Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Pediatrics is the field of medicine that centers on physical, social, and mental health from birth to the end of adolescence. Nanotechnology has received enthusiasm among the scientific community, particularly in medicine and pharmaceutical fields, due to its potential to incorporate diagnostic and treatment tools in the same nanocarrier, enhance targetability to specific organs, decrease toxicity, and potentially reduce treatment schedules. At the same time, it provides a tool to increase patient compliance, which is an essential task concerning the pediatric population.
- pediatrics
- nanoparticles
1. Introduction
Pediatrics is the field of medicine that centers on physical, social, and mental health from birth to the end of adolescence [1].
The pediatric population can be subcategorized, according to the “International Council for Harmonization” (ICH) topic E11 (CPMP/ICH/2711/99) and the ICH E11(R1), as preterm newborn infants (from the day of birth to the expected date of birth plus 27 days), term and post-term newborn infants (aged from 0 to 27 days), infants and toddlers (with 28 days to 23 months), children (aged between 2 and 11 years old), and adolescents (with age ranges from 12 to 16–18 years old, depending on region).
However, a considerable overlap can exist across the age subcategories, namely in physical, cognitive, and psychosocial development. Moreover, no consensus seems to exist on the upper age limit of pediatric patients, which may hamper the evaluation and development of age-appropriate treatment plans [2]. In particular, according to the American Academy of Pediatrics (AAP), the upper age limit of pediatrics is considered 21 years, with a proposed subcategorization of adolescence into three main groups: (1) early, represented by adolescents from 11 to 14 years old; (2) middle, for adolescents with ages between 15 and 17 years old; and (3) late adolescence ranging from 18 to 21 years old. However, this age limit has been questioned as increasing evidence has demonstrated that brain development only reaches adult levels of functioning by the third decade of life, which may contribute to the increase in complexity when addressing age-related pathologies and treatments [3].
Historically, the intrinsic heterogeneity in the pediatric population and the reduced number of individuals that can be included per each subcategory in clinical trials may have constituted fatal reasons to dub children as “therapeutic orphans” and for the “off-label” prescription of adult medication to pediatric patients. However, this paradigm has been shifting as it is well recognized that children cannot be considered mini-adults, since the developmental, physiological, and metabolic stages across these two age segments are critically different [4]. The impact on the pharmacokinetics (PK) and pharmacodynamics (PD) of the Active Pharmaceutical Ingredients (API) makes it unreasonable to translate dosage forms and dosage strengths straightforwardly from adults to children [5][6][7].
Therefore, a strategic workforce has been constructed to appropriately reply to disease burden across childhood, addressing the therapeutic deficit and developing age-appropriate formulations, in order to maximize efficacy and design quality, promote safety, minimize risks, and increase patient adherence to treatments [8][9].
Considering the route of administration, the most favored is the oral one. In contrast, the parenteral route remains reserved for more acute conditions, mainly when a quick onset is required [9]. Planning a pediatric oral formulation is challenging, and involves the choice of excipients, dosage form, and palatability [10]. For instance, the choice of dosage form for oral administration depends on the gut function and, thus, on both age and clinical condition [11]. Moreover, the choice of excipients for pediatric drug formulation has been questioned as certain excipients used in adult drug formulation are not adequate for pediatric use, with toxicological risks and safety issues in children [12]. Therefore, the collaboration of the European and the United States Pediatric Formulation Initiatives (PFIs) has resulted in the creation of the “Safety and Toxicity of Excipients for Pediatrics” (STEP) database that aims for the screening of excipients that can appropriately fit pediatric drug formulation [12][13][14]. Furthermore, a set of potentially inappropriate drugs for pediatric use has been released by the “Key Potentially Inappropriate Drugs in Pediatrics” tool, or “KIDs” List, with the primary goal of anticipating risks for adverse drug reactions (ADRs), decreasing severe ADRs, improving the quality of care, decreasing costs, and identifying subjects that need research in the pediatric population [15].
Despite the efforts made in the development of pediatric drug formulation, as well as in age-appropriate medical devices, clinical trials and approved drugs for the pediatric population remain constrained [16][17][18].
Nanotechnology has received enthusiasm among the scientific community, particularly in medicine and pharmaceutical fields, due to its potential to incorporate diagnostic and treatment tools in the same nanocarrier, enhance targetability to specific organs, decrease toxicity, and potentially reduce treatment schedules. At the same time, it provides a tool to increase patient compliance, which is an essential task concerning the pediatric population [19][20][21].
2. Nanomedicine for Pediatric Healthcare
Nanomedicine has emerged through the conjugation of two main fields, namely nanotechnology and medicine. The European Technology Platform on Nanomedicine (ETPN) defined the term nanomedicine as the use of nanotechnology to achieve advances in healthcare by exploiting unique bio and physicochemical properties of materials at the nano scale [22]. On the other hand, the EMA refers to nanomedicine as the application of nanosized components with specific advantageous properties, such as better targeting and bioavailability of therapeutics, new modes of therapeutic action, and nanostructured surfaces/scaffolds for engineered tissues [23]. Among the most studied nanoparticles intended for the prophylaxis, diagnosis, and treatment of diseases are inorganic, lipid-based, and polymeric-based nanoparticles (Figure 1) [19].
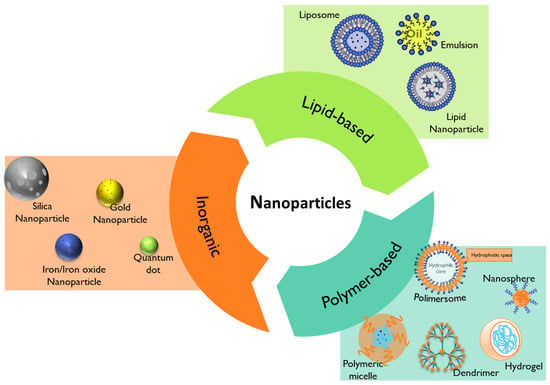
Figure 1. Summary of the different types of nanoparticles that can be used in nanomedicine.
In the field of pediatric medicine, the use of nanomedicine has offered innovative solutions for the diagnosis and treatment of various conditions, particularly in cancer [24][25][26], infection [27], dentistry [28], dermatology [29], and nutrition [30].
2.1. Lipid-Based Nanoparticles
Lipid-based nanoparticles comprise liposomes, lipid nanoparticles, and emulsions (Figure 1) [31]. Their advantageous properties, like biocompatibility, formulation simplicity, and payload flexibility, make them the most highly approved nanomedicines by the FDA [19][31].
Liposomes are typically composed of phospholipids, which can form unilamellar and multilamellar vesicular structures which allow the delivery of hydrophilic, hydrophobic, and lipophilic drugs in the same system. Liposomes can be modified to extend their circulation and enhance delivery, avoiding rapid detection from the reticuloendothelial system (RES) [31].
Nano-emulsions are heterogeneous oil-in-water or water-in-oil emulsions mainly formed by oil droplets containing the API, stabilized by surfactants and cosurfactants and dispersed in an aqueous external phase [19]. They are usually prepared using Generally Recognized as Safe (GRAS)-grade excipients approved by the FDA [32], and possess high loading capacity for lipophilic APIs with some thermodynamically reported instabilities [33].
The development of next-generation lipid nanoparticles, namely solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), has emerged to overcome some limitations of the conventional lipid-based nanosystems [19][34]. Lipid-based nanoparticles like SLN and NLCs can offer the targeted delivery of drugs, increase the bioavailability of hydrophobic drugs, and protect sensitive active compounds [19].
Lipid-based nanoparticles have been widely investigated for various applications, namely in cancer [35][36] and more recently in the formulations of the mRNA COVID-19 nano-vaccines [37], with some of them approved by the FDA for different therapeutic purposes
Among these, liposomes are the most widely studied in pediatrics, and transversal variations in the PK parameters have been registered between the adult and the pediatric populations [38][39].
Furthermore, significant differences between the participation of children (birth–17 years) versus adults in clinical trials using liposomes (clinicalTrial.gov database, data collected by 7 August 2023) have been registered. In fact, of 285 clinical trials that are currently recruiting or not yet recruiting, only 31 include liposomes in pediatrics (birth–17 years), with the majority of them addressing cancer treatment.
Other types of lipid nanoparticles, such as in situ self-assembly nanoparticles (ISNPs), have been investigated. For example, child-friendly Lopinavir/Ritonavir pediatric granules utilizing ISNPs were developed. In vivo pre-clinical data demonstrated that the orally administered formulation improved lopinavir bioavailability and concentration in the brain and lymphoid tissues, the target sites of the HIV [40]. In another study, Rodríguez-Nogales et al. formulated nano-assemblies using squalenoyl-gemcitabine and alkyl-lysophospholipid edelfosine with a nanoprecipitation method. Their results revealed that the 50 nm nanoparticles presented a high uptake by human osteosarcoma cells, resulting in antitumoral activity and enhanced gemcitabine and edelfosine pharmacokinetic profiles [41].
2.2. Polymer-Based Nanoparticles
Polymer-based nanoparticles are colloidal systems made up of natural, semi-synthetic, or synthetic polymers (Figure 2), allowing for a wide variety of possible architectures and characteristics [19][39]. They include dendrimers, polymeric micelles, polymersomes, nanospheres, and nanogels (Figure 1) with diverse clinical applications [19]. Usually, natural polymers present fewer toxic effects than synthetic polymers [42].
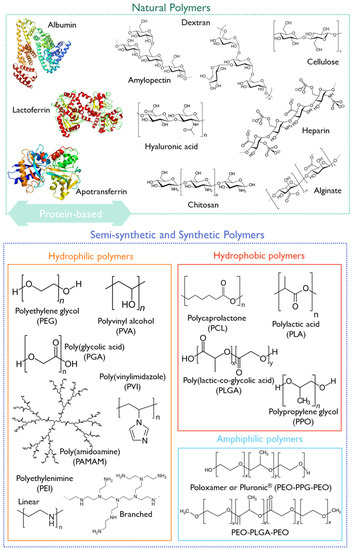
Figure 2. Structural representation of some natural, semi-synthetic, and synthetic polymers.
They can be biodegradable or non-biodegradable. As biodegradable polymers undergo biodegradation in vivo through enzymatic or non-enzymatic pathways producing biocompatible or harmless by-products, they have been preferred in nanomedicine, particularly for pediatrics [39]. The performance of polymeric biodegradable formulations can be improved by (1) using FDA-approved biodegradable polymers, (2) administering the formulations in situ, (3) using combined therapies, such as immunotherapy or radiotherapy, and (4) applying the on-demand delivery of molecularly targeted agents [43].
Some examples of biodegradable polymers are polysaccharides, such as hyaluronic acid, chitosan, dextrin, or alginate (Figure 2).
Chitosan is a natural biocompatible and biodegradable cationic polymer with low toxicity. It is based on deacetylated chitin [44] obtained from crustaceans, insects, squibs-centric diatoms, or fungi [45]. At an acidic pH, chitosan presents a high density of positive charges that deliver mucoadhesive properties, and a suitable environment for complexing anionic polymers or nucleic acids [46]. Moreover, it can entrap poorly water-soluble drugs, combining antimicrobial, anti-inflammatory, and wound-healing effects [47]. This polymer has been classified by the FDA as GRAS [48], and is approved as a biomaterial for use in tissue engineering and drug delivery applications [49].
However, concerns regarding the source, purity, and immunogenicity of chitosan have hampered its approval for pharmaceutical applications [49].
Hyaluronic acid (HA) is a mucopolysaccharide present in the extracellular matrix, synovial fluid, and connective tissues, consisting of D-glucuronic acid and (1-b-3) N-acetyl-D-glucosamine alternating units (Figure 2) [43]. HA is biocompatible, non-immunogenic, and biodegradable, and presents a viscoelastic nature, making it suitable for nanomedicine applications [43]. Cluster of differentiation-44 (CD44) is a main receptor of HA and is overexpressed in solid tumors, making it suitable for cancer-targeting purposes [50]. Due to its versatile properties, HA has been studied for pediatric drug formulations, aiming at increased patient compliance through the modification of the dosage form or by decreasing the dosing frequency [51][52][53]. Moreover, HA has already undergone clinical trials, with 91 registered entries addressing the pediatric population (birth to 17 years).
Another group of natural polymers is the protein-based biomaterials, such as albumin, lactoferrin, or apotransferrin (Figure 2).
Albumin is a water-soluble globular protein present in ca. 50% of the total plasma body mass. Due to its hemocompatibility, albumin has been applied for intravenous gene and drug delivery. Consequently, an albumin-based nanosystem for the delivery of paclitaxel (Abraxane®) received FDA approval in 2005. According to the information approved by the FDA in 2020 (Reference ID: 4661467), the safety and effectiveness of Abraxane® have not been established in pediatric patients so far. However, in 2013, a Phase 1/2 clinical trial (NCT01962103) was begun aiming to find the safe dose of nab-paclitaxel, Abraxane®, in children with solid tumors, and to see if it could constitute a treatment for children and young adults with solid tumors (1 ≤ 18 years old in Phase 1 and 2 ≤ 24 years old in Phase 2).
Lactoferrin (LF) is a natural cationic iron-binding glycoprotein present in milk, with antiviral, anti-inflammatory, antioxidant, anti-cancer, and immune-stimulating effects [54][55]. LF receptors are known to be overexpressed in cancer and endothelial brain cells, making them suitable for active tumor targeting or crossing the blood–brain barrier (BBB) via receptor-mediated transcytosis for brain delivery. In addition, LF-based nanocarriers were found to have a pH-dependent release profile. At an acidic pH, a faster drug release is observed, which could increase drug release in acidic sites such as the tumor tissue microenvironment and could enhance the therapeutic efficacy of the encapsulated hydrophobic active molecules [54][56]. Commercial preparations of bovine lactoferrin, recognized as GRAS by the FDA, are commonly used in in vitro and in vivo testing. Recently, recombinant human lactoferrin has also become available [57]. Ahmed et al. [58] developed LF-based nanoparticles containing carboplatin to address retinoblastoma in children. Apotransferrin-based nanoparticles were also prepared as they are also implicated in iron transport [58][59]. In another study, Narayana et al. developed carboplatin and etoposide-loaded LF nanoparticles to address retinoblastoma treatment in vitro [60].
Semi-synthetic or synthetic polymers have also been exploited for pediatric applications. The FDA-approved synthetic polymer PEG is widely used due to its biocompatibility and biodegradability [39][61]. It is often combined with other more hydrophobic polymers or other API nanocarriers since it provides stealth properties and improves the pharmacological properties of nanomedicines. However, some allergic reactions were reported when using PEG as an excipient in pediatric drug formulation, which may limit its use.
Polycaprolactone (PCL) is recognized as non-toxic and suitable for controlled/sustained drug and vaccine delivery owing to its high permeability in relation to drugs [43]. Conjugates of PLC with PEG have recently been reviewed [62]. Krishnan et al. produced PEG-PCL nanoparticles using the nanoprecipitation method, aiming at treating leukemia in the pediatric population. The in vivo results have demonstrated improved life quality and survival in mice in the dexamethasone-loaded nanoparticles group compared to the free drug group [63].
The FDA-approved polymer poly lactic-co-glycolic acid (PLGA) has shown suitable properties for drug delivery, with improved circulation time and permeability. PLGA is an aliphatic polyester polymer that comprises a synthetic copolymer of lactic acid (α-hydroxy propanoic acid) and glycolic acid (hydroxy acetic acid) with demonstrated potential for drug delivery and tissue engineering scaffolds [64]. The 50:50 ratio of lactic to glycolic acid monomers and molecular weight PLGA (3–9 kDa) have been associated with decreased half-time and fastest degradation [38]. PLGA-PEG nanoparticles have been synthesized and decorated with a CD133 aptamer to target salinomycin delivery to CD133+ pediatric osteosarcoma cancer stem cells [65].
Other synthetic polymers (Figure 2), such as polyethyleneimine (PEI), poly(vinylimidazole) (PVI), or poly(amidoamine) (PAMAM).
Due to their versatility, the arrangement of different polymers can result in different nanoparticle architectures. The following sections will give a brief overview of the use of polymeric micelles and dendrimers in pediatric nanomedicine.
2.2.1. Polymeric Micelles
Polymeric micelles (Figure 1) exhibit versatile features as drug carriers and as active ingredients [66][67]. Polymeric micelles are usually characterized as a core–shell structures developed through the self-assembly of amphiphilic block copolymers in an aqueous solution, with attractive flexibility for functionalization [68]. For instance, the use of amphiphilic-block co-polymers, such as Pluronic® (Figure 2) and Tetronic® surfactants, can form polymeric micelles above the critical micellar concentration/temperature with singular features [68][69]. The use of Pluronic® mixed micelles based on F127 and P123 surfactants was reported for curcumin incorporation to treat pediatric osteosarcoma [70].
To date, some polymeric micelles-based nanomedicines have reached the market, such as Genexol-PM®, Nanoxel-PMTM, and Paclical® [71][72]. Genexol-PM® is a polymeric micellar formulation of paclitaxel, composed of the low-molecular-weight amphiphilic diblock copolymer, monomethoxy poly (ethylene glycol)-block-poly(D,L-lactide) (mPEG-PDLLA) [73], that was approved for the treatment of metastatic breast cancer, non-small cell lung cancer (NSCLC), and ovarian cancer in South Korea, Philippines, India, and Vietnam [71]. On the other hand, NanoxelTM, DO/NDR/02, is a micellar formulation that consists of a di-block copolymer (poly-(vinylpyrrolidone)-b–poly-(N-isopropyl acrylamide) (PVP-b-PNIPAAM) with paclitaxel as the API [72]. It is a liquid formulation approved for storage at 2 to 8 °C, while Genexol-PM® is commercially available as a lyophilized powder [72]. NanoxelTM has been approved by the Drug Controller General of India since 2006, for the treatment of metastatic breast cancer, NSCLC, and AIDS-related Kaposi Sarcoma patients [74]. Paclical®, in certain countries Apealea®, is a CremophorEL-free paclitaxel formulation based on a XR17 micelle platform technology. It received market authorization from the EMA in November 2018 (EMA/791927/2018) to treat women with ovarian cancer.
2.2.2. Dendrimers
Dendrimers (Figure 1) are hyperbranched three-dimensional polymeric nanostructures with functional moieties in the cavities and at the surface [43]. Polyester dendrimers are termed “smart carriers” for drug delivery applications, as they can be tailored for the complete release of their payloads in a specific environment, reducing the side-effects [75].
Dendrimers can be used for transdermal drug delivery as a substitute route of administration due to the reported unpleasant feedback when taken in oral dosage forms and for nauseated and unconscious patients [43]. Dendrimer uptake was analyzed 24 h after intravenous administration in rabbits, and less than 5% of the injected dose remained in circulation, with over 90% cleared out. G4-OH dendrimers are 4 nm in size and are expected to clear out via the kidney. In this model, dendrimers were not seen in the glomerulus 24 h after administration [38]. The use of ruthenium-terminated carbosilane dendrimers (CRD) significantly decreased the viability of pediatric leukemia cells (1301) with low toxicity for non-cancer cells (peripheral blood mononuclear cells—PBMCs) [76]. Moreover, Chittasupho et al. [77] formulated a CXCR4-targeted PAMAM dendrimer that decreased the migration and viability of an established B-cell-precursor-leukemia cell line derived from an adolescent male (NALM-6).
2.3. Inorganic Nanoparticles
Inorganic nanoparticles encompass metal nanoparticles (iron, gold, silver, and zinc) or rare-earth metal nanoparticles (lanthanum oxide, La2O3 or ytterbium oxide, Yb2O3) and silica nanoparticles, among others [19]. They have been widely used to diagnose and treat atherosclerosis or cancer [19]. The FDA has approved some inorganic nanoparticles intended for iron replacement therapies or for treating anemia and associated diseases. Among them, Venofer® and Ferrlecit® have been studied for pediatric interventions. Venofer® is an iron oxide nanoparticle coated with sucrose used for the slow dissolution of iron following intravenous injection, preventing a rapid and toxic increase in free iron in the blood. Ferrlecit® is a stable macromolecular complex of sodium ferric gluconate in sucrose [38].
Ongoing research in this field has highlighted the possible application of inorganic nanoparticles in diagnosing, treating, and monitoring pediatric brain tumors [78] and other pathologies [79]. Moreover, the application of hybrid nanoparticles has also revealed promising features [80]. For example, the use of Angiopep-2 (An)-PEG-doxorubicin (DOX)-gold nanoparticles (AuNPs) could penetrate the BBB and target glioma cells [80].
2.4. Challenges in Using Nanotherapy in Pediatrics
As reviewed by us previously, the bright side of the coin in the application of nanotechnology in medicine may obscure dark shadows and it should further evolve as an auxiliary to circumvent troubleshooting in nanomedicine [19]. These challenges may impact not only the adult population, but particularly the pediatric population, as limited information for this age group is available [19][81]. Moreover, most preclinical studies to assess the impact of the physicochemical properties of nanosystems are conducted in adult models after intravenous administration, while the preferential route of administration for pediatrics is p.o. [82]. Additionally, the evaluation of the PK parameters of the nanoformulations could also be hindered, as reviewed elsewhere [38]. Other issues regarding the application of nanotherapies in pediatrics are transversal to those present for different dosage forms. However, here it is more evident because the topic of nanomedicine is more recent, and there is a vast unknown to explore [83].
In Figure 3, a snapshot of the main issues that remain to be overcome in using nanotherapies in the pediatric age is presented.
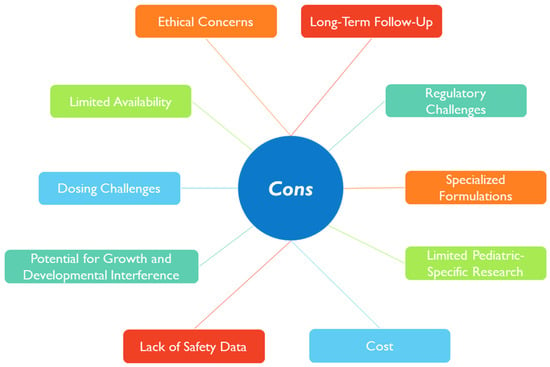
Figure 3. Summary of some issues that remain in developing nanotherapies for pediatric patients.
When designing a nanomedicine intended for pediatric application, it would be beneficial to consider some of these points, particularly regarding the safety and efficacy that could contribute to long-term effects [84]. It would also be relevant to study how environmental exposure to nanoparticles could impact children’s health, development, and their treatment response [85].
Moreover, ethical concerns regarding informed consent in this age group for enrollment in clinical trials, the lack of public understanding of nanotechnology, and socioeconomic issues may also limit the studies using nanoparticles in children [79]. The pros and cons of nanomedicine should cross all stages during the nanomedicine design and development, focusing on the well-being and the best interest of children.
Taking into account potential benefits, nanomedicine has been dubbed, together with ATMPs, as the “therapies for the future” by the European Parliament [86].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15102431
References
- Rimsza, M.E.; Hotaling, C.A.J.; Keown, M.E.; Marcin, J.P.; Moskowitz, W.B.; Sigrest, T.D.; Simon, H.K. Definition of a Pediatrician. Pediatrics 2015, 135, 780–781.
- Sawyer, S.M.; McNeil, R.; Francis, K.L.; Matskarofski, J.Z.; Patton, G.C.; Bhutta, Z.A.; Esangbedo, D.O.; Klein, J.D. The age of paediatrics. Lancet Child Adolesc. Health 2019, 3, 822–830.
- Hardin, A.P.; Hackell, J.M.; Simon, G.R.; Boudreau, A.D.A.; Baker, C.N.; Barden, G.A.; Meade, K.E.; Moore, S.B.; Richerson, J.; Brown, O.W.; et al. Age limit of pediatrics. Pediatrics 2017, 140, e20172151.
- Maheshwari, M.; Sanwatsarkar, S.; Katakwar, M. Pharmacology related to paediatric anaesthesia. Indian J. Anaesth. 2019, 63, 698.
- Ernest, T.B.; Elder, D.P.; Martini, L.G.; Roberts, M.; Ford, J.L. Developing paediatric medicines: Identifying the needs and recognizing the challenges. J. Pharm. Pharmacol. 2010, 59, 1043–1055.
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418.
- O’Brien, F.; Clapham, D.; Krysiak, K.; Batchelor, H.; Field, P.; Caivano, G.; Pertile, M.; Nunn, A.; Tuleu, C. Making medicines baby size: The challenges in bridging the formulation gap in neonatal medicine. Int. J. Mol. Sci. 2019, 20, 2688.
- Walsh, J.; Schaufelberger, D.; Iurian, S.; Klein, S.; Batchelor, H.; Turner, R.; Gizurarson, S.; Boltri, L.; Alessandrini, E.; Tuleu, C. Path towards Efficient Paediatric Formulation Development Based on Partnering with Clinical Pharmacologists and Clinicians, a Conect4children Expert Group white Paper; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2021; p. bcp.14989.
- Vieira, I.; Sousa, J.J.; Vitorino, C. Paediatric Medicines—Regulatory Drivers, Restraints, Opportunities and Challenges. J. Pharm. Sci. 2021, 110, 1545–1556.
- Ogbonna, J.D.N.; Cunha, E.; Attama, A.A.; Ofokansi, K.C.; Ferreira, H.; Pinto, S.; Gomes, J.; Marx, Í.M.G.; Peres, A.M.; Lobo, J.M.S.; et al. Overcoming Challenges in Pediatric Formulation with a Patient-Centric Design Approach: A Proof-of-Concept Study on the Design of an Oral Solution of a Bitter Drug. Pharmaceuticals 2022, 15, 1331.
- World Health Organization (WHO). Development of Paediatric Medicines: Points to Consider in Formulation. Available online: https://www.who.int/publications/m/item/trs970-annex-5-development-of-paediatric-medicines-points-to-consider-in-formulation (accessed on 12 July 2023).
- Salunke, S.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database. Part 1—A need assessment study. Int. J. Pharm. 2012, 435, 101–111.
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the paediatric population: A review. Pharmaceutics 2021, 13, 387.
- Salunke, S.; Brandys, B.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: Part 2—The pilot version. Int. J. Pharm. 2013, 457, 310–322.
- Meyers, R.S.; Thackray, J.; Matson, K.L.; McPherson, C.; Lubsch, L.; Hellinga, R.C.; Hoff, D.S. Key potentially inappropriate drugs in pediatrics: The KIDs list. J. Pediatr. Pharmacol. Ther. 2020, 25, 175–191.
- Rose, K.; Grant-Kels, J.M. The Meanings of “Pediatric Drug Development”. Ther. Innov. Regul. Sci. 2019, 53, 767–774.
- Nishiwaki, S.; Ando, Y. Gap between pediatric and adult approvals of molecular targeted drugs. Sci. Rep. 2020, 10, 17145.
- Espinoza, J.C. The Scarcity of Approved Pediatric High-Risk Medical Devices. JAMA Netw. Open 2021, 4, e2112760.
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano 2022, 16, 9994–10041.
- Marques, M.S.; Lima, L.A.; Poletto, F.; Contri, R.V.; Kulkamp Guerreiro, I.C. Nanotechnology for the treatment of paediatric diseases: A review. J. Drug Deliv. Sci. Technol. 2022, 75, 103628.
- Pires, L.R.; Vinayakumar, K.B.; Turos, M.; Miguel, V.; Gaspar, J. A Perspective on Microneedle-Based Drug Delivery and Diagnostics in Paediatrics. J. Pers. Med. 2019, 9, 49.
- Fornaguera, C.; García-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12.
- Ehmann, F.; Sakai-Kato, K.; Duncan, R.; Pérez De La Ossa, D.H.; Pita, R.; Vidal, J.M.; Kohli, A.; Tothfalusi, L.; Sanh, A.; Tinton, S.; et al. Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine 2013, 8, 849–856.
- Yan, H.; Zhai, B.; Yang, F.; Chen, Z.; Zhou, Q.; Paiva-Santos, A.C.; Yuan, Z.; Zhou, Y. Nanotechnology-Based Diagnostic and Therapeutic Strategies for Neuroblastoma. Front. Pharmacol. 2022, 13, 908713.
- Rodríguez-Nogales, C.; González-Fernández, Y.; Aldaz, A.; Couvreur, P.; Blanco-Prieto, M.J. Nanomedicines for pediatric cancers. ACS Nano 2018, 12, 7482–7496.
- Yang, S.; Wallach, M.; Krishna, A.; Kurmasheva, R.; Sridhar, S. Recent Developments in Nanomedicine for Pediatric Cancer. J. Clin. Med. 2021, 10, 1437.
- Rubey, K.M.; Brenner, J.S. Nanomedicine to fight infectious disease. Adv. Drug Deliv. Rev. 2021, 179, 113996.
- Acharya, S.; Godhi, B.S.; Saha, S.; Singh, B.; Dinsa, K.; Bhagchandani, J.; Gautam, A. Use of nanoparticles in pediatric dentistry: A narrative review. J. Int. Oral Health 2022, 14, 357.
- Delouise, L.A. Applications of Nanotechnology in Dermatology. J. Investig. Dermatol. 2012, 132, 964–975.
- Trandafir, L.M.; Dodi, G.; Frasinariu, O.; Luca, A.C.; Butnariu, L.I.; Tarca, E.; Moisa, S.M. Tackling Dyslipidemia in Obesity from a Nanotechnology Perspective. Nutrients 2022, 14, 3774.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M.M. Nanoemulsions in Translational Research—Opportunities and Challenges in Targeted Cancer Therapy. AAPS PharmSciTech 2014, 15, 694.
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821.
- Basso, J.; Mendes, M.; Cova, T.; Sousa, J.; Pais, A.; Fortuna, A.; Vitorino, R.; Vitorino, C. A Stepwise Framework for the Systematic Development of Lipid Nanoparticles. Biomolecules 2022, 12, 223.
- Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating Nanotechnology into Cancer Care. ACS Nano 2019, 13, 7370–7376.
- Wang, G.; Zannikou, M.; Lofchy, L.; Li, Y.; Gaikwad, H.; Balyasnikova, I.V.; Simberg, D. Liposomal Extravasation and Accumulation in Tumors as Studied by Fluorescence Microscopy and Imaging Depend on the Fluorescent Label. ACS Nano 2021, 15, 11880–11890.
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles from Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015.
- Yellepeddi, V.K.; Joseph, A.; Nance, E. Pharmacokinetics of nanotechnology-based formulations in pediatric populations. Adv. Drug Deliv. Rev. 2019, 151–152, 44–55.
- Nieto González, N.; Obinu, A.; Rassu, G.; Giunchedi, P.; Gavini, E. Polymeric and Lipid Nanoparticles: Which Applications in Pediatrics? Pharmaceutics 2021, 13, 670.
- Pham, K.; Li, D.; Guo, S.; Penzak, S.; Dong, X. Development and in vivo evaluation of child-friendly lopinavir/ritonavir pediatric granules utilizing novel in situ self-assembly nanoparticles. J. Control Release 2016, 226, 88–97.
- Rodríguez-Nogales, C.; Mura, S.; Couvreur, P.; Blanco-Prieto, M.J. Squalenoyl-gemcitabine/edelfosine nanoassemblies: Anticancer activity in pediatric cancer cells and pharmacokinetic profile in mice. Int. J. Pharm. 2020, 582, 119345.
- Bhatia, S. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–225.
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191.
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975.
- Kaur, M.; Sharma, A.; Puri, V.; Aggarwal, G.; Maman, P.; Huanbutta, K.; Nagpal, M.; Sangnim, T. Chitosan-Based Polymer Blends for Drug Delivery Systems. Polymers 2023, 15, 2028.
- Verma, A.; Sharma, G.; Jain, A.; Tiwari, A.; Saraf, S.; Panda, P.K.; Katare, O.P.; Jain, S.K. Systematic optimization of cationic surface engineered mucoadhesive vesicles employing Design of Experiment (DoE): A preclinical investigation. Int. J. Biol. Macromol. 2019, 133, 1142–1155.
- Nerli, G.; Gonçalves, L.M.D.; Cirri, M.; Almeida, A.J.; Maestrelli, F.; Mennini, N.; Mura, P.A. Design, Evaluation and Comparison of Nanostructured Lipid Carriers and Chitosan Nanoparticles as Carriers of Poorly Soluble Drugs to Develop Oral Liquid Formulations Suitable for Pediatric Use. Pharmaceutics 2023, 15, 1305.
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313.
- Kantak, M.N.; Bharate, S.S. Analysis of clinical trials on biomaterial and therapeutic applications of chitosan: A review. Carbohydr. Polym. 2022, 278, 118999.
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618.
- Pereira, M.; Silva, F.C.; Simões, S.; Ribeiro, H.M.; Almeida, A.J.; Marto, J. Innovative, Sugar-Free Oral Hydrogel as a Co-administrative Vehicle for Pediatrics: A Strategy to Enhance Patient Compliance. AAPS PharmSciTech 2022, 23, 691936.
- Laffleur, F. Novel adhesive hyaluronic acid based solid dosage form for pediatric application. J. Drug Deliv. Sci. Technol. 2018, 44, 213–219.
- Di Cicco, M.; Peroni, D.; Sepich, M.; Tozzi, M.G.; Comberiati, P.; Cutrera, R. Hyaluronic acid for the treatment of airway diseases in children: Little evidence for few indications. Pediatr. Pulmonol. 2020, 55, 2156–2169.
- Sabra, S.; Agwa, M.M. Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int. J. Biol. Macromol. 2020, 164, 1046.
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.Y.; Elkhodairy, K.A. Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 2020, 263, 120355.
- Guzmán-Mejía, F.; Godínez-Victoria, M.; Molotla-Torres, D.E.; Drago-Serrano, M.E. Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus. Pharmaceuticals 2023, 16, 214.
- Janicka, M.; Tomaszewska, M.; Ranoszek-Soliwoda, E.; Celichowski, K.; Grobelny, G.; Szymanski, J.; Conte, M.P.; Rosa, L.; Krzyzowska, M.; Janicka, M.; et al. Lactoferrin-Conjugated Nanoparticles as New Antivirals. Pharmaceutics 2022, 14, 1862.
- Ahmed, F.; Ali, M.J.; Kondapi, A.K. Carboplatin loaded protein nanoparticles exhibit improve anti-proliferative activity in retinoblastoma cells. Int. J. Biol. Macromol. 2014, 70, 572–582.
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology for Pediatric Retinoblastoma Therapy. Pharmaceuticals 2022, 15, 1087.
- Narayana, R.V.L.; Jana, P.; Tomar, N.; Prabhu, V.; Nair, R.M.; Manukonda, R.; Kaliki, S.; Coupland, S.E.; Alexander, J.; Kalirai, H.; et al. Carboplatin- and Etoposide-Loaded Lactoferrin Protein Nanoparticles for Targeting Cancer Stem Cells in Retinoblastoma In Vitro. Investig. Ophthalmol. Vis. Sci. 2021, 62, 13.
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone Nanoparticles as Promising Candidates for Nanocarriers in Novel Nanomedicines. Pharmaceutics 2021, 13, 191.
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control Release 2017, 260, 46–60.
- Krishnan, V.; Xu, X.; Barwe, S.P.; Yang, X.; Czymmek, K.; Waldman, S.A.; Mason, R.W.; Jia, X.; Rajasekaran, A.K. Dexamethasone-loaded Block Copolymer Nanoparticles Induce Leukemia Cell Deathand Enhances Therapeutic Efficacy: A Novel Application in PediatricNanomedicine. Mol. Pharm. 2013, 10, 2199.
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377.
- Ni, M.Z.; Xiong, M.; Zhang, X.C.; Cai, G.P.; Chen, H.W.; Zeng, Q.M.; Yu, Z.C. Poly(lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. Int. J. Nanomed. 2015, 10, 2537–2554.
- Jarak, I.; Varela, C.L.; Tavares da Silva, E.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. Eur. J. Med. Chem. 2020, 206, 112526.
- De Castro, K.C.; Coco, J.C.; dos Santos, É.M.; Ataide, J.A.; Martinez, R.M.; do Nascimento, M.H.M.; Prata, J.; da Fonte, P.R.M.L.; Severino, P.; Mazzola, P.G.; et al. Pluronic® triblock copolymer-based nanoformulations for cancer therapy: A 10-year overview. J. Control Release 2023, 353, 802–822.
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700.
- Domingues, C.; Alvarez-Lorenzo, C.; Concheiro, A.; Veiga, F.; Figueiras, A. Nanotheranostic Pluronic-Like Polymeric Micelles: Shedding Light into the Dark Shadows of Tumors. Mol. Pharm. 2019, 16, 4757–4774.
- Khodaei, A.; Jahanmard, F.; Madaah Hosseini, H.R.; Bagheri, R.; Dabbagh, A.; Weinans, H.; Amin Yavari, S. Controlled temperature-mediated curcumin release from magneto-thermal nanocarriers to kill bone tumors. Bioact. Mater. 2022, 11, 107–117.
- Jia, Y.; Jiang, Y.; He, Y.; Zhang, W.; Zou, J.; Magar, K.T.; Boucetta, H.; Teng, C.; He, W. Approved Nanomedicine against Diseases. Pharmaceutics 2023, 15, 774.
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495.
- Werner, M.E.; Cummings, N.D.; Sethi, M.; Wang, E.C.; Sukumar, R.; Moore, D.T.; Wang, A.Z. Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 463.
- Ranade, A.A.; Joshi, D.A.; Phadke, G.K.; Patil, P.P.; Kasbekar, R.B.; Apte, T.G.; Dasare, R.R.; Mengde, S.D.; Parikh, P.M.; Bhattacharyya, G.S.; et al. Clinical and economic implications of the use of nanoparticle paclitaxel (Nanoxel) in India. Ann. Oncol. 2013, 24, v6–v12.
- Huang, D.; Wu, D. Biodegradable dendrimers for drug delivery. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 90, 713–727.
- Michlewska, S.; Ionov, M.; Szwed, A.; Rogalska, A.; Del Olmo, N.S.; Ortega, P.; Denel, M.; Jacenik, D.; Shcharbin, D.; de la Mata, F.J.; et al. Ruthenium Dendrimers against Human Lymphoblastic Leukemia 1301 Cells. Int. J. Mol. Sci. 2020, 21, 4119.
- Chittasupho, C.; Aonsri, C.; Imaram, W. Targeted dendrimers for antagonizing the migration and viability of NALM-6 lymphoblastic leukemia cells. Bioorg. Chem. 2021, 107, 104601.
- Guido, C.; Baldari, C.; Maiorano, G.; Mastronuzzi, A.; Carai, A.; Quintarelli, C.; De Angelis, B.; Cortese, B.; Gigli, G.; Palamà, I.E. Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers. Diagnostics 2022, 12, 173.
- Mfoafo, K.; Tuleu, C.; Hanning, S.; Omidian, H.; Mfoafo, K. Exploring the Potential of Nanotechnology in Pediatric Healthcare: Advances, Challenges, and Future Directions. Pharmaceutics 2023, 15, 1583.
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435.
- Sosnik, A.; Carcaboso, A.M. Nanomedicines in the future of pediatric therapy. Adv. Drug Deliv. Rev. 2014, 73, 140–161.
- Morford, L.L.; Bowman, C.J.; Blanset, D.L.; Bøgh, I.B.; Chellman, G.J.; Halpern, W.G.; Weinbauer, G.F.; Coogan, T.P. Preclinical safety evaluations supporting pediatric drug development with biopharmaceuticals: Strategy, challenges, current practices. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2011, 92, 359–380.
- Rasool, M.; Malik, A.; Waquar, S.; Arooj, M.; Zahid, S.; Asif, M.; Shaheen, S.; Hussain, A.; Ullah, H.; Gan, S.H. New challenges in the use of nanomedicine in cancer therapy. Bioengineered 2022, 13, 759.
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.S.; Buerki-Thurnherr, T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part. Fibre Toxicol. 2020, 17, 31.
- Ahmad, A. Safety and Toxicity Implications of Multifunctional Drug Delivery Nanocarriers on Reproductive Systems In Vitro and In Vivo. Front. Toxicol. 2022, 4, 895667.
- Scientific Foresight (STOA). Therapies for the Future—Advanced Therapies & Nanomedicine. Available online: https://epthinktank.eu/2017/11/22/therapies-for-the-future-advanced-therapies-nanomedicine/ (accessed on 17 July 2023).
This entry is offline, you can click here to edit this entry!
