been described in mouse embryonic stem cells) [143]. At any rate, telomere alteration occurs much less frequently in stem than in committed cells.
However, there are rare cases of telomerase oncogenic pathways starting directly in stem cells (primary defects of the telomere complex). One such case is represented by Beckwith–Wiedemann syndrome, which is associated with an 800-fold increased risk of childhood neoplasms, where multiple tumors arise in different organs or even in the same organ. Overexpression of telomerase reverse transcriptase (TERT), defective TGFβ signaling with epigenetic silencing of β2 spectrin, an SMAD adaptor for TGFβ signaling, are involved in the etiology of this syndrome [9]. Another case in point in the area of molecular cancer modeling were the reports by Passegué et al. [10] and Santaguida et al.11] that JunB inactivation recapitulates aspects of human malignancies, including myelogenous leukemia. It was described that JunB-deficient HSCs had impaired reconstitution ability compared to control HSCs owing to a lower fraction of quiescent cells. When transplanting equal numbers of CD34−/Flk2− LSK cells into irradiated recipients, those from JunB-deficient mice lost reconstitution ability due to their rapid expansion and differentiation into progenitors. After isolating HSCs to near purity using CS150+/CD48−/Flk2−, it was observed that the HSPCs compartment of JunB-deficient mice was expanded by an increase in the CD48+/Flk2− population. JunB-deficient CD150+/CD48−/Flk2− displayed higher repopulation ability than controls, which was apparently caused by an increased production of myeloid cells that ultimately led to the appearance of MPD. Here, it is interesting to point out how rapidly stem cells differentiate into progenitors and therefore lose their indefinite self-renewal ability, and they almost immediately transfer this capacity for indefinite self-renewal to their myeloid progeny. It is intriguing that the expanded tumor population has a granulocyte-macrophage (GM) phenotype [10,11] rather than the preceding common myeloid phenotype, suggesting that transformation does not arise directly at the stem cell stage. Nevertheless, the MPD could only be transplanted to secondary recipients by HSCs; therefore, this was considered true stem cell leukemia even though it had the GMP phenotype and transformation could have occurred in the immediate post-stem cell stage. JunB deficiency was associated with significant reduced expression of Pten, Hoxb4 and Hoxb9, Notch1, and Hes1 and Hes5 in Flk2− LSK cells, but only Hes1, a downstream target of the TGFβ pathway, remained significantly decreased in the LT-HSC population (Hes1 is a downstream target of TGFβ and Notch). When JunB-deficient cells were incubated in OP9 stromal cells expressing the Notch ligand delta-1, Hes1 was not significantly induced. Incubation of JunB-deficient cells with recombinant TGFβ resulted in significant reduced expression of three direct transcriptional targets of the TGFβ pathway (Hes1, Smad7, and the CKI p57). It has been shown that the oncogenic effects of telomerase re-expression can be mediated by reduced TGFβ expression [142]. This indicates that JunB deficiency engages downstream signaling similar to Beckwith–Wiedemann syndrome and telomerase itself. Therefore, these arguments can be extended to many other oncogenic pathways mediated by JunB deficiency or activator protein 1 (AP-1) that engages and/or shares final downstream targets with the telomerase pathway. On the other hand, it would not be surprising that future research unveils common pathways of preneoplastic telomere alteration in these tumors (here referred to as true stem cell tumors) and a subset of childhood/immature tumors.
According to the above criteria, there are two main oncogenic routes: one initially triggered by DNA damage reactions that converge on telomere alterations ultimately responsible for oncogenic signaling (this mechanism would be preferentially associated with tumors originating in relatively mature cells); and another one that is initiated by mechanism directly related to telomere maintenance represented by tumors of true stem cell origin. (Fig.2) However, this may be a simplification because there are oncogenes that may potentially act through both routes. For instance, overexpression of Tcl1 has been shown to cause B-CLL by inhibiting AP-1 (containing JunB) and enhancing NF-κB [,144] Fanconi anemia is a recessive genetic disorder that manifest early in life and evolves with bone marrow failure due to decrease of hematopoietic stem cells which is frequently followed by acute leykemia. These clinicopathological features meet the criterio set above for inclusion in the group of childhood/immature cancers and as the above discusión indicates is a component of the DDR pathway that may directly cause telomere dysfunction
(Figure 2). Different routes to malignant transformation are followed by normal stem and committed cells.
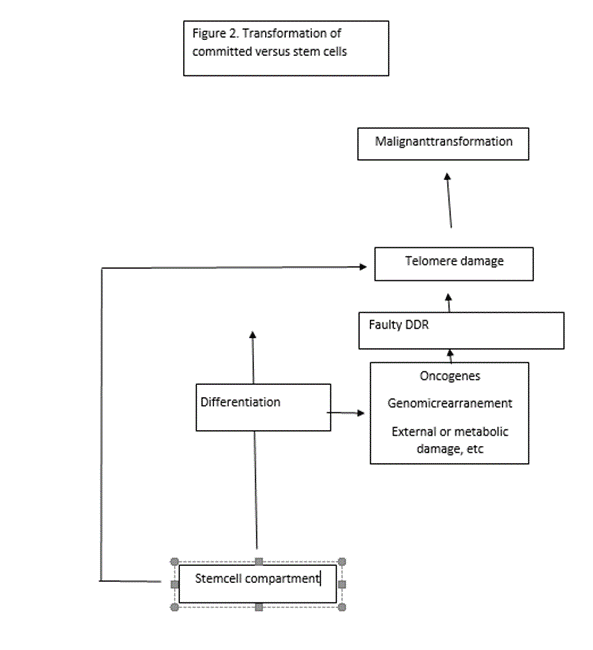
.
Future Directions
The former observations raise an enigmatic question: How can these pathways that can be initiated by the telomerase pathway (including its downstream targets such as JunB) ultimately give rise to telomerase expression as it is re-expressed by the cancer stem cell? A clue to this question was provided by Chen et al. According to their study in Beckwith–Wiedemann syndrome tumors and the murine TGFβ/Smad3 disruption tumorigenic model, formation of a β2SP/SMAD3/CTCF protein complex is TGFβ-dependent and its disruption in these tumors increases TERT levels. Overexpression of ectopic β2SP and SMAD3 significantly decreased TERTmRNA levels in SNU398 cells and a TGFβ inhibitor blocked this effect, suggesting that β2SP/SMAD3 decreases TERTmRNA levels in a TGFβ-dependent manner and vice versa [9].
- DNA–telomere interaction is a two-way road. Anomalous DNA damage responses result in telomere damage and the emergence of new telomere maintenance mechanisms. Afterwards, signaling arising from modified telomeres/ALT, independent of telomere maintenance, may contribute to the survival of the cancer stem cell. Although we discussed some examples of the role of telomeric proteins in oncogenic pathways, such as Rap1 induction of NF-κB nuclear translocation, this second type of interaction is largely unexplored.
- An alternative and non-mutually exclusive explanation for the resistance of stem cells to direct cancer transformation is that it would not be dependent (at least not only) on its more efficient telomere maintenance of telomere integrity but might also be linked to the self-renewal capacity of normal stem cells. Indefinite self-renewal is an exclusive property of both normal stem and cancer stem cells. A clear gradient can be traced in repopulation potential from long-term HSCs (LT-HSCs) to short-term HSCs to multipotent progenitors (MPPs), and diminished repopulating ability has been found to be associated with the propensity for tumorigenesis. A population of HSCs called dormant HSCs (dHSCs), which contains the highest number of quiescent cells within this compartment, has been identified recently. Retinoic acid (RA) metabolism is characteristically enriched in this population compared to that in downstream multipotent progenitors. Peptide GPCR and prostaglandin synthesis were also enriched in dHSCs. These cells transition more slowly than other HSCs toward active HSCs (aHSCs) and show a higher expression of retinoic-acid-induced genes. All-transretinoic acid (ATRA) treatment maintained the dHSCs compartment and increased quiescence. Thus, according to the concept that loss of HSC quiescence facilitates tumorigenesis, treatment of HSCs with ATRA and/or prostaglandin E2 should exhibit tumor suppressor activity [145]. This prediction has already been fulfilled after the discovery of Pin1 inhibition by ATRA [146]. Pin1 enhances cell proliferation by induction of cyclinD1 [147] and regulates the expression of numerous mitosis-specific phosphoproteins [148] whose levels change during the cell cycle in normal cells but not in cancer cells [149]. Moreover, ATRA blocks multiple cancer-driving pathways and suppresses the growth of hepatocellular and breast cancer [160,149]. These observations provide an explanation for the association of enhanced stem cell quiescence, preservation of repopulation potential, and cancer suppression. It is tempting to speculate that telomere protection mechanisms of stem cells might, indirectly, provide antitumorigenic protection to their progeny. This type of anticancer resistance might underlie the resistance of immature stem cells to transformation induced by PTEN-loss reported by Xue et al. [149,141].
Antonio Torres-Montaner.
Funding: This theoretical study did not involve resources other than the authors’ expenses and did not receive external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: All material used for this study is within the list of references. Acknowledgments: The author gratefully acknowledges the help of librarian Visitacion Ortega for her constant supply of literature and data search. He is also indebted to Marisa Peleato and Esmeralda Francés
Conflicts of Interest: The author declares that he does not have any conflict of interest
REFERENCES
- Torres-Montaner, A & Hugues D. A hypothetical anti-neoplastic mechanism associated to reserve cells. J. Theoretical Biolgy (2004), 231, 239-248
- Cancer origin in committed versus stem cells: Hypothetical antineoplastic mechanisms associated with stem cells. Critical Rev. Oncol./Hematol. 2011, 80, 209–224.
- Chin, K.; Ortiz de Solorzano, C.; Knowles, D.; Jones, A.; et al. In situ analysis of genome instability in breast cancer. Genet. 2004, 36, 984–988
- Ventura-Ferreira, M.S.; Crysandt, M.; Ziegler, P.; Hummel, S.; et al. Evidence for a pre-existing telomere deficit in non-clonal hematopoietic stem cells in patients with acute leukemia. Hematol. 2017, 96, 1457–1461.
- Engelhardt, M.; Mackenzie, K.; Drullinsky, P.; Silver, R.T. Telomerase activity and telomere length in acute and chronic leukemia, pre- and post- ex vivo culture. Cancer Res. 2000, 60, 610–617.
- Wilson, A.; Murphy, M.J.; Oskarsson, T.; Kaloulis, K.; et al. c-Myc controls the balance between stem cell self-renewal and differentiation. Dev. 2004, 18, 2747–2763.
- Wang, J.; Xie, L.Y.; Allan, S.; Beach, D.; et al. Myc activates telomerase. Genes Dev. 1998, 12, 1769–1774.
- Yamanaka, S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell 2020, 27, 23–531.
- Chen, J.; Tsukamoto, H.; Mushra, L.; Chen, J.; et al. Suppression in human stem cell disorder Beckwith-Wiedemann syndrome find the latest version: TGF-β/β2-spectrin/CTF-regulated tumor suppression in human stem cell disorder Beckwith-Wiedemann syndrome. Clin. Investig. 2016, 126, 527–542.
- Passegué, E.; Wagner, E.F.; Weissman, I.L. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 2004, 119, 431–443.
- Santaguida, M.; Schepers, K.; King BSabnis, A.J.; et al. JunB protects against myeloid malignancies by limiting hematopoietic stem cell proliferation and differentiation without affecting self-renewal. Cancer Cell 2009, 15, 341–352.
- Shiloh Y. ATM and related protein kinases Nat. Rev. Cancer (2003) 3, 155- doi: 10.1038/nrc1011)
- Pearce AK and Humphrey TC. Integrating stress-response and cell cycle checkpoint pathways. Trends in cell biolgy (2001) 11, 426-433)
- Lieberman, H.B.; Panigtahi, S.K.; Hopkins, K.M. p53 and Rad9, the DNA damage response and regulation of transcription networks. Res. 2017, 187, 424–432.
- Hayashi, M.T.; Cesare, A.J.; Fitzpatrick, J.A.J.; et al. A telomere dependent DNA damage checkpoint induced by prolonged mitotic arrest. Struct. Mol. Biol. 2012, 19, 387–38.
- Trkova, M.; Prochazkova, K.; Krutilkova, V. Telomere length in peripheral blood cells of germline TP53 mutation carriers is shorter than that of normal individuals of corresponding age. Cancer 2007, 110, 694–702.
- Tabori, U.; Nauda, S.; Druker, H. Younger age of cancer initiation is associated with shorter telomere length in Li-Fraumeni syndrome. Cancer Res. 2007, 67, 1415–1418.
- Broustas, C.G.; Lieberman, H.B.J Contributions of Rad9 to tumorigenesis. Cell. 2012, 113, 742–751. https://doi.org/10.1002/jbc.2342.
- Barroso-González, J.; García-Expósito, L.; Hoang, S.M.; et al. Rad51AP1is an essential mediator of alternative lengthening of telomeres. Cell 2019, 76, 11–26.e7. https://doi.org/10.1016/j.molcel.2019-060043.
- Bridges, A.E.; Ramachandran, S.; Tamizhmani, K.; Parwal, U.; Lester, A.; Rajpurohit, P.; Morera, D.S.; Hasanali, S.L.; Arjunan, P.; Jedeja, R.N.; et al. Rad51 loss attenuates colon cancer stem cell self-renewal and sensitizes to chemotherapy. Cancer Res. 2021, 19, 1486–1497. https://doi.org/10.1158/1541-7786.MCR-20-0780.
- Wu, Y.; Wang, H.; Qiao, L. Silencing of Rad51AP1 suppresses epithelial-mesenchymal transition and metastasis in non-small cell lung cancer. Cancer 2019, 10, 1748–1763. https://doi.org/10.1111/1759-7714.131.
- Zhang, J.M.; Yadav, T.; Ouyang, J. Alternative lengthening of telomeres through two distinct break induced replication pathways. Cell Rep. 2019, 26, 955–968.c3. https://doi.org/10.1016/j.celrep.2018.12.102.
- Hamid, A.B.; Frank, L.E.; Bouley, R.A. Pan-cancer analysis of co-occurring mutations between Rad52 and the BRCA1-BRCA2-PALB axis in human cells. PLoS ONE 2022, 17, e0273736. https://doi.org/10.1371.
- Wahhiby, A.I.; Wong, S.; Slijepcevic, P. Shortened telomeres in murine Scid cells expressing mutant hRad54 coincide with reduction in recombination at telomeres. Res. 2005, 578, 132–142. https://doi.org/10.1016/j.mrfmm.2005.040008.
- Li, H.; Zhuang, H.; Gu, T.; et al. Rad54L promotes progression of hepatocellular carcinoma via the homologous recombination repair pathway. Integr. Genom. 2023, 23, 128. https://doi.org/10.1007/s10142-023-01060.
- Zheng, S.; Yao, L.; Li, F. Homologous recombination repair pathway and Rad54L in early stage lung adenocarcinoma. PeerJ 2021, 9, e10680. https://doi.org/10.7717/peerj.10680.
- Burger, K.; Schlackow, M.; Gullerova, M. c-ABL couples RNA polII transcription to DNA double strand breaks. Nucleic Acids Res. 2019, 47, 3467–3484. https://doi.org/10.1093/narlgk2024.
- Misri, S.; Pandita, S.; Kumar, R.; Pandita, T. Telomeres, histone code and DNA damage response. Genome Res. 2008, 122, 297–307. https://doi.org/10.1159/000167816.
- Goga, A.; McLaughlin, J.; Pendergast, A.M. Oncogenic activation of c-Abl by mutation within its last exon. Olecular Cell. Biol. 1993, 13, 4967–4975.
- Vohodina, J.; Goerhring, L.J.; Liu, B. BRCA1 binds TERRA RNA and suppresses R-loop based telomeric DNA damage. Commun. 2021, 12, 3542. https://doi.org/10.1038/s41467-021-23716-6.
- Gunnarsdottir, S.R.; Bjarnason, H.; Thorvaldsdottir, B. BRCA2 haploinsufficiency in telomere maintenance. Genes 2021, 13, 83. https://doi.org/10.3390/genes13010083.
- Lee, E.Y.; Muller, W.J. Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a003236.
- Slijecepvic, P. The role of DNA damage response proteins at telomeres-an integrative model. DNA Repair 2006, 5, 1299–1306.
- Laspata, N.; Kaur, P.; Mersaoui, S.Y.; et al. PARP1 associates with R-loops to promote their resolution and genome stability. Nucleic Acids Res. 2023, 51, 2215–2237.
- Pazzaglia, S.; Pioli, C. Multifaceted roles of PARP1 in DNA repair and inflammarion: Pathological and therapeutic implications in cancer and non-cancer diseases. Cells 2020, 9, 41. https://doi.org/10.3390/cells9010041.
- Puentes-Pardo, J.D.; Moreno-San Juan, S.; Casado, J.; et al. PARP-1 expression influences cancer stem cell phenotype in colorectal cancer depending on p53. J. Mol. Sci. 2023, 24, 4787. https://doi.org/10.3390/ijms24054787.
- Sui, J.; Zhang, S.; Chen, B.P.C. DNA-dependent protein kinase in telomere maintenance and protection. Mol. Biol. Lett. 2020, 25, 2. https://doi.org/10.1186/s11658-020-0199-0.
- Hsu, F.M.; Zhang, S.; Chen, B.P.C. Role of DNA-dependent protein kinase catalytic subunit in cancer development and treatment. Cancer Res. 2012, 1, 22–34. https://doi.org/10.3978/j.issn.2218-676x.2012.04.01.
- Yasaei, H.; Slijepcevic, P. Defective Artemis causes mild telomere dysfunction. Genome Integr. 2010, 1, 3.
- Jacobs, C.; Huang, Y.; Masud, T. A hypomorphic artemis human disease allele causes aberrant chromosomal rearrangement and tumorigenesis. Mol. Genet. 2011, 20, 806–819. https://doi.org/10.1093/hmg/ddgq524.
- Gan, W.; Liu, P.; Wei, W. Akt promotes tumorigenesis in part through modulating genomic instability via pohosphorylating XLF. Nucleus 2015, 6, 261–265. https://doi.org/10.1080/19491034-2015.1074365.
- Zahid, S.; El Dahan, M.S.; Iehl, F.; Fernandez-Varela, P. The multifaceted roles of Ku70/Ku80. J. Mol. Sci. 2021, 22, 4134. https://doi.org/10.3390/ijms22084134.
- Fell, V.L.; Schild-Poulter, C. The Ku heterodimers: Function in DNA repair and beyond. Res. Rev. Mutat. Res. 2015, 763, 15–29. https://doi.org/10.1016/j.mrrev.2014.06.002.
- Bae, S.J.; Wang, S.Y.; Lu, H. Ablating putativeKu70 phosphorylation site results in defective DNA damage repair and spontaneous induction of hepatocellular carcinoma. Nucleic Acids Res. 2021, 49, 9836–9850. https://doi.org/10.1093/nar/gkab743.
- Felgentreff, K.; Baxi, S.N.; Lee, Y.N. Ligase-4 deficiency causes distinctive immune abnormalities in asymptomatic individuals. Clin. Immunol. 2016, 36, 341–353. https://doi.org/10.1007/s10875-016-02266-5.
- Sharma, R.; Lewis, S.; Wlodarski, M.W. DNA repair syndromes and cancer: Insights into genetics and phenotype patterns. Pediatr. 2020, 8, 570084. https://doi.org/10.3389/fped.2020.570084.
- Adelfalk, C.; Lorenz, M.; Serra, V.; et al. Accelerated telomere shortening in Fanconi anemia fibroblasts- a longitudinal study. FEBS Lett. 2001, 506, 22–26.
- Stroik, S.; Kurtz, K.; Hendrickson, E.A. CtIP is essential for telomere replication. Nucleic Acids Res. 2019, 47, 8927–8940. https://doi.org/10.1093/nar/gkz652.
- Mozaffari, N.L.; Pagliarulo, F.; Sartori, A.A. Human CtIP: A double agent in DNA repair and tumorigenesis. Cell Dev. Biol. 2021, 113, 47–56.
- Paull, T.P.; Lee, J.-H. Rad17, the clamp loader that loads more than clamps. EMBO J. 2014, 33, 783–785. https://doi.org/10.1002/embj.201488223.
- Sun, J.; Lin, W.; Wang, Q. The cell cycle checkpoint gene, Rad17rs 1045051, is associated with prostate cancer risk. Acta Medica Okoyama 2021, 75, 415–421. https://doi.org/10.18926/AMO/62379.
- Yasuda, Y.; Sakai, A.; Ito S Genetic polymorphism at codón 546 of the human Rad17 contributes to the risk of esophageal squamous cell carcinoma. J. Mol. Epidemiol. Genet. 2016, 7, 58–66.
- Nabetani, A.; Yokoyama, O.; Ishikawa, F. Localization of hRad9, hHus1, hRad1 and hRad17 and caffeine-sensitive DNA replication at the alternative lengthening of telomeres-associated promyelocytic leukemia body. Biol. Chem. 2004, 279, 25849–25859. https://doi.org/10.1074/jbc.M312652200.
- Khorrami, A.; Bagheri, M.S.; Tavalleri, M. The functional significance of 14-3-3 proteins in cancer: Focus on lung cancer. Mol. Biol. Clin. Investig. 2017, 32, 20170032. https://doi.org/10.1515/hmbci.2017-0032.
- Stewart, J.A.; Wang, Y.; Ackerson, S.M.; et al. Emerging roles of CST in maintaining genome stability and human disease. Biosci.(Landmark edition) 2018, 23, 1564–1586.
- Arantes dos Santos, G.; Viana, N.; Pimenta, R.; et al. Pan-cancer analysis reveals that CTC1-STN1-TEN1 (CST) complex may have a key position in oncology. Cancer Genet. 2022, 262–263, 80–90. https://doi.org/10.1016/j.cancergen.2022. 01.006.
- Tummala, H.; Kirwan, M.; Walne, A.J. ERCC6L2 mutations link a distinct bone marrow syndrome to DNA repair and mitochondrial function. Am. Hum. Genet. 2014, 94, 246–256. https://doi.org/10.1016/j.ajhg2014.01.007.
- Mendez-Bermudez, A.; Royle, N.J. Deficiency in DNA mismatch repair increases the rate of telomere shortening in normal human cells. Mutat. 2011, 32, 939–946. https://doi.org/10.1002/humu.21522.
- Du, X.; Shen, J.; Kugan, N. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Cell. Biol. 2004, 24, 8437–8446. https://doi.org/10.1128/MCB.24.19.8437-8446.2004,.
- Gosh, A.K.; Rossi, M.L.; Singh, K.; et al. RECQL4, the protein mutated in Rothmund-Thompson syndrome functions in telomere maintenance. Biol. Chem. 2012, 287, 196–209.
- Tanaka, A.; Weinel, S.; Nagy, N.; et al. Germline mutation in ATR in autosomal dominant oropharyngeal cancer syndrome. Am. J. Hum. Genet. 2012, 90, 511–517.
- Flynn, R.L.; Zou, L. ATR: A master conductor of cellular responses to DNA replication stress. Trends Biochem. 2011, 36, 133–140.
- Ruzankina, Y.; Pinzon-Guzman, C.; Asare, A.; et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 2007, 1, 113–126. https://doi.org/10.1016/j.stem.2007.03.002.
- Epanchintsev, A.; Costanzo, F.; Rauschendorf, M.-A.; et al. Cockayne’s syndrome A and B proteins regulate transcription arrest after genotoxic stress by promoting ATF3 degradation. Cell 2017, 68, 1054–1066. https://doi.org/10.1016/j.molcel.2017.11.009.
- Iyama, T.; Wilson, D. III. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 2013, 12, 620–636. https://doi.org/10.1016/j.dnarep.2013.04.015.
- Lee, J.-H.; Mand, M.R.; Deshpande, R.A. Ataxia-telangiectasis mutated (ATM) kinase activity is regulated by ATP-driven conformational changes in the Mre11/Rad50/NBs1(MRN) complex. Biol. Chem. 2013, 288, 12840–12851.
- Uziel, T.; Lerenthal, Y.; Moyal, L.; Andegeko, Y.; et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003, 22, 5612–5621.
- Matei, I.R.; Guidos, C.J.; Danska, J.S. ATM-dependent DNA damage surveillance in T-cell development and leukemogenesis: The DSB connection. Rev. 2006, 209, 142–158.
- Tauchi, H.; Matsuura, S.; Kobayashi, J.; Sakamoto, S.; Komatsu, K. Nijmegen breakage syndrome gene, NSB1, and molecular links to factors for genome stability. Oncogene 2002, 21, 8967–8980.
- Otahalova, B.; Volkova, Z.; Soukupova, J.; Kleiblova, P.; et al. Importance of germ line and somatic alterations in human MRE11, RAS50 and NBN genes coding for MRN complex. J. Mol.Sci. 2023, 24, 5612. https.//doi.org/10.3390/ijms24065612.
- Spehalski, E.; Capper, K.M.; Smith, C.J.; Morgan, M.J.; et al. MRE11 promotes tumorigenesis by facilitating resistance to oncogene-induced replication stress. Cancer Res. 2017, 77, 5327–5338. https://doi.org/10.1158/0008-5472CAN-17-1355).
- Theunissen, J.-W.F.; Kaplan, M.I.; Hunt, P.A.; Williams, B.R.; et al. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11ATLD1/ATLD1 Mol. Cell 2003, 12, 1511–1523.
- Attwooll, C.L.; Akpmar, M.; Petrini, J.H.J. The Mre11 complex and the response to dysfunctional telomeres. Cell. Biol. 2009, 29, 5540–5551.
- Liao M-J, Zhang X-X, Hill R, Gao J, Qumsiyeh MB, Nichols W, van Dyke T. No requirement for V(D)J recombination in p53-deficient thymic lymphoma. Mol Cell Biol 18(6), 3495-3501
- Ragamin, A.; Yigit, G.; Bousset, K.; Beleggia, F.; et al. Human Rad50 deficiency: Confirmation of a distinctive phenotype.J. Med. Genet. Part A 2020, 182, 1378–1386.
- Lombard, D.B.; Chua, K.F.; Mostoslavsky, R.; Franco, S.; et al. DNA repair, genome stability and aging. Cell 2005, 120, 497–512.
- Sherman, M.H.; Kuraishy, A.; Deshpande, C.H.; Hong, J.S.; Cacalano, N.A.; Gatti, R.A.; et al. AID-induced genotoxic stress promotes B cell differentiation in the germinal center via ATM and LKB1 signaling. Mol. Cell 2010, 39, 873–885.
- Torres-Montaner. The telomere complex and the origin of the cáncer stem cell. Res. 2021, 9, 1–30. https://doi.org/10.1186/s40364-021-00339-z
- Allman D, Karnell, F.G.; Punt, J.A.; Bakkour, S.; et al. Separation of Notch1 promoted lineage commitment and expansion/transformation in developing T cells. Exp. Med. 2001, 194, 99–106.
- Ciofani, M.; Schmitt, T.M.; Ciofani, A.; Michie, A.M.; et al. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. Immunol. 2004, 172, 5230–5239.
- Campese AFGarge, A.I.; Zhang, F.; Grassi, F.; et al. Notch1-dependent lymphomagenesis is assisted by but does not essentially require pre-TCR signaling. Blood 2006, 108, 305–310.
- Pusapati, R.V.; Rounbehler, R.J.; Hong, S.; Powers, J.T.; Yan, M.; et al. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Natl. Acad. Sci. USA 2006, 103, 1446–1451.
- Petiniot, L.K.; Weaver, Z.; Barlow, C.; Shen, R.; et al. Recombinase-activating gene (RAG) 2-mediated V(D)J recombination is not essential for tumorigenesis in Atm-deficient mice. Natl. Acad. Sci. USA 2000, 97, 6664–6669.
- von Boehmer, H. Developmental biology of T cells in T cell-receptor transgenic mice. Rev. Immunol. 1990, 8, 531–556.
- Gong, Y.; Liu, Y. R-loops at chromosome ends: From formation, regulation and cellular consequences. Cancers 2023, 15, 2178 https://doi.org/10.3390/cancers15072178.
- Bayona-Feliu, A.; Barroso, S.; Muñoz, S. The SWI/SNF chromatin remodeling complex helps resolve R-loop-mediated transcription-replication conflicts. Genet. 2021, 53, 1050–1063. https://doi.org/10.1038/s41588-021-00867-2.
- Schaefer, I.-M.; Hornick, J.L. SWI/SNF complex-deficient soft tissue neoplasms: An update. Diagn. Pathol. 2021, 38, 222–231.
- Goos, J.A.C.; Vogel, W.K.; Micochova, H.; et al. A de novo substitution in Bcl11b leads to loss of interaction with transcriptional complexes and craniosynostosis. Mol. Genet. 2019, 28, 2501–2513. https://doi.org/10.1093/hmg/ddz072.
- Yang, S.F.; Sun, A.A.; Shi, Y.; et al. Structural and functional characterization of RBBP4-ZNF827 interaction and its role in NuRD recruitment to telomeres. Biochem. 2018, 475, 2667–2679. https://doi.org/10.1042/BCJ20180310.
- Conomos, D.; Reddel, R.R.; Picket, H.A. NuRD-ZNF827 recruitment to telomeres creates a molecular scaffold for homologous recombination. Struct. Mol. Biol. 2014, 21, 760–770. https://doi.org/10.1038/nsmb.2877.
- Montefiori, L.E.; Mulligan, C.G. Redefining the biological basis of lineage-ambiguous leukemia through genomics: BCL11B deregulation in acute leukemias of ambiguous lineage. Best Pract. Res. Clin. Haematol. 2021, 34, 101329. https://doi.org/10.1016/j.beha.2021.101329.
- Lennon, M.J.; Jones, S.P.; Lovelace, M.D.; et al. Bcl11b-A critical neurodevelopmental transcription factor- Roles in health and disease. Neurosci. 2017, 11, 89. https://doi.org/10.3389/fncel. 2017.00089
- Li, F.; Deng, Z.; Zhang, L.; et al. ATRX loss induces telomere dysfunction and necessitates induction of alternative lengthening of telomeres during human cell immortalization. EMBO J. 2019, 38, e96659. https://doi.org/10.15252/emboj-201796659
- Bester, A.C.; Roniger, M.; Oren, Y.S.; Im, M.M.; Sarni, D.; Chaoat, M.; Bensimon, A.; Zamir, G.; Shewach, D.S.; Kerem, B. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 2011, 145, 435–446.
- Saldivar, J.C.; Miuma, S.; Bene, J.; Hossein, S.A.; Dhibata, H.; et al. Initiation of genome instability and preneoplastic processes through loss of Fhit expression. PLoS Genet. 2012, 8, e1003077.
- Hemanth Tummala, Amanda Walne, Roberto Buccafusca, Jenna Alnajar, Anita Szabo et al. Germline thymidylate synthase deficiency impacts nucleotide metabolism and causes dyskeratosis congenita The American Journal of Human Genetics (2022) 109, 1472– 1483 http://doi.org/10.1016/j.ajhg.2022.06.014
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801
- Lopez-Contreras AJ., Specks J, Barlow JH, Ambrogio C et al Increased Rrm2 gene dosaje reduces fragile site breakage and prolongs survival of ATR mutant mice. Genes Dev (2015) 29, 690-695
- Wilhelm, T.; Said, M.; Naim, V. DNA replication stres and chromosomal instability: Dangerous liaisons. Genes 2020, 11, 642. https://doi.org/10.3390/genes11060642.
- Hewitt G, Jurk D, Marques FDM, Correia-Melo C et al. Nature communications (2012) 3, 708- doi: 10.1038/ncomms1708
- Garcia de Teresa B, Rodriguez A, Frias S. Chromosome instability in Fanconi Anemia: from breaks to phenotypic consequences. Genes (2020)11, 1528 doi: 10.3390/genes11121528
- Helbling-Leclerc, C. Garcin, F. Rosselli Beyond DNA repair and chromosome instability-Fanconi anaemia as a cellular senescence-associated syndrome.. Cell Death& Differentiation (2021) 28:1159-1173
- Fernandes P, Miotto B, Saint-Ruf C Said M et al. FANCD2 modulates the mitochondrial stress response to prevent common fragile site instability. Nature Commun (2021) 4, 127/http://doi.org/10.1038/s42003-021-01647-8.
- Casper AM, Nghiem P, Arit MF, and Glower TW ATR regulates fragil site stability. Cell (2002) 111, 779-789
- Sfeir A, Kosiyatrakul, Hockemeyer D, MacRae S.L. et al. Mammalian telomeres resemble fragile sitesand require TRF1 for efficient replication. Cell (2009) 138, 90-103).
- J. Collis, A. Ciccia, A.J. Deans, Z. Horejsi, J.S. Martin, et al. FANCM and FAPP24 function in ATR-mediated checkpoint signaling independently of the Fanconi anemia core complex. Molecular Cell, (2008 )32: 313-324
- Leteurtre F, Li X, Guardiola P, Le Roux G et al. Accelerated telomere shortening and telomerase activation in Fanconi anemia. British Journal of Haematology (1999), 105, 883-893
- Horejsi, S.J. Collis & S.J. Boulton. FANCM-FAAP24 and HCLK2: Roles in ATR signaling and the Fanconi Anemia pathway. Cell Cycle (2009) 8:8: 1133-1137)
- Runge K.W. et al. TEL2, an essential gene required for telomere length regulation and telomere position effect in saccharmyces cerevisiae. Mol Cell Biol (1996)16:3094-3105
- C. Fan, F-W. Chang, J-D. Tsai, K-M Lin, C-M Chen et al .Telomeres and cancer(2021) Life 11, 1405) doi.org/10.3390/life11121405
- Blanco, R.; Muñoz, P.; Flores, J.M.; Klatt, P.; Blasco, M.A. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev. 2007, 21, 206–220.
- Muñoz R, Blasco, M.A. Blasco Role of the TRF2 telomeric protein in cancer and aging. Cell Cycle 2006, 5, 718–721.
- Imran, S.A.M.; Yazid, M.D.; Cui, W.; Lokanathan, Y. The intra and extratelomeric role of TRF2 in the DNA damage response. J. Mol. Sci. 2021, 22, 9900. https://doi.org/10.3390/ijms22189900
- Bozulic, L.; Surucu, B.; Hynx, D.; Hemmings, B.A. PKBα/Akt1 acts downstream of DNA-PK in the DNA double strand break reponse and promotes survival. Cell 2008, 30, 203–213.
- CBassi; Fortin, J.; Dnow, B.E.; Wakeham, A.; et al. The PTEN and ATM axis controls the G1/S cell cycle checkpoint and tumorigenesis in HER2-positive breast cancer. Cell Deth Differ. 2021, 28, 3036–3051. https://doi.org/10.1038/s41418-021-00799-8.
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Rev. Drug Discov. 2014, 13, 140–156. https://doi.org/10.1038/nrd4204.
- Shen, W.H.; Balajee, A.S.; Wang, J.; Wu, H. et al. Essential role for nuclear Pten in maintaining chromosomal integrity. Cell 2007, 128, 157–170.
- Planchon, S.M.; Waite, K.A.; Eng, C. The nuclear affairs of Ptem. Cell Sci. 2008, 121, 249–253.
- Skeen, J.E.; Bhaskar, P.T.; Chen, C.-C.; Chen, W.C.; et al. AKT deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and TORC1-dependent manner. Cancer Cell 2006, 10, 269–280.
- Hannan, K.M.; Brandengurger, Y.; Jenkins, A.; Sharkey, K.; et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Cell. Biol. 2003, 23, 8862–8877.
- Torres-Montaner, A.; Bolivar, J.; Astola, A.; Gimenez-Mas, J.A.; Brieva JA and Valdivia MM. Immunohistochemical detection of ribosomal transcription factor UBF and AgNOR staining identify apoptotic events in neoplastic cells od Hodgkin`s disease and other lymphoid cells. Histochem. Cytochem. 2000, 48, 1521–1530.
- (Hoshii T, Tadokoro Y, Naka K, OOshio T et al. mTORC1 is essential for leukemia propagation but not stem cell self-renewal. Journal of clinical investigation (2012) 122(6), 2114-2129
- Chen, C.; et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. Exp. Med. 2008, 205, 2397–2408.
- Lee, J.Y.; Nakada, D.; Yilmaz, O.H.; Tothova, Z.; Joseph, N.M.; Lim, M.S.; Gilliland, D.G.; Morrison, S.J. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell 2010, 7, 593–605.
- Kharas, M.G.; Okabe, R.; Ganis, J.J.; Gozo, M. et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood 2010, 115, 1406–1415.
- Huang, J.; Nguyen-McCarty, M.; Hexner, E.O.; Danet-Desnoyers, G.; et al. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Med. 2012, 18, 1778–1785.
- Huang, J.; Zhang, Y.; Bersenev, A.; O’Brien, W.T.; et al. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J. Investig. 2009, 119, 3519–3529.
- Murtaza, G.; Khan, A.K.; Rashid, R.; Muneer, S.; Hasan, S.M.F.; Chen, J. Foxo transciptional factors and long-term living. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. https://doi.org/10.1155/2017/3494289.
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; et al. Ablation in mice of the mTORC components raptor, rictor or mLST8 reveals that mTORC2 is required for signaling to AKT_FOXO and PKC alpha but not S6K1. Dev. Cell 2006, 11, 859–871. https://doi.org/10.1016/j.devcel2006.10.007.
- Miyamoto, K.; Araki, K.Y.; Naka, K.; Arai, F.; et al. Foxo3a is essential for the maintenance of the hematopoietic stem cell pool. Cell Stem Cell 2007, 1, 101–112.
- Finlay, D.K.; Sinclair, L.V.; Feijoo, C.; Waugh, C.M.; et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in Pten-null lymphocytes. Exp. Med. 2009, 206, 2441–2454.
- Guertin, D.A.; Stevens, D.M.; Saitoh, M.; Kinkel, S.; Crosby, K.; Sheen, J.-H.; Mullholland, D.J.; Magnuson, M.A.; Wu, H.; Sabatini, D.M. The mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 2009, 15, 148–159.
- Magee, J.A.; Ikenoue, T.; Nakada, D.; Lee, J.Y.; et al. Temporal changes in Pten and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell 2012, 11, 415–428.
- Duronio, V. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. J. 2008, 415, 333–344.
- Sykes, S.M.; Lane, S.W.; Bullinger, N.; Kalaitzidis, D.; et al. AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemia. Cell 2011, 146, 697–70.
- Hu, M.C.T.; Lee, D.-F.; Xia, W.; Golfman, L.S.; et al. IκB kinase promotes tumorigenesis through inhibition of Forkhead FOXO3a. Cell 2004, 117, 225–237.
- Cai, Y.; Kandula, V.; Ye, X.; Irwin, M.G.; Xia, Z. Decoding telomere protein Rap1: Its telomeric and nontelomeric functions and potential implications in diabetic cardiomyopathy. Cell Cycle 2017, 16, 1765–1773.
- Li, N.; Banin, S.; Ouyang, H.; Li, G.C.; et al. ATM is required for IκB kinase (IKK) activation in response to DNA double strand breaks. Biol. Chem. 2001, 270, 8898–8903.
- Shats, I.; Gatza, M.L.; Liu, B.; Angus, S.P.; You, L.; Nevins, J.R. FOXO transcription factors control E2F1 transcriptional specificity and apoptotic function. Cancer Res. 2013, 73, 6056–6067.
- Ryo, A.; Liou, Y.-C.; Wulf, G.; Nakamura, M.; et al. Pin1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Cell. Biol. 2002, 22, 5281–5295.
- Xue L, Nolla H, Suzuki A, Mak TWand Winoto. Normal development is an integral parto f tumorigenesis in T-cell specific PTEN-deficient mice. PNAS (2008), 105(6), 2022-2027] Puc, J.; Keniry, M.; Li, H.D.; Pandita, T.K.; et al. Lack of Pten sequesters CHK1 and initiates genetic instability. Cancer Cell 2005, 7, 193–204. https://doi.org/10.1016/j.ccr. 2005.01.009.
- Geserick, C.; Tejera, A.; Gonzalez-Suarez, E.; Klatt, P.; et al. Expression of mTert in primary murine cells links the growth-promoting effects of telomerase to transforming growth factor-β signaling. Oncogene 2006, 25, 4310–4319.
- Sprung, C.N.; Reynolds, G.E.; Jasin, M.; Murnane, J.P. Chromosome healing in mouse embryonic stem cells. Natl. Acad. Sci. USA 1999, 96, 6781–6786.
- Pekarsky, Y.; Palamarchuk, A.; Maximov, V.; Efanov, E.; et al. Tcl1 functions as a transcriptional regulator and is directly involved in the pathogenesis of CLL. Natl. Acad. Sci. USA 2008, 105, 19643–19648.
- Cabezas-Wallscheid, N.; Buettner, F.; Sommerkamp, P.; Klimmeck, D.; et al. Vitamin A-Retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell 2017, 169, 807–823.
- Liao, X.-H.; Zhang, A.L.; Zheng, M.; Li, M.-Q.; et al. Chemical or genetic Pin1 inhibition exerts potent anticancer activity against hepatocellular carcinoma by blocking multiple cancer-driving pathways. Rep. 2017, 7, srep43639 https://doi.org/10.1038/srep43639.
- Liou, Y.-C.; Ryo, A.; Huang, H.-K.; Lu, P.-J.; et al. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Natl. Acad. Sci. USA 2002, 99, 1335–1340.
- Shen, M.; Stuckenberg, T.; Kirschner, M.; et al. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Dev. 1998, 12, 706–720. https://doi.org/10.1101/gad.12.5.706.
- Bao, L.; Kimzey, A.; Sauter, G.; Sowadski, J.M.; et al. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. J. Pathol. 2004, 164, 1727–1737.
This entry is adapted from the peer-reviewed paper 10.3390/cimb45090478
