Cholangiocarcinoma (CCA) is the most common malignancy in patients with primary sclerosing cholangitis (PSC), accounting for 2–8% of cases and being the leading cause of death in these patients. The majority of PSC-associated CCAs (PSC-CCA) develop within the first few years after PSC diagnosis. Older age and male sex, as well as concomitant inflammatory bowel disease (IBD) or high-grade biliary stenosis, are some of the most relevant risk factors. PSC-CCA pathogenesis is characterised by peculiar molecular and genetic features, being a distinct disease from the novo CCA. There has been a significant push to develop innovative strategies for PSC-CCA early diagnosis and surveillance.
- cholangiocarcinoma
- primary sclerosing cholangitis
- pathogenesis
- diagnosis
1. Introduction
2. Pathogenesis
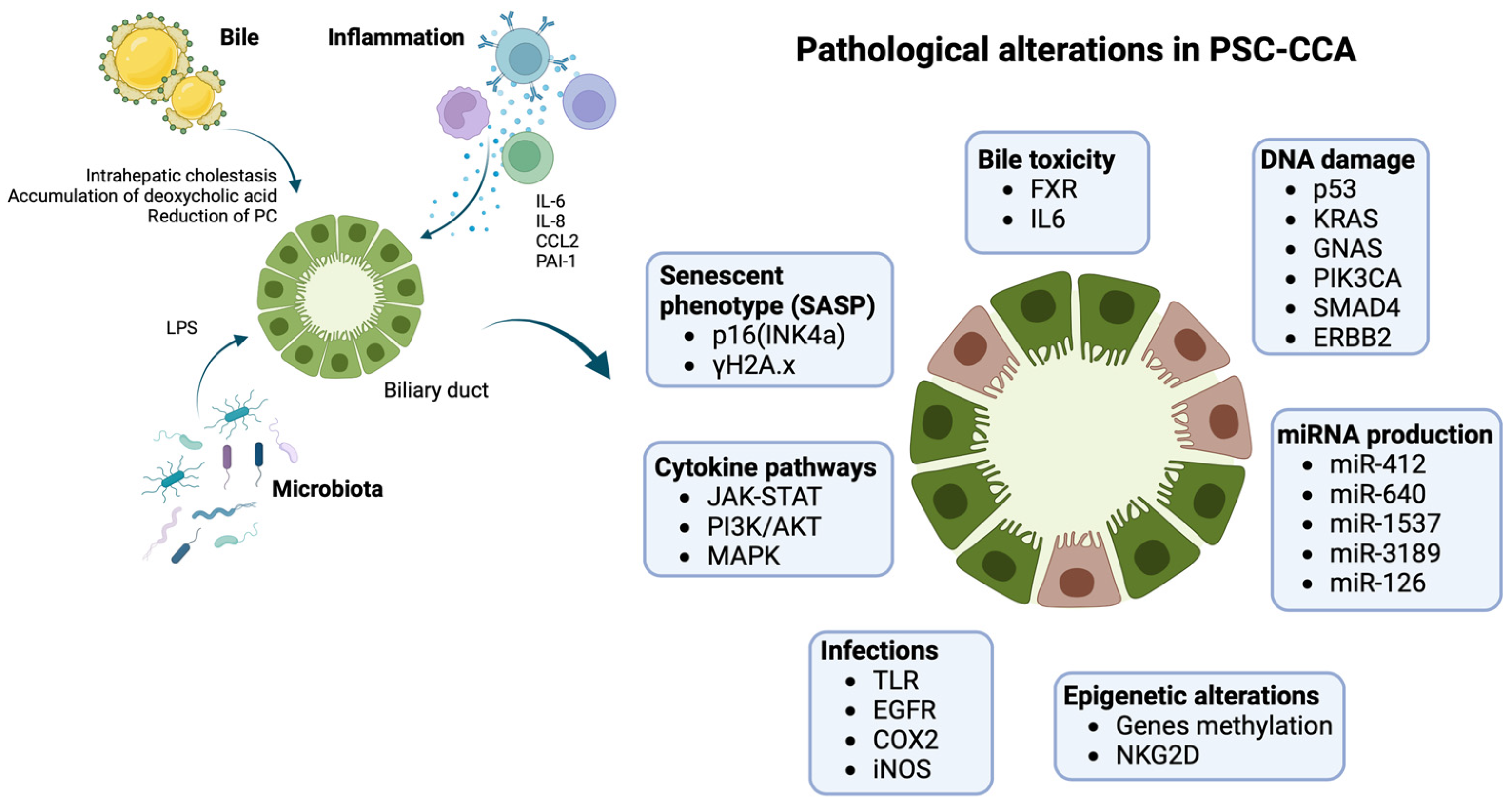
3. Surveillance of CCA in PSC
4. Future Perspective: Next-Generation Biomarkers for PSC-CCA Diagnosis and Surveillance
This entry is adapted from the peer-reviewed paper 10.3390/cancers15204947
References
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.-J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma Landscape in Europe: Diagnostic, Prognostic and Therapeutic Insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121.
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588.
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global Trends in Mortality from Intrahepatic and Extrahepatic Cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114.
- Kaewpitoon, N. Opisthorchis Viverrini: The Carcinogenic Human Liver Fluke. WJG 2008, 14, 666.
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk Factors for Intrahepatic and Extrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Hepatol. 2020, 72, 95–103.
- Wongjarupong, N.; Assavapongpaiboon, B.; Susantitaphong, P.; Cheungpasitporn, W.; Treeprasertsuk, S.; Rerknimitr, R.; Chaiteerakij, R. Non-Alcoholic Fatty Liver Disease as a Risk Factor for Cholangiocarcinoma: A Systematic Review and Meta-Analysis. BMC Gastroenterol. 2017, 17, 149.
- Tyson, G.L.; El-Serag, H.B. Risk Factors for Cholangiocarcinoma. Hepatology 2011, 54, 173–184.
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary Sclerosing Cholangitis—A Comprehensive Review. J. Hepatol. 2017, 67, 1298–1323.
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and Risk Factors. Liver Int. 2019, 39, 19–31.
- Lewis, J.T.; Talwalkar, J.A.; Rosen, C.B.; Smyrk, T.C.; Abraham, S.C. Precancerous Bile Duct Pathology in End-Stage Primary Sclerosing Cholangitis, with and without Cholangiocarcinoma. Am. J. Surg. Pathol. 2010, 34, 27–34.
- Fleming, K.A.; Boberg, K.M.; Glaumann, H.; Bergquist, A.; Smith, D.; Clausen, O.P.F. Biliary Dysplasia as a Marker of Cholangiocarcinoma in Primary Sclerosing Cholangitis. J. Hepatol. 2001, 34, 360–365.
- Bergquist, A.; Glaumann, H.; Stal, P.; Wang, G.-S.; Broome, U. Biliary Dysplasia, Cell Proliferation and Nuclear DNA-Fragmentation in Primary Sclerosing Cholangitis with and without Cholangiocarcinoma. J. Intern. Med. 2001, 249, 69–75.
- Chung, B.K.; Karlsen, T.H.; Folseraas, T. Cholangiocytes in the Pathogenesis of Primary Sclerosing Cholangitis and Development of Cholangiocarcinoma. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 1390–1400.
- de Vries, A.B. Distinctive Inflammatory Bowel Disease Phenotype in Primary Sclerosing Cholangitis. WJG 2015, 21, 1956.
- Hirschfield, G.M.; Karlsen, T.H.; Lindor, K.D.; Adams, D.H. Primary Sclerosing Cholangitis. Lancet 2013, 382, 1587–1599.
- Özdirik, B.; Müller, T.; Wree, A.; Tacke, F.; Sigal, M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci. 2021, 22, 6975.
- Saab, M.; Mestivier, D.; Sohrabi, M.; Rodriguez, C.; Khonsari, M.R.; Faraji, A.; Sobhani, I. Characterization of Biliary Microbiota Dysbiosis in Extrahepatic Cholangiocarcinoma. PLoS ONE 2021, 16, e0247798.
- Jia, X.; Lu, S.; Zeng, Z.; Liu, Q.; Dong, Z.; Chen, Y.; Zhu, Z.; Hong, Z.; Zhang, T.; Du, G.; et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 71, 893–906.
- Reich, M.; Spomer, L.; Klindt, C.; Fuchs, K.; Stindt, J.; Deutschmann, K.; Höhne, J.; Liaskou, E.; Hov, J.R.; Karlsen, T.H.; et al. Downregulation of TGR5 (GPBAR1) in Biliary Epithelial Cells Contributes to the Pathogenesis of Sclerosing Cholangitis. J. Hepatol. 2021, 75, 634–646.
- Hohenester, S.; Maillette de Buy Wenniger, L.; Paulusma, C.C.; van Vliet, S.J.; Jefferson, D.M.; Oude Elferink, R.P.; Beuers, U. A Biliary HCO3− Umbrella Constitutes a Protective Mechanism against Bile Acid-Induced Injury in Human Cholangiocytes. Hepatology 2012, 55, 173–183.
- Gauss, A. Biliary Phosphatidylcholine and Lysophosphatidylcholine Profiles in Sclerosing Cholangitis. WJG 2013, 19, 5454.
- Henriksen, E.K.K.; Viken, M.K.; Wittig, M.; Holm, K.; Folseraas, T.; Mucha, S.; Melum, E.; Hov, J.R.; Lazaridis, K.N.; Juran, B.D.; et al. HLA Haplotypes in Primary Sclerosing Cholangitis Patients of Admixed and Non-European Ancestry. HLA 2017, 90, 228–233.
- The International IBD Genetics Consortium (IIBDGC); International Genetics of Ankylosing Spondylitis Consortium (IGAS); International PSC Study Group (IPSCSG); Genetic Analysis of Psoriasis Consortium (GAPC); Psoriasis Association Genetics Extension (PAGE); Ellinghaus, D.; Jostins, L.; Spain, S.L.; Cortes, A.; Bethune, J.; et al. Analysis of Five Chronic Inflammatory Diseases Identifies 27 New Associations and Highlights Disease-Specific Patterns at Shared Loci. Nat. Genet. 2016, 48, 510–518.
- Folseraas, T.; Liaskou, E.; Anderson, C.A.; Karlsen, T.H. Genetics in PSC: What Do the “Risk Genes” Teach Us? Clin. Rev. Allergy Immunol. 2015, 48, 154–164.
- The UK-PSCSC Consortium; The International IBD Genetics Consortium; The International PSC Study Group; Liu, J.Z.; Hov, J.R.; Folseraas, T.; Ellinghaus, E.; Rushbrook, S.M.; Doncheva, N.T.; Andreassen, O.A.; et al. Dense Genotyping of Immune-Related Disease Regions Identifies Nine New Risk Loci for Primary Sclerosing Cholangitis. Nat. Genet. 2013, 45, 670–675.
- Alvaro, D.; Gigliozzi, A.; Attili, A.F. Regulation and Deregulation of Cholangiocyte Proliferation. J. Hepatol. 2000, 33, 333–340.
- Raven, A.; Lu, W.-Y.; Man, T.Y.; Ferreira-Gonzalez, S.; O’Duibhir, E.; Dwyer, B.J.; Thomson, J.P.; Meehan, R.R.; Bogorad, R.; Koteliansky, V.; et al. Cholangiocytes Act as Facultative Liver Stem Cells during Impaired Hepatocyte Regeneration. Nature 2017, 547, 350–354.
- Carpino, G.; Cardinale, V.; Renzi, A.; Hov, J.R.; Berloco, P.B.; Rossi, M.; Karlsen, T.H.; Alvaro, D.; Gaudio, E. Activation of Biliary Tree Stem Cells within Peribiliary Glands in Primary Sclerosing Cholangitis. J. Hepatol. 2015, 63, 1220–1228.
- DiPaola, F.; Shivakumar, P.; Pfister, J.; Walters, S.; Sabla, G.; Bezerra, J.A. Identification of Intramural Epithelial Networks Linked to Peribiliary Glands That Express Progenitor Cell Markers and Proliferate after Injury in Mice. Hepatology 2013, 58, 1486–1496.
- Guicciardi, M.E.; Trussoni, C.E.; LaRusso, N.F.; Gores, G.J. The Spectrum of Reactive Cholangiocytes in Primary Sclerosing Cholangitis. Hepatology 2020, 71, 741–748.
- Carpino, G.; Nevi, L.; Overi, D.; Cardinale, V.; Lu, W.; Di Matteo, S.; Safarikia, S.; Berloco, P.B.; Venere, R.; Onori, P.; et al. Peribiliary Gland Niche Participates in Biliary Tree Regeneration in Mouse and in Human Primary Sclerosing Cholangitis. Hepatology 2020, 71, 972–989.
- Clerbaux, L.-A.; Manco, R.; Van Hul, N.; Bouzin, C.; Sciarra, A.; Sempoux, C.; Theise, N.D.; Leclercq, I.A. Invasive Ductular Reaction Operates Hepatobiliary Junctions upon Hepatocellular Injury in Rodents and Humans. Am. J. Pathol. 2019, 189, 1569–1581.
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430.
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.-P.; Schwabe, R.F. Fate Tracing Reveals Hepatic Stellate Cells as Dominant Contributors to Liver Fibrosis Independent of Its Aetiology. Nat. Commun. 2013, 4, 2823.
- Volckaert, T.; De Langhe, S. Lung Epithelial Stem Cells and Their Niches: Fgf10 Takes Center Stage. Fibrogenes. Tissue Repair. 2014, 7, 8.
- Tabibian, J.H.; O’Hara, S.P.; Splinter, P.L.; Trussoni, C.E.; LaRusso, N.F. Cholangiocyte Senescence by Way of N-Ras Activation Is a Characteristic of Primary Sclerosing Cholangitis: Hepatology. Hepatology 2014, 59, 2263–2275.
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118.
- O’Hara, S.P.; Splinter, P.L.; Trussoni, C.E.; Guicciardi, M.E.; Splinter, N.P.; Al Suraih, M.S.; Nasser-Ghodsi, N.; Stollenwerk, D.; Gores, G.J.; LaRusso, N.F. The Transcription Factor ETS1 Promotes Apoptosis Resistance of Senescent Cholangiocytes by Epigenetically Up-Regulating the Apoptosis Suppressor BCL2L1. J. Biol. Chem. 2019, 294, 18698–18713.
- Boberg, K.M.; Schrumpf, E.; Bergquist, A.; Broomé, U.; Pares, A.; Remotti, H.; Schjölberg, A.; Spurkland, A.; Clausen, O.P.F. Cholangiocarcinoma in Primary Sclerosing Cholangitis: K-Ras Mutations and Tp53 Dysfunction Are Implicated in the Neoplastic Development. J. Hepatol. 2000, 32, 374–380.
- Kamp, E.J.; Dinjens, W.N.; Doukas, M.; Van Marion, R.; Verheij, J.; Ponsioen, C.Y.; Bruno, M.J.; Groot Koerkamp, B.; Trivedi, P.J.; Peppelenbosch, M.P.; et al. Genetic Alterations during the Neoplastic Cascade towards Cholangiocarcinoma in Primary Sclerosing Cholangitis. J. Pathol. 2022, 258, 227–235.
- Goeppert, B.; Folseraas, T.; Roessler, S.; Kloor, M.; Volckmar, A.; Endris, V.; Buchhalter, I.; Stenzinger, A.; Grzyb, K.; Grimsrud, M.M.; et al. Genomic Characterization of Cholangiocarcinoma in Primary Sclerosing Cholangitis Reveals Therapeutic Opportunities. Hepatology 2020, 72, 1253–1266.
- Ahrendt, S. Diagnosis and Management of Cholangiocarcinoma in Primary Sclerosing Cholangitis. J. Gastrointest. Surg. 1999, 3, 357–368.
- Yamada, D.; Rizvi, S.; Razumilava, N.; Bronk, S.F.; Davila, J.I.; Champion, M.D.; Borad, M.J.; Bezerra, J.A.; Chen, X.; Gores, G.J. IL-33 Facilitates Oncogene-induced Cholangiocarcinoma in Mice by an Interleukin-6-sensitive Mechanism. Hepatology 2015, 61, 1627–1642.
- Meng, F.; Yamagiwa, Y.; Ueno, Y.; Patel, T. Over-Expression of Interleukin-6 Enhances Cell Survival and Transformed Cell Growth in Human Malignant Cholangiocytes. J. Hepatol. 2006, 44, 1055–1065.
- Frampton, G.; Invernizzi, P.; Bernuzzi, F.; Pae, H.Y.; Quinn, M.; Horvat, D.; Galindo, C.; Huang, L.; McMillin, M.; Cooper, B.; et al. Interleukin-6-Driven Progranulin Expression Increases Cholangiocarcinoma Growth by an Akt-Dependent Mechanism. Gut 2012, 61, 268–277.
- Jaiswal, M.; LaRusso, N.F.; Burgart, L.J.; Gores, G.J. Inflammatory Cytokines Induce DNA Damage and Inhibit DNA Repair in Cholangiocarcinoma Cells by a Nitric Oxide-Dependent Mechanism. Cancer Res. 2000, 60, 184–190.
- Ehlken, H.; Schramm, C. Primary Sclerosing Cholangitis and Cholangiocarcinoma: Pathogenesis and Modes of Diagnostics. Dig. Dis. 2013, 31, 118–125.
- Finzi, L.; Shao, M.X.G.; Paye, F.; Housset, C.; Nadel, J.A. Lipopolysaccharide Initiates a Positive Feedback of Epidermal Growth Factor Receptor Signaling by Prostaglandin E2 in Human Biliary Carcinoma Cells. J. Immunol. 2009, 182, 2269–2276.
- Lozano, E.; Sanchez-Vicente, L.; Monte, M.J.; Herraez, E.; Briz, O.; Banales, J.M.; Marin, J.J.G.; Macias, R.I.R. Cocarcinogenic Effects of Intrahepatic Bile Acid Accumulation in Cholangiocarcinoma Development. Mol. Cancer Res. 2014, 12, 91–100.
- Melum, E.; Karlsen, T.H.; Schrumpf, E.; Bergquist, A.; Thorsby, E.; Boberg, K.M.; Lie, B.A. Cholangiocarcinoma in Primary Sclerosing Cholangitis Is Associated with NKG2D Polymorphisms. Hepatology 2007, 47, 90–96.
- Yang, H.; Li, T.W.H.; Peng, J.; Tang, X.; Ko, K.S.; Xia, M.; Aller, M. A Mouse Model of Cholestasis-Associated Cholangiocarcinoma and Transcription Factors Involved in Progression. Gastroenterology 2011, 141, 378–388.e4.
- Voigtländer, T.; Gupta, S.K.; Thum, S.; Fendrich, J.; Manns, M.P.; Lankisch, T.O.; Thum, T. MicroRNAs in Serum and Bile of Patients with Primary Sclerosing Cholangitis and/or Cholangiocarcinoma. PLoS ONE 2015, 10, e0139305.
- Bergquist, A.; Weismüller, T.J.; Levy, C.; Rupp, C.; Joshi, D.; Nayagam, J.S.; Montano-Loza, A.J.; Lytvyak, E.; Wunsch, E.; Milkiewicz, P.; et al. Impact on Follow-up Strategies in Patients with Primary Sclerosing Cholangitis. Liver Int. 2023, 43, 127–138.
- Majeed, A.; Castedal, M.; Arnelo, U.; Söderdahl, G.; Bergquist, A.; Said, K. Optimizing the Detection of Biliary Dysplasia in Primary Sclerosing Cholangitis before Liver Transplantation. Scand. J. Gastroenterol. 2018, 53, 56–63.
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People with Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020, 159, 915–928.
- Ali, A.H.; Tabibian, J.H.; Nasser-Ghodsi, N.; Lennon, R.J.; DeLeon, T.; Borad, M.J.; Hilscher, M.; Silveira, M.G.; Carey, E.J.; Lindor, K.D. Surveillance for Hepatobiliary Cancers in Patients with Primary Sclerosing Cholangitis. Hepatology 2018, 67, 2338–2351.
- Eaton, J.E.; Welle, C.L.; Bakhshi, Z.; Sheedy, S.P.; Idilman, I.S.; Gores, G.J.; Rosen, C.B.; Heimbach, J.K.; Taner, T.; Harnois, D.M.; et al. Early Cholangiocarcinoma Detection with Magnetic Resonance Imaging versus Ultrasound in Primary Sclerosing Cholangitis. Hepatology 2021, 73, 1868–1881.
- Chazouilleres, O.; Beuers, U.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Samyn, M.; Schramm, C.; Trauner, M. EASL Clinical Practice Guidelines on Sclerosing Cholangitis. J. Hepatol. 2022, 77, 761–806.
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD Practice Guidance on Primary Sclerosing Cholangitis and Cholangiocarcinoma. Hepatology 2023, 77, 659–702.
- Nicoletti, A.; Maurice, J.B.; Thorburn, D. Guideline Review: British Society of Gastroenterology/UK-PSC Guidelines for the Diagnosis and Management of Primary Sclerosing Cholangitis. Frontline Gastroenterol. 2021, 12, 62–66.
- Villard, C.; Friis-Liby, I.; Rorsman, F.; Said, K.; Warnqvist, A.; Cornillet, M.; Kechagias, S.; Nyhlin, N.; Werner, M.; Janczewska, I.; et al. Prospective Surveillance for Cholangiocarcinoma in Unselected Individuals with Primary Sclerosing Cholangitis. J. Hepatol. 2023, 78, 604–613.
- Aabakken, L.; Karlsen, T.H.; Albert, J.; Arvanitakis, M.; Chazouilleres, O.; Dumonceau, J.-M.; Färkkilä, M.; Fickert, P.; Hirschfield, G.M.; Laghi, A.; et al. Role of Endoscopy in Primary Sclerosing Cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J. Hepatol. 2017, 66, 1265–1281.
- von Seth, E.; Arnelo, U.; Enochsson, L.; Bergquist, A. Primary Sclerosing Cholangitis Increases the Risk for Pancreatitis after Endoscopic Retrograde Cholangiopancreatography. Liver Int. 2015, 35, 254–262.
- Rupp, C.; Hippchen, T.; Bruckner, T.; Klöters-Plachky, P.; Schaible, A.; Koschny, R.; Stiehl, A.; Gotthardt, D.N.; Sauer, P. Effect of Scheduled Endoscopic Dilatation of Dominant Strictures on Outcome in Patients with Primary Sclerosing Cholangitis. Gut 2019, 68, 2170–2178.
- Bowlus, C.L.; Lim, J.K.; Lindor, K.D. AGA Clinical Practice Update on Surveillance for Hepatobiliary Cancers in Patients with Primary Sclerosing Cholangitis: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 2416–2422.
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. Diagnosis and Management of Primary Sclerosing Cholangitis. Hepatology 2010, 51, 660–678.
- Taghavi, S.A.; Eshraghian, A.; Niknam, R.; Sivandzadeh, G.R.; Bagheri Lankarani, K. Diagnosis of Cholangiocarcinoma in Primary Sclerosing Cholangitis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 575–584.
- Wannhoff, A.; Brune, M.; Knierim, J.; Weiss, K.H.; Rupp, C.; Gotthardt, D.N. Longitudinal Analysis of CA19-9 Reveals Individualised Normal Range and Early Changes before Development of Biliary Tract Cancer in Patients with Primary Sclerosing Cholangitis. Aliment. Pharmacol. Ther. 2019, 49, 769–778.
- Jendrek, S.T.; Gotthardt, D.; Nitzsche, T.; Widmann, L.; Korf, T.; Michaels, M.A.; Weiss, K.-H.; Liaskou, E.; Vesterhus, M.; Karlsen, T.H.; et al. Anti-GP2 IgA Autoantibodies Are Associated with Poor Survival and Cholangiocarcinoma in Primary Sclerosing Cholangitis. Gut 2017, 66, 137–144.
- Saffioti, F.; Roccarina, D.; Vesterhus, M.; Hov, J.R.; Rosenberg, W.; Pinzani, M.; Pereira, S.P.; Boberg, K.M.; Thorburn, D. Cholangiocarcinoma Is Associated with a Raised Enhanced Liver Fibrosis Score Independent of Primary Sclerosing Cholangitis. Eur. J. Clin. Investig. 2019, 49, e13088.
- Cuenco, J.; Wehnert, N.; Blyuss, O.; Kazarian, A.; Whitwell, H.J.; Menon, U.; Dawnay, A.; Manns, M.P.; Pereira, S.P.; Timms, J.F. Identification of a Serum Biomarker Panel for the Differential Diagnosis of Cholangiocarcinoma and Primary Sclerosing Cholangitis. Oncotarget 2018, 9, 17430–17442.
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Pombeiro, I.; Leyh, C.; Benz, F.; Vucur, M.; Longerich, T.; Koch, A.; Braunschweig, T.; et al. Elevated Levels of Circulating Osteopontin Are Associated with a Poor Survival after Resection of Cholangiocarcinoma. J. Hepatol. 2017, 67, 749–757.
- Navaneethan, U.; Parsi, M.A.; Lourdusamy, V.; Bhatt, A.; Gutierrez, N.G.; Grove, D.; Sanaka, M.R.; Hammel, J.P.; Stevens, T.; Vargo, J.J.; et al. Volatile Organic Compounds in Bile for Early Diagnosis of Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis: A Pilot Study. Gastrointest. Endosc. 2015, 81, 943–949.e1.
- Navaneethan, U.; Parsi, M.A.; Gutierrez, N.G.; Bhatt, A.; Venkatesh, P.G.K.; Lourdusamy, D.; Grove, D.; Hammel, J.P.; Jang, S.; Sanaka, M.R.; et al. Volatile Organic Compounds in Bile Can Diagnose Malignant Biliary Strictures in the Setting of Pancreatic Cancer: A Preliminary Observation. Gastrointest. Endosc. 2014, 80, 1038–1045.
- Navaneethan, U.; Parsi, M.A.; Lourdusamy, D.; Grove, D.; Sanaka, M.R.; Hammel, J.P.; Vargo, J.J.; Dweik, R.A. Volatile Organic Compounds in Urine for Noninvasive Diagnosis of Malignant Biliary Strictures: A Pilot Study. Dig. Dis. Sci. 2015, 60, 2150–2157.
- Ishikawa, A.; Sasaki, M.; Sato, Y.; Ohira, S.; Chen, M.-F.; Huang, S.-F.; Oda, K.; Nimura, Y.; Nakanuma, Y. Frequent P16ink4a Inactivation Is an Early and Frequent Event of Intraductal Papillary Neoplasm of the Liver Arising in Hepatolithiasis. Human. Pathol. 2004, 35, 1505–1514.
- Nakamoto, S.; Kumamoto, Y.; Igarashi, K.; Fujiyama, Y.; Nishizawa, N.; Ei, S.; Tajima, H.; Kaizu, T.; Watanabe, M.; Yamashita, K. Methylated Promoter DNA of CDO1 Gene and Preoperative Serum CA19-9 Are Prognostic Biomarkers in Primary Extrahepatic Cholangiocarcinoma. PLoS ONE 2018, 13, e0205864.
- Amornpisutt, R.; Proungvitaya, S.; Jearanaikoon, P.; Limpaiboon, T. DNA Methylation Level of OPCML and SFRP1: A Potential Diagnostic Biomarker of Cholangiocarcinoma. Tumor Biol. 2015, 36, 4973–4978.
- Kim, B.; Cho, N.-Y.; Shin, S.H.; Kwon, H.-J.; Jang, J.J.; Kang, G.H. CpG Island Hypermethylation and Repetitive DNA Hypomethylation in Premalignant Lesion of Extrahepatic Cholangiocarcinoma. Virchows Arch. 2009, 455, 343–351.
- Andresen, K.; Boberg, K.M.; Vedeld, H.M.; Honne, H.; Jebsen, P.; Hektoen, M.; Wadsworth, C.A.; Clausen, O.P.; Lundin, K.E.A.; Paulsen, V.; et al. Four DNA Methylation Biomarkers in Biliary Brush Samples Accurately Identify the Presence of Cholangiocarcinoma. Hepatology 2015, 61, 1651–1659.
- Vedeld, H.M.; Grimsrud, M.M.; Andresen, K.; Pharo, H.D.; von Seth, E.; Karlsen, T.H.; Honne, H.; Paulsen, V.; Färkkilä, M.A.; Bergquist, A.; et al. Early and Accurate Detection of Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis by Methylation Markers in Bile. Hepatology 2022, 75, 59–73.
- Klump, B.; Hsieh, C.-J.; Dette, S.; Holzmann, K.; Kiebetalich, R.; Jung, M.; Sinn, U.; Ortner, M.; Porschen, R.; Gregor, M. Promoter Methylation of INK4a/ARF as Detected in Bile-Significance for the Differential Diagnosis in Biliary Disease. Clin. Cancer Res. 2003, 9, 1773–1778.
- Shin, S.-H.; Lee, K.; Kim, B.-H.; Cho, N.-Y.; Jang, J.-Y.; Kim, Y.-T.; Kim, D.; Jang, J.J.; Kang, G.H. Bile-Based Detection of Extrahepatic Cholangiocarcinoma with Quantitative DNA Methylation Markers and Its High Sensitivity. J. Mol. Diagn. 2012, 14, 256–263.
- Sriraksa, R.; Zeller, C.; El-Bahrawy, M.A.; Dai, W.; Daduang, J.; Jearanaikoon, P.; Chau-in, S.; Brown, R.; Limpaiboon, T. CpG-Island Methylation Study of Liver Fluke-Related Cholangiocarcinoma. Br. J. Cancer 2011, 104, 1313–1318.
- Wasenang, W.; Chaiyarit, P.; Proungvitaya, S.; Limpaiboon, T. Serum Cell-Free DNA Methylation of OPCML and HOXD9 as a Biomarker That May Aid in Differential Diagnosis between Cholangiocarcinoma and Other Biliary Diseases. Clin. Epigenet. 2019, 11, 39.
- Branchi, V.; Schaefer, P.; Semaan, A.; Kania, A.; Lingohr, P.; Kalff, J.C.; Schäfer, N.; Kristiansen, G.; Dietrich, D.; Matthaei, H. Promoter Hypermethylation of SHOX2 and SEPT9 Is a Potential Biomarker for Minimally Invasive Diagnosis in Adenocarcinomas of the Biliary Tract. Clin. Epigenet. 2016, 8, 133.
- Letelier, P.; Riquelme, I.; Hernández, A.; Guzmán, N.; Farías, J.; Roa, J. Circulating MicroRNAs as Biomarkers in Biliary Tract Cancers. Int. J. Mol. Sci. 2016, 17, 791.
- Marin, J.J.G.; Bujanda, L.; Banales, J.M. MicroRNAs and Cholestatic Liver Diseases. Curr. Opin. Gastroenterol. 2014, 30, 303–309.
- Li, L.; Masica, D.; Ishida, M.; Tomuleasa, C.; Umegaki, S.; Kalloo, A.N.; Georgiades, C.; Singh, V.K.; Khashab, M.; Amateau, S.; et al. Human Bile Contains MicroRNA-Laden Extracellular Vesicles That Can Be Used for Cholangiocarcinoma Diagnosis. Hepatology 2014, 60, 896–907.
- Bernuzzi, F.; Marabita, F.; Lleo, A.; Carbone, M.; Mirolo, M.; Marzioni, M.; Alpini, G.; Alvaro, D.; Boberg, K.M.; Locati, M.; et al. Serum microRNAs as Novel Biomarkers for Primary Sclerosing Cholangitis and Cholangiocarcinoma. Clin. Exp. Immunol. 2016, 185, 61–71.
- Loosen, S.H.; Lurje, G.; Wiltberger, G.; Vucur, M.; Koch, A.; Kather, J.N.; Paffenholz, P.; Tacke, F.; Ulmer, F.T.; Trautwein, C.; et al. Serum Levels of miR-29, miR-122, miR-155 and miR-192 Are Elevated in Patients with Cholangiocarcinoma. PLoS ONE 2019, 14, e0210944.
- Correa-Gallego, C.; Maddalo, D.; Doussot, A.; Kemeny, N.; Kingham, T.P.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Betel, D.; Klimstra, D.; et al. Circulating Plasma Levels of MicroRNA-21 and MicroRNA-221 Are Potential Diagnostic Markers for Primary Intrahepatic Cholangiocarcinoma. PLoS ONE 2016, 11, e0163699.
- Miolo, G.; Muraro, E.; Caruso, D.; Crivellari, D.; Ash, A.; Scalone, S.; Lombardi, D.; Rizzolio, F.; Giordano, A.; Corona, G. Pharmacometabolomics Study Identifies Circulating Spermidine and Tryptophan as Potential Biomarkers Associated with the Complete Pathological Response to Trastuzumab-Paclitaxel Neoadjuvant Therapy in HER-2 Positive Breast Cancer. Oncotarget 2016, 7, 39809–39822.
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164, 681–694.
- Banales, J.M.; Iñarrairaegui, M.; Arbelaiz, A.; Milkiewicz, P.; Muntané, J.; Muñoz-Bellvis, L.; La Casta, A.; Gonzalez, L.M.; Arretxe, E.; Alonso, C.; et al. Serum Metabolites as Diagnostic Biomarkers for Cholangiocarcinoma, Hepatocellular Carcinoma, and Primary Sclerosing Cholangitis. Hepatology 2019, 70, 547–562.
- Metzger, J.; Negm, A.A.; Plentz, R.R.; Weismüller, T.J.; Wedemeyer, J.; Karlsen, T.H.; Dakna, M.; Mullen, W.; Mischak, H.; Manns, M.P.; et al. Urine Proteomic Analysis Differentiates Cholangiocarcinoma from Primary Sclerosing Cholangitis and Other Benign Biliary Disorders. Gut 2013, 62, 122–130.
- Lankisch, T.O.; Metzger, J.; Negm, A.A.; Voβkuhl, K.; Siwy, J.; Weismüller, T.J.; Schneider, A.S.; Thedieck, K.; Baumeister, R.; Zürbig, P.; et al. Bile Proteomic Profiles Differentiate Cholangiocarcinoma from Primary Sclerosing Cholangitis and Choledocholithiasis. Hepatology 2011, 53, 875–884.
- Voigtländer, T.; Metzger, J.; Schönemeier, B.; Jäger, M.; Mischak, H.; Manns, M.P.; Lankisch, T.O. A Combined Bile and Urine Proteomic Test for Cholangiocarcinoma Diagnosis in Patients with Biliary Strictures of Unknown Origin. United Eur. Gastroenterol. J. 2017, 5, 668–676.
- Betesh, L.; Comunale, M.A.; Wang, M.; Liang, H.; Hafner, J.; Karabudak, A.; Giama, N.H.; Moser, C.D.; Miyoshi, E.; Roberts, L.R.; et al. Identification of Fucosylated Fetuin-A as a Potential Biomarker for Cholangiocarcinoma. Prot. Clin. Appl. 2017, 11, 1600141.
- Lapitz, A.; Arbelaiz, A.; Olaizola, P.; Aranburu, A.; Bujanda, L.; Perugorria, M.J.; Banales, J.M. Extracellular Vesicles in Hepatobiliary Malignancies. Front. Immunol. 2018, 9, 2270.
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Extracellular Vesicles in Liver Pathobiology: Small Particles with Big Impact. Hepatology 2016, 64, 2219–2233.
- Arbelaiz, A.; Azkargorta, M.; Krawczyk, M.; Santos-Laso, A.; Lapitz, A.; Perugorria, M.J.; Erice, O.; Gonzalez, E.; Jimenez-Agüero, R.; Lacasta, A.; et al. Serum Extracellular Vesicles Contain Protein Biomarkers for Primary Sclerosing Cholangitis and Cholangiocarcinoma. Hepatology 2017, 66, 1125–1143.
- Lapitz, A.; Arbelaiz, A.; O’Rourke, C.J.; Lavin, J.L.; Casta, A.L.; Ibarra, C.; Jimeno, J.P.; Santos-Laso, A.; Izquierdo-Sanchez, L.; Krawczyk, M.; et al. Patients with Cholangiocarcinoma Present Specific RNA Profiles in Serum and Urine Extracellular Vesicles Mirroring the Tumor Expression: Novel Liquid Biopsy Biomarkers for Disease Diagnosis. Cells 2020, 9, 721.
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid Biopsy-Based Protein Biomarkers for Risk Prediction, Early Diagnosis, and Prognostication of Cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108.
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary Tract Cancer. Lancet 2021, 397, 428–444.
- Singhi, A.D.; Nikiforova, M.N.; Chennat, J.; Papachristou, G.I.; Khalid, A.; Rabinovitz, M.; Das, R.; Sarkaria, S.; Ayasso, M.S.; Wald, A.I.; et al. Integrating Next-Generation Sequencing to Endoscopic Retrograde Cholangiopancreatography (ERCP)-Obtained Biliary Specimens Improves the Detection and Management of Patients with Malignant Bile Duct Strictures. Gut 2020, 69, 52–61.
- Arechederra, M.; Rullán, M.; Amat, I.; Oyon, D.; Zabalza, L.; Elizalde, M.; Latasa, M.U.; Mercado, M.R.; Ruiz-Clavijo, D.; Saldaña, C.; et al. Next-Generation Sequencing of Bile Cell-Free DNA for the Early Detection of Patients with Malignant Biliary Strictures. Gut 2022, 71, 1141–1151.
