In the Indian subcontinents, Euphorbia neriifolia Linn. (EN) is one of the valuable plants from the big family of Euphorbiaceae, which is usually found in rocky and hilly areas. E. neriifolia was found to be useful in curing tumors, abdominal swelling, bronchial infection, hydrophobia, earache, cough and cold, asthma, leprosy, gonorrhea, spleen enlargement, leucoderma, snake bites, scorpion stings, and causing appetite improvement, etc. Different in vitro and in vivo experimental studies were performed to determine the antioxidant, anti-diabetic, immunomodulatory, anti-inflammatory, anti-arthritic, wound healing, anti-atherosclerosis, radioprotective, anti-anxiety, anti-convulsant, anti-psychotic, anti-thrombotic, dermal irritation, hemolytic, analgesic, anti-fertility, diuretic, anti-microbial, anti-diarrheal, and anti-carcinogenic activities of the various parts of EN. Several bioactive compounds, such as euphol, nerifoliol, taraxerol, euphonerins A–G, lectin, etc., were isolated from E. neriifolia and need to be investigated further for various biological activities (cardiovascular and neuronal diseases). In the pharmaceutical sector, E. neriifolia was selected for the development of new drugs due to its broad pharmacological activities.
- Euphorbia neriifolia
- diseases
- triterpenes
1. Introduction
2. Medicinal Uses of Different Parts of Euphorbia neriifolia
| Plant Parts | Used in | Applications | References |
|---|---|---|---|
| Whole plant | Anemia, fever, ulcer, inflammation, loss of consciousness, piles, delirium, bronchitis, and tumor | Whole plant juice as alexipharmic, carminative, and laxative | [9] |
| Vata-dosha disorders such as constipation, neuroglia, bloating, paralysis, induction of severe purgation, and for improving the strength of digestion | Whole plant | [19] | |
| Anal fistula | Whole plant as rubefacient and aphrodisiac | [19] | |
| Anorexia, fatigue, vomiting, weakness, spree syndrome, arthritis, and digestive tract disorder | Whole plant as one of the components of Dashmoolarishtam | [19] | |
| Insecticide | As a spray to kill insects | [27] | |
| For fencing | As it is covered with spines | [27] | |
| Leaves | Asthma | Succus administration comprising leaf juice and simple syrup in a minimum dosage of 10–20 mL three times a day | [9] |
| Earache | Leaf juice | [9] | |
| Wound healing | Steamed leaves paste on the affected area for 4–5 days | [9][16] | |
| Arthritis, skin wart | Leaf juice | [17] | |
| Bleeding piles, ano-rectal fistula, bronchitis, cold, and cough | As a diuretic and aphrodisiac | [28] | |
| Stem | Direct expectoration of phlegm | Stem juice at a small dosage with honey and borax | [28] |
| Hydrophobia | Pulp of the stem mixed with fresh ginger | [28] | |
| Piles and fistula | Stem juice | [28] | |
| Chronic respiratory problem | Stem juice with black pepper | [27] | |
| Latex | Drastic cathartic condition | Latex juice | [15] |
| Piles | Latex juice with turmeric | [15] | |
| Warts | Latex juice | [20] | |
| Skin warts, arthritis, and earache. | Latex juice | [16] | |
| Opthalmia | Milky juice in combination with shoot | [15][16][29] | |
| Rheumatic infection | Milky juice in combination with margosa oil | [15][16][29] | |
| Unhealthy ulcer, glandular swelling, and scabies | EN latex juice with fresh butter | [15][16][21][29] | |
| Cracks in their foot soles | Boiling latex with castor oil and salt | [15][16][29] | |
| Wounds and burns | Milk of E. neriifolia latex | [15][16][29] | |
| Reduce swelling in piles, pain, and itching | Lukewarm extract | [15][16][29] | |
| Asthma | Five drops of latex juice containing gokaran root, agaba root, and madar flower with honey | [9] | |
| Vitiligo, fistula, and syphilis | Latex juice | [15][25][26][30] | |
| Leprosy, general anasarca, dropsy, syphilis, spleen and liver enlargement | Trivit root, chebulic myrobalans, long-peppers, clove soaked in latex juice for a month and then dried to form pills | [9] | |
| Ascites, anasarea, and tympanitis | Latex juice with chebulic myrobalan, trivit root, and long pepper | [15] | |
| Protection against herbivorous insects | Latex | [31] | |
| Bark | Semen passing with urine | Mixture of bark and leaves of Piper betle L. | [32] |
| Roots | Snake bites and scorpion stings | Root of E. neriifolia in combination with black pepper | [9][15][32] |
| Blood pressure | A small dosage may increase blood pressure and a high dosage may decrease blood pressure | [9][15][32] | |
| Dropsy | Boiled mixture of root-bark in water | [9][15] |
3. Phytochemical Composition
3.1. Terpenes
3.2. Flavonoids
3.3. Saponins
| Plant part | Extracts | Secondary Metabolite | Types | Structure | Pharmacological Activity | References |
|---|---|---|---|---|---|---|
| Whole plant | Methanol extract | Diterpenoids |
|
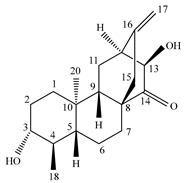 Eupnerias G 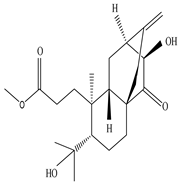 Eupnerias H 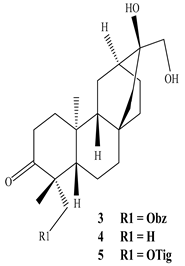 Eupnerias I, ent-16α,17-dihydroxyatisan-3-one, Eurifoloid R 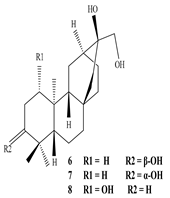 Ent-atisane-3α,16α,17-triol, Ent-atisane-3β,16α,17-triol, Ent-atisane-1β,16α,17-triol 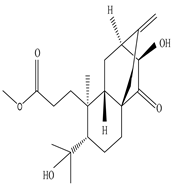 4,13β-Dihydroxy-14-oxo-3,4-secoatis-16-en-3-oic acid methyl ester 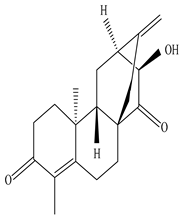 Eurifoloid M 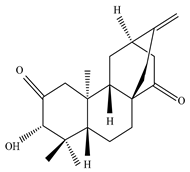 Ent-3S-hydroxyatis-16(17)-en-1,14-dione 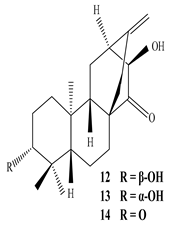 Ent-3α,13S-dihydroxyatis-16-en-14-one, Ent-3β,13S-dihydroxyatis-16-en-14-one, Ent-13S-hydroxyatis-16-ene-3,14-dione 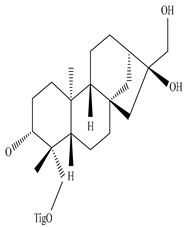 (4R,5S,8S,9R,10S,13R,16S)-Ent-16α,17-dihydroxy-19-tigloyloxykauran-3-one |
Anti-HIV effect was shown by compound 4 and 5 with EC50 values of 6.6 ± 3.2 and 6.4 ± 2.5 μg/mL, Moderate cytotoxic activity was exhibited by compound 1 and 6 against HepG2/Adr and HepG2 cells with IC50 values of 13.70 and 15.57 μM, and compound 15 was reported to exhibit cytotoxic activity (IC50 = 0.01µM) against HepG2 but not against HepG2/Adr cell line |
[11] |
| Whole plant | Hexane extract | Triterpene and Triterpene alcohol |
|
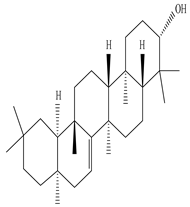 Taraxerol 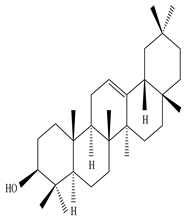 β-Amyrin |
Taraxerol exhibits anti-cancer activity via Nf-kB signalling pathway inhibition or by induction of apoptosis in case of middle ear epithelial cholesteatoma cells | [45][46] |
| Leaves | Hydroethanolic extract | Flavonoids |
|
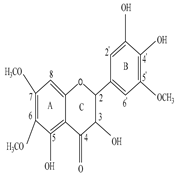 2-(3,4-dihydroxy-5-methoxy-phenyl)-3,5-dihydroxy-6,7-dimethoxychromen-4-one (C18H18O9) |
Exhibits anti-cancer activity due to its ability to scavenge reactive oxygen species and to inhibit lipid peroxidation | [41] |
| Leaves | Ethanolic extract |
|
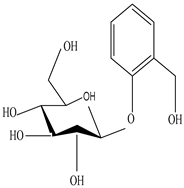 Glycosides |
Inhibits the proliferation of Plamodium falciparum with IC50 values of 5.4, 4.1, and 1.1 µg/mL, and shows cytotoxic activity against KN3-1 human epidermoid cancer cells | [47] | |
| Leaves | Ethanolic extract | Triterpenoids
|
|
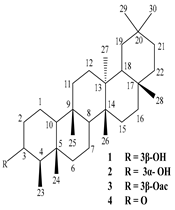 3β-Friedelanol, 3α-Friedelanol, 3β-Acetoxy fridelane, Friedelin 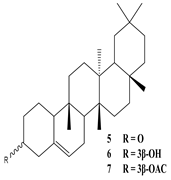 Glutinone, Glutin-5-en-3β-ol, Glutinol acetate 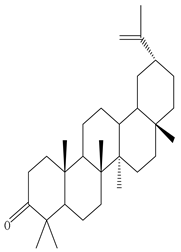 Lupenone 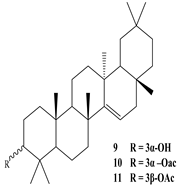 Epitaraxerol, Epitaraxeryl acetate, Taraxeryl acetate 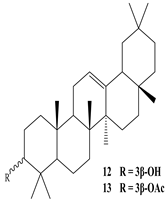 β-Amyrin, β-Amyrin acetate 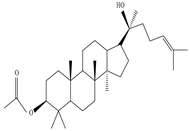 Dammarenediol II acetate 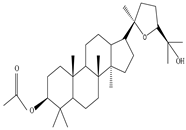 Cabraleadiol monoacetate 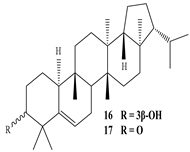 3β-Simiarenol, Simiarenone 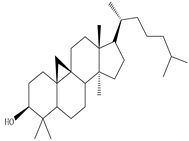 Cycloartenol 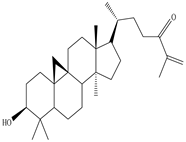 24-Oxocycloart-25-en-3β-ol 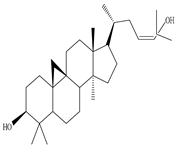 (23Z)-Cycloart-23-ene-3β,25-diol 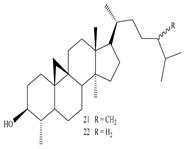 29-Norcycloartanol 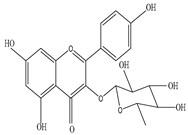 Afzelin |
Triterpenoids showed anti-viral activity in comparison to actinomycin D, Found to be effective against the herpes virus and inhibits replication of SARS-COV by binding to its 3CL pro proteases | [27][36][47] |
| Leaves | Aqueous extract | Flavonoid |
|
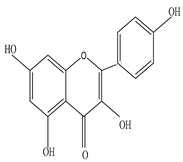 Kaempferol |
Kaempferol modulates metastasis, inflammation, angiogenesis, and apoptosis, and provides protection against chronic diseases by activating the body antioxidant defense mechanism against free reactive species | [48][49] |
| Leaves | Ethyl acetate extract | Diterpenoids (Eurifoloids A–R) |
|
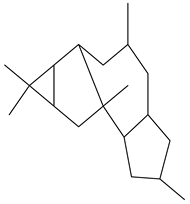 Ingenane (1&2)  Abietane  Isopimarane  Ent-atisane |
Various diterpenoids such as ingenanae, abietane, isopimarane, and ent-atisane exhibit anti-HIV activity | [50] |
| Leaves | Methanol extract | Cycloartane terpenoids |
|
 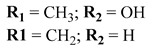 Neriifolins A, Neriifolins B 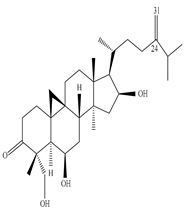 Neriifolins C |
Neriifolins A–C showed cytotoxicity against MCF breast cancer cell line with IC50 value of 9.50, 7.12, and 13.14 µM | [51] |
| Leaves | Methanol extract | Pachypodol |
|
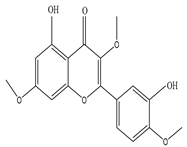 5,40-Dihydroxy-3,7,30-trimethoxyflavone |
Inhibits the proliferation of Psudomonas aeruginosa, Escherichia coli, Streptococcus faecalis, Streptococcus aureus, Bacillus subtilis, Candida glabrata, Candida krusei, and Candida albicans | [27] |
| Leaf | Chloroform and ethanol extract |
|
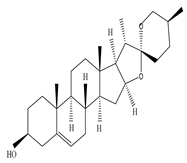 Saponin 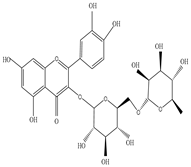 Rutin 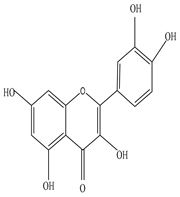 Quercetin |
Saponin shows anti-microbial activity against Staphylococcus aeruginosa, Escherichia coli, and Pseudomonas aeruginosa; Rutin inhibits the activity of Cryptococcus gattii, and Cryptococcus neoformans; and Quercetin found to be effective against Candida albicans |
[9][27][44][52][53] | |
| Leaves | _ | Diterpenoids |
|
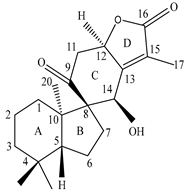 Phorneroids A 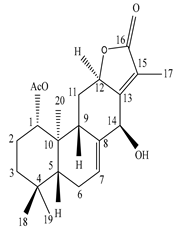 Phorneroids B 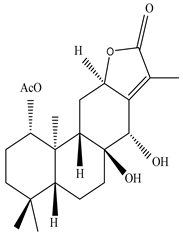 Phorneroids C 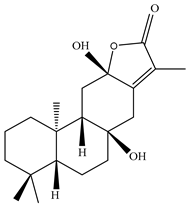 Phorneroids D 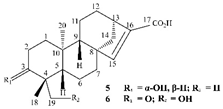 Phorneroids E, F 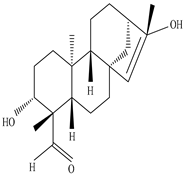 Phorneroids G 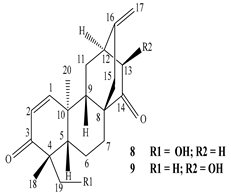 Phorneroids H, I 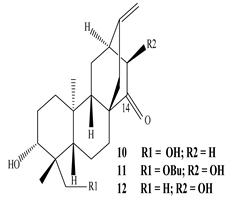 Phorneroids J, K, L  Phorneroids M  Compound I 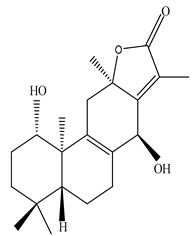 Compound II 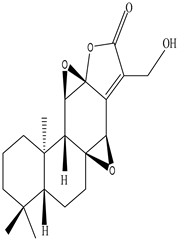 Compound III |
Phorneroids A–M, and three known compounds exhibit moderate cytotoxic activity against HL-60 and A549 cancer cell line | [54] |
| Leaves | _ | Ingenane and ingol diterpenoids |
|
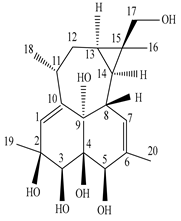 Phonerilins A 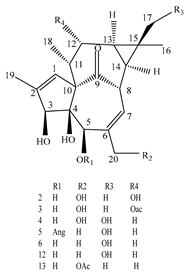 Phonerilins B, Phonerilins C, Phonerilins D, Phonerilins E, Phonerilins F, Phonerilins G, and 2 analogues 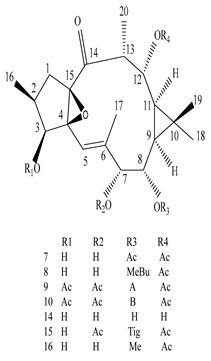 Phonerilins G, Phonerilins H, Phonerilins I, Phonerilins J, Phonerilins K, and three analogues |
Phonerilins A–K, and five known analogues exhibit moderate cytotoxic activity against HL-60 and A549 cancer cell line | [55] |
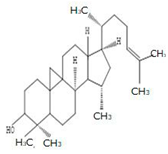
| Stem | Ethanolic extract | Euphane and tirucallane triterpenes |
|
 Neritriterpenols A, Neritriterpenols B  Neritriterpenols C  Neritriterpenols D 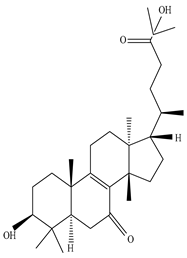 Neritriterpenols E 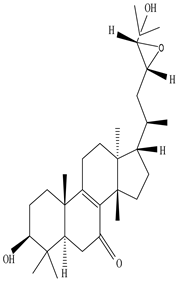 Neritriterpenols F 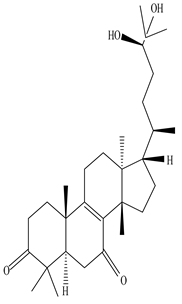 Neritriterpenols G 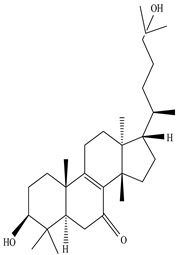 Triterpene 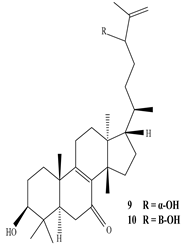 Triterpenes 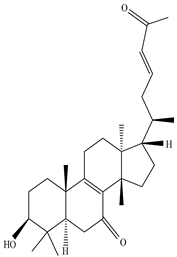 Triterpenes |
Neritriterpenols A–G, and four known triterpene exhibit anti-proliferative and anti-inflammatory activities | [12] |
| Stem bark | Ethyl acetate extract | Ingenane-type diterpenoids |
|
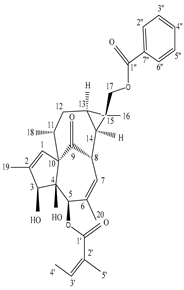 Eurifoloid E 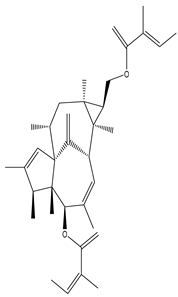 Euphorneroid A |
Eurifoloid E and Euphorneroid A inhibit pro-inflammatory mediators such as iNOS, IL-6, IL-1β, and NO in cases of LPS-induced RAW264.7 macrophage |
[56] |
| Stem bark | Methanol extract | Diterpenes |
|
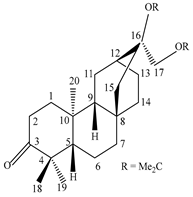 Ent-3-oxoatis-16α,17-acetonide |
Exhibits anti-HIV activity with EC50 value of 8.7 µg/mL | [57] |
| Stem and Bark | Methanol extract | Diterpenes |
|
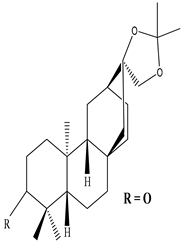 Ent-3-oxoatisan-16α, 17-acetonide 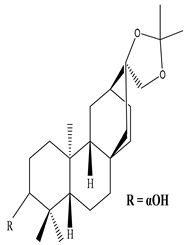 Euphorneroid D |
Ent-3-oxoatisan-16α, 17-acetonide and Euphorneroid D exhibit anti-HIV activity with EC50 value of 24 and 34 mM | [58] |
| Stem and Bark | Ethanol and petroleum ether extract | Anthocyanin |
|
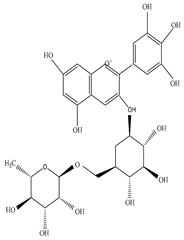 Tulipanin 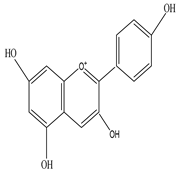 Pelargonin |
Tulipanin inhibits the proliferation of Bacillus subtilis, E. coli, P. aeruginosa, and Staphylococcus aureus | [27][45] |
| Stem and Bark | Methanol extract | Triterpene alcohol |
|
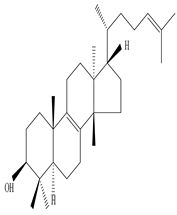 Euphol |
Euphol exhibits an anti-nociceptive effect in neuropathic pain and inflammatory conditions | [45][59] |
| Stem barks | Ethyl acetate extract | Ent-isopimarane diterpenoids |
|
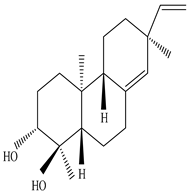 Eupneria J 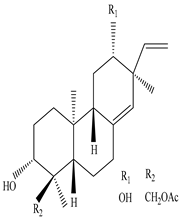 Eurifoloid H 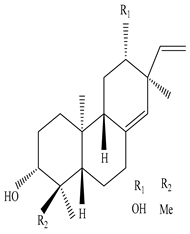 Ent-isopimara-8(14),15-dien-3β,12β-diol |
Eupneria J, Eurifoloid H, and ent-isopimara-8(14),15-dien-3β,12β-diol exhibit anti-HIV activity with an IC50 value of 6.70 and 0.31 µg/mL and anti-influenza activity with an IC50 value of 3.86 µg/mL | [60] |
| Stem bark | Ethyl acetate extract | Ent-abietane diterpenoids |
|
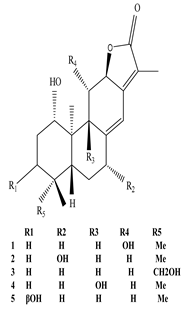 Eupnerias A–E  Eupnerias F |
Exhibit anti-influenza and anti-inflammatory activities | [61] |
| Bark | Petroleum ether extract | Diterpenes |
|
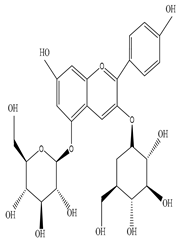 Pelargonin-3,5-diglucoside 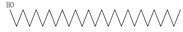 n-Hexacosanol 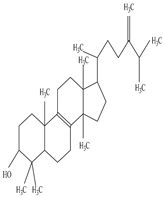 Euphorbol |
n-Hexacosanol was found to be effective to treat diabetic ileum by ameliorating the overexpression of M(3) and M(2) mRNA receptor | [45][62] |
| Fresh Latex from stem | Crude extract |
|
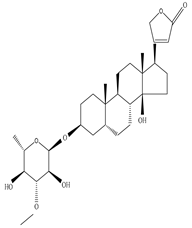 Neriifolin-S |
Neriifolin-S exhibits milk clotting activity | [22] | |
| Dried Latex | Methanol extract | Triterpene and Triterpene alcohol |
|
 Cycloartenol 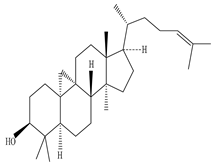 Nerifolione 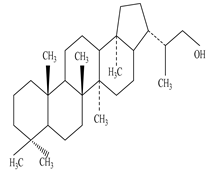 Nerifoliol |
Cycloartenol, Nerifolione, and Nerifoliol inhibit p38 MAP kinase phosphorylation and migration of glioma cells |
[33][45][63] |
| Root | Petroleum ether extract | Diterpenes |
|
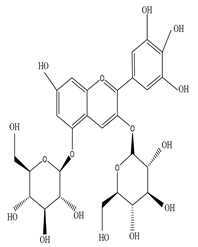 Delphin 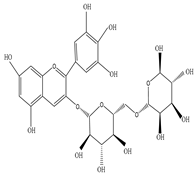 Tulipanin |
Delphinidin 3,5-O-diglucoside and Tulipanin exhibit antioxidant activity by suppressing the formation of reactive oxygen species from lacrimal gland tissue that preserves tear secretion | [45][64][65] |
| Root | Ethanol extract |
Triterpene alcohol |
|
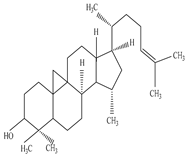 Cycloartenol 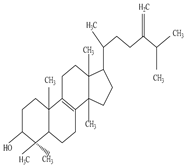 Euphorbol |
Cycloartenol exhibits antioxidant activity | [45][66] |
| Root | Methanolic extract | Diterpenes |
|
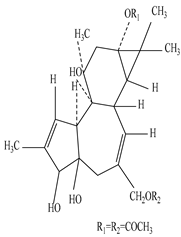 12-Deoxyphorbhol-13, 20-diacetate 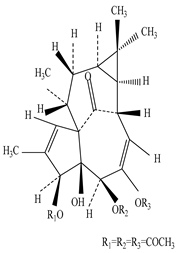 Ingenol triacetate |
12-Deoxyphorbhol-13, 20-diacetate and Ingenol triacetate exhibit anti-HIV activity in the case of MT-4 cells at 0.65 and 0.051 mM concentration, and are useful to treat the skin condition, actinic keratosis | [45][67][68] |
| Fresh Root | Protease fraction | Diterpenes |
|
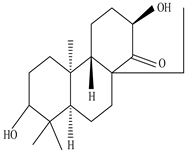 Neriifolene 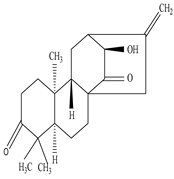 Atisine diterpene anti-quorin |
Atisine diterpene anti-quorin exhibits wound healing activity by inducing the intercellular signaling and aggregation of platelets vis PAR-1 | [45][69] |
This entry is adapted from the peer-reviewed paper 10.3390/su15021225
References
- Petrovska, B.B. Historical review of medicinal plants′ usage. Pharmacogn. Rev. 2012, 6, 1–5.
- Aktar, K.; Foyzun, T. Phytochemistry and Pharmacological Studies of Citrus macroptera: A Medicinal Plant Review. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–7.
- WHO. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/index.html (accessed on 13 February 2007).
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid.-Based Complement. Altern. Med. 2011, 2011, 680354.
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177.
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293.
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336.
- Chaudhary, P.; Janmeda, P. Comparative pharmacognostical standardization of different parts of Euphorbia neriifolia Linn. Vegetos-Int. J. Plant Res. 2022.
- Mali, P.Y.; Panchal, S.S. Euphorbia neriifolia L.: Review on botany, ethnomedicinal uses, phytochemistry and biological activities. Asian Pac. J. Trop. Med. 2017, 10, 430–438.
- Hasan, M.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Protective effect of Euphorbia neriifolia extract on experimentally induced thrombosis in murine model. Niger. J. Exp. Clin. Biosci. 2014, 2, 86–89.
- Li, J.; Feng, X.; Liu, D.; Zhang, Z.; Chen, X.; Li, R.; Li, H. Diterpenoids from Euphorbia neriifolia and Their Related Anti-HIV and Cytotoxic Activity. Chem. Biodivers. 2019, 16, e1900495.
- Chang, S.S.; Huang, H.-T.; Lin, Y.-C.; Chao, C.-H.; Liao, G.-Y.; Lin, Z.-H.; Huang, H.-C.; Kuo, J.C.-L.; Liaw, C.-C.; Tai, C.-J.; et al. Neritriterpenols A-G, euphane and tirucallane triterpenes from Euphorbia neriifolia L. and their bioactivity. Phytochemistry 2022, 199, 113199.
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991.
- Mohan, V.; Pradeepa, R. Epidemiology of type 2 diabetes in India. Indian, J. Ophthalmol. 2021, 69, 2932.
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula; Crown Agents for the Colonies: London, UK, 1936; Volume 1–2.
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; International Book Distributors: Dehradun, India, 1996; Volume II, p. 1581.
- Sharma, D.K. Bioprospecting for Drug Research and functional foods for the prevention of diseases- Role of flavonoids in drug development. J. Sci. Indust. Res. 2006, 65, 391–401.
- Al Mahtab, M.; Akbar, S.M.F.; Pramanik, E.A.; Miah, M.M.Z.; Ahmed, I.; Hossain, A.M.; Ali, M.N.; Haque, J.; Islam, A.M.; Jahan, R.A.; et al. Euphorbia neriifolia Leaf Juice on Mild and Moderate COVID-19 Patients: Implications in OMICRON Era. Euroasian J. Hepato-Gastroenterol. 2022, 12, 10–18.
- Pullaiah, T. Medicinal Plants of India; Department of Botany, Sri Krishnsdevaraya University: Anantapur, India, 2002; Volume 1, pp. 245–246.
- Burkill Ivor, H.; Haniff, M. Malay Village Medicine. Gard. Bull. Straits Settl. 1930, 6, 167–282.
- Oudhia, P.; Medicinal Herbs of Chhattisgarh. India Having Less Known Traditional Uses, VII. Thura (Euphorbia neriilofia, family: Euphorbiaceae), Research Note. 2003. Available online: http://www.botanical.com (accessed on 9 May 2005).
- Yadav, R.P.; Patel, A.K.; Jagannadham, M. Purification and biochemical characterization of a chymotrypsin-like serine protease from Euphorbia neriifolia Linn. Process Biochem. 2011, 46, 1654–1662.
- Raza, A.K. Tazkeratul Hind (Yadgare Razayee); Shamshul Islam Press: Hyderabad, India, 1938; pp. 300–302.
- Azam, R.M.; Ibraheem, M.; Khana, K.; Taraqqi, A. Urdu Bazar, Jama Masjid; Delhi Press: Delhi, India, 1895; Volume 2, pp. 264–279.
- Ghani, M.N. Khazaenul Advia; Barqi Press Lahore: Lahore, Pakistan, 1926; Volume 2,4,5, p. 1002.
- Jagannath, K.R. Ayurvedic Pharmacopea. Edara-e-mat-boat-e-sulaimani, Rehmani Printers; Ghazni street Urdu Bazar: Lahore, Pakistan, 1983; Volume 288, pp. 1–202.
- Sultana, A.; Hossain, J.; Kuddus, R.; Rashid, M.A.; Zahan, M.S.; Mitra, S.; Roy, A.; Alam, S.; Sarker, M.R.; Mohamed, I.N. Ethnobotanical Uses, Phytochemistry, Toxicology, and Pharmacological Properties of Euphorbia neriifolia Linn. against Infectious Diseases: A Comprehensive Review. Molecules 2022, 27, 4374.
- Ahmed, S.A.; Nazim, S.; Siraj, S.; Siddik, P.M.; Wahid, A.C. Euphorbia neriifolia Linn: A phytopharmacological review. Int. Res. J. Pharm. 2011, 2, 41.
- Anonymous. The Wealth of India, A Dictionary of Indian Raw Materials and Industrial Products (Raw materials). Central Institute of Medicinal and Aromatic Plants: New Delhi, India, 2003; Volume III (D–E), pp. 226–228.
- Gray, S.F. A Supplement to the Pharmacopeia, and Treatise on Pharmacology in General: Including not Only the Drugs and Preparations Used by Practitioners of Medicine, but Also Most of Those Employed in the Chemical Art: Together with a Collection of the Most Useful Medical Formula. Materia Medica. longman, Rees, Orme, Brown, Green, and Longman, London, Great Britain, 6th ed.; 1836; Available online: https://www.biodiversitylibrary.org/item/76371 (accessed on 6 June 2019).
- Pracheta, S.V. Microscopic studies and preliminary pharmacognostical evaluation of Euphorbia neriifolia L. leaves. Indian J. Nat. Prod. Resour. 2013, 4, 348–357.
- Rahman, M.B.; Talukdar, S.N.; Paul, S.; Rajbongshi, S. Evaluation of pharmacognostical, phytochemical, and ethnobotanical properties of Euphorbia neriifolia. Bioj Sci. Technol. 2015, 2, 1–18. Available online: https://bio-journal.com/content/article/2015/09/evaluation-of-pharmacognostical-properties-of-euphorbia (accessed on 6 June 2019).
- Ilyas, M.; Parveen, M.; Amin, K.M.Y. Neriifolione, a triterpene from Euphorbia neriifolia. Phytochemistry 1998, 48, 561–563.
- Kemboi, D.; Peter, X.; Langat, M.; Tembu, J. A Review of the Ethnomedicinal Uses, Biological Activities, and Triterpenoids of Euphorbia Species. Molecules 2020, 25, 4019.
- Magozwi, D.; Dinala, M.; Mokwana, N.; Siwe-Noundou, X.; Krause, R.; Sonopo, M.; McGaw, L.; Augustyn, W.; Tembu, V. Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure–Activity Relationships. Pharmaceuticals 2021, 14, 428.
- Thorat, B.R.; Bolli, V. Review on Euphorbia neriifolia Plant. Biomed. J. Sci. Tech. Res. 2017, 1, 001–0010.
- Yeoh, H.H.; Wee, Y.C.; Paesawat, C.; Khng, Y.W. Characterising latex enzyme profiles of tropical species using the Api Zym system. Physiol. Mol. Biol. Plants 1995, 1, 187–189.
- Seshagirirao, K.; Prasad, M. Purification and partial characterization of a lectin from E. neriifolia latex. Biochem. Mol. Biol. Int. 1995, 35, 1199–1204.
- Mallavadhani, U.V.; Satyanarayana, K.; Mahapatra, A.; Sudhakar, A. A new tetracyclic triterpene from the latex of Euphorbia nerifolia. Nat. Prod. Res. 2004, 18, 33–37.
- Bigoniya, P.; Shukla, A.; Singh, C.S. Dermal irritation and sensitization study of Euphorbia neriifolia latex and its anti-inflammatory efficacy. Int. J. Phytomed. 2010, 2, 240–254.
- Sharma, V.; Janmeda, P. Protective assessment of Euphorbia neriifolia and its isolated flavonoid against N-nitrosodiethylamine-induced hepatic carcinogenesis in male mice: A histopathological analysis. Toxicol. Int. 2014, 21, 38–43.
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A review on phytochemicals, pharmacological activities, drug interactions, and associated toxicities of licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485.
- Bigoniya, P. Hemolytic and in-vitro antioxidant activity of saponin isolated from Euphorbia neriifolia leaf. In Recent Progress in Medicinal Plants: Natural Products-II, 1st ed.; Govil, J.N., Ed.; Studium Press LLC: Houston, TX, USA, 2006; Chapter 20.
- Bigoniya, P.; Rana, A.C. Protective effect of Euphorbia neriifolia saponin fraction on CCl4- induced acute heapatotoxicity. Afr. J. Biotechnol. 2010, 9, 7148–7156.
- Bigoniya, P. Immunomodulatory activity of Euphorbia neriifolia leaf hydro-alcoholic extract in rats. Indian Drug. 2008, 45, 90–97.
- Liao, J.; Wu, F.; Lin, W.; Huang, Z. Taraxerol exerts potent anticancer effects via induction of apoptosis and inhibition of Nf-kB signalling pathway in human middle ear epithelial cholesteatoma cells. Trop. J. Pharm. Res. 2018, 17(6), 1011–1017.
- Chang, F.R.; Yen, C.T.; Ei-Shazly, M.; Lin, W.H.; Yen, M.H.; Lin, K.H.; Wu, Y.C. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod. Commun. 2012, 7, 1415–1417.
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107.
- Mali, P.Y.; Goyal, S. HPTLC Densitometric Quantification of Kaempferol from Leaves of Euphorbia neriifolia. Indian J. Pharm. Educ. Res. 2020, 54, s586–s592.
- Zhao, H.; Sun, L.; Kong, C.; Mei, W.; Dai, H.; Xu, F.; Huang, S. Phytochemical and pharmacological review of diterpenoids from the genus Euphorbia Linn (2012–2021). J. Ethnopharmacol. 2022, 298, 115574.
- Choodej, S.; Pudhom, K. Cycloartane triterpenoids from the leaves of Euphorbia neriifolia. Phytochem. Lett. 2019, 35, 1–5.
- Batiha, G.E.S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374.
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2016, 25, 149–164.
- Gao, Y.; Zhou, J.-S.; Liu, H.-C.; Zhang, Y.; Yin, W.-H.; Liu, Q.-F.; Wang, G.-W.; Zhao, J.-X.; Yue, J.-M. Phorneroids A–M, diverse types of diterpenoids from Euphorbia neriifolia. Phytochemistry 2022, 198, 113142.
- Gao, Y.; Zhou, J.-S.; Liu, H.-C.; Zhang, Y.; Yin, W.-H.; Liu, Q.-F.; Wang, G.-W.; Zhao, J.-X.; Yue, J.-M. Phonerilins A–K, cytotoxic ingenane and ingol diterpenoids from Euphorbia neriifolia. Tetrahedron 2022, 123, 132955.
- Jiang, M.; Xue, Y.; Li, J.; Rao, K.; Yan, S.; Li, H.; Chen, X.; Li, R.; Liu, D. PKCδ/MAPKs and NF-κB Pathways are Involved in the Regulation of Ingenane-Type Diterpenoids from Euphorbia neriifolia on Macrophage Function. J. Inflamm. Res. 2021, 14, 2681–2696.
- Yan, S.-L.; Li, Y.-H.; Li, R.-T.; Chen, X.-Q. Diterpenes from stem bark of Euphorbia neriifolia. Chin. Tradit. Herb. Drugs 2017, 48, 3698–3704.
- Yan, S.-L.; Li, Y.-H.; Chen, X.-Q.; Liu, D.; Chen, C.-H.; Li, R.-T. Diterpenes from the stem bark of Euphorbia neriifolia and their in vitro anti-HIV activity. Phytochemistry 2018, 145, 40–47.
- Dutra, R.C.; da Silva, K.A.B.S.; Bento, A.F.; Marcon, R.; Paszcuk, A.F.; Meotti, F.C.; Pianowski, L.F.; Calixto, J.B. Euphol, a tetracyclic triterpene produces antinociceptive effects in inflammatory and neuropathic pain: The involvement of cannabinoid system. Neuropharmacology 2012, 63, 593–605.
- Li, J.-C.; Dai, W.-F.; Liu, D.; Jiang, M.-Y.; Zhang, Z.-J.; Chen, X.-Q.; Chen, C.-H.; Li, R.-T.; Li, H.-M. Bioactive ent-isopimarane diterpenoids from Euphorbia neriifolia. Phytochemistry 2020, 175, 112373.
- Li, J.-C.; Zhang, Z.-J.; Yang, T.; Jiang, M.-Y.; Liu, D.; Li, H.-M.; Li, R.-T. Six new ent-abietane-type diterpenoids from the stem bark of Euphorbia neriifolia. Phytochem. Lett. 2019, 34, 13–17.
- Shinbori, C.; Saito, M.; Kinoshita, Y.; Satoh, I.; Kono, T.; Hanada, T.; Nanba, E.; Adachi, K.; Suzuki, H.; Yamada, M.; et al. N-hexacosanol reverses diabetic induced muscarinic hypercontractility of ileum in the rat. Eur. J. Pharmacol. 2006, 545, 177–184.
- Toume, K.; Nakazawa, T.; Hoque, T.; Ohtsuki, T.; Arai, M.; Koyano, T.; Kowithayakorn, M.; Ishibashi, T. Cycloartane triterpenes and ingol diterpenes isolated from Euphorbia neriifolia in a screening program for death-receptor expression-enhancing activity. Planta Med. 2012, 78, 1370–1377.
- Jaiswal, Y.S.; Guan, Y.; Moon, K.H.; Williams, L.L. Anthocyanins: Natural Sources and Traditional Therapeutic Uses. In Flavonoids-A Coloring Model for Cheering up Life; Badria, F.A., Ananga, A., Eds.; IntechOpen: London, UK, 2019.
- Nakamura, S.; Tanaka, J.; Imada, T.; Shimoda, H.; Tsubota, K. Delphinidin 3,5-O-diglucoside, a constituent of the maqui berry (Aristotelia chilensis) anthocyanin, restores tear secretion in a rat dry eye model. J. Funct. Foods 2014, 10, 346–354.
- Sawale, J.A.; Patel, J.A.; Kori, M.L. Antioxidant properties of cycloartenol isolated from Euphorbia neriifolia leaves. Indian J. Nat. Prod. 2019, 33, 60–64.
- Fujiwara, M.; Ijichi, K.; Tokuhisa, K.; Katsuura, K.; Shigeta, S.; Konno, K.; Wang, G.Y.; Uemura, D.; Yokota, T.; Baba, M. Mechanism of selective inhibition of human immunodeficiency virus by ingenol triacetate. Antimicrob. Agents Chemother. 1996, 40, 271–273.
- Hammadi, R.; Kúsz, N.; Dávid, C.; Behány, Z.; Papp, L.; Kemény, L.; Hohmann, J.; Lakatos, L.; Vasas, A. Ingol and Ingenol-Type Diterpenes from Euphorbia trigona Miller with Keratinocyte Inhibitory Activity. Plants 2021, 10, 1206.
- Urs, A.P.; Manjuprasanna, V.N.; Rudresha, G.V.; Hiremath, V.; Sharanappa, P.; Rajaiah, R.; Vishwanath, B.S. Thrombin-like serine protease, antiquorin from Euphorbia antiquorum latex induces platelet aggregation via PAR1-Akt/p38 signaling axis. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2020, 1868, 118925.
