Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pediatrics
Puberty identifies the transition from childhood to adulthood. Precocious puberty is the onset of signs of pubertal development before age eight in girls and before age nine in boys, it has an incidence of 1/5000–1/10,000 with an F:M ratio ranging from 3:1 to 20:1. Precocious puberty can be divided into central, also known as gonadotropin-dependent precocious puberty or true precocious puberty, and peripheral, also recognized as gonadotropin-independent precocious puberty or precocious pseudopuberty.
- precocious puberty
- pseudopuberty
- children
- pediatrician
- GnRh analogues
1. Introduction
Puberty is the transitional period from childhood to adulthood characterized by major physical and psychological modifications leading to the development of secondary sexual characteristics, the maturation of the gonads and the achievement of reproductive capacity [1]. Puberty is a complex process characterized by environmental, genetic, geographical and metabolic factors [2]. The mechanism underlying pubertal activation remains unknown, although the following have been identified as possible causes: adrenal activation, physical and psychological stress, an abundance of adipose tissue and the inflammation of the intestinal tract [3,4].
Puberty begins with the activation of the hypothalamic-pituitary-gonadal axis (HPG), which already occurs during fetal life, but is usually silenced in the final period of pregnancy and then reactivated immediately after birth [5,6]. This post-natal transitory activation is defined as mini-puberty and lasts up to 6 months in boys and up to 2 years in girls, until the blockage of gonadotropin-releasing hormone (GnRH) secretion, which will resume during puberty [7,8].
The beginning of puberty is determined by the secretion of GnRH at the hypothalamic level, which in turn activates the production of two hormones by the pituitary gland, the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [9]. LH and FSH act on the gonads, promoting gametogenesis [10]. Pubertal development is considered physiological when it begins between the ages of 8 and 13 in females and between 9 and 14 years in males, although it is a variable process within each individual, lasting on average between 3 and 5 years [2,10].
1.1. Precocious Puberty
Precocious puberty (PP) is the onset of secondary sexual features before the age of eight in girls and before the age of nine in boys. Specifically, the first sign of pubertal activation is represented by thelarche in females and an increase in testicular volume in males greater than or equal to 4 mL [1,10]. It is a relatively rare condition affecting 1:5000–1:10,000 children, with an F:M ratio ranging from 3:1 to 20:1 [11,12]. There has been an increase in diagnoses of precocious puberty partly attributable to the SARS-CoV-2 pandemic, probably related to a sedentary lifestyle characterized by being overweight, the use of electronic devices and stress-related symptoms acting as endocrine disruptors [13,14,15,16,17].
PP can be classified as follows:
- -
-
central or true precocious puberty (CPP), if it is determined by early activation of the HPG axis with the production of gonadotropins;
- -
-
peripheral or precocious pseudopuberty (PPP), unrelated to the production of gonadotropins.
1.2. Central Precocious Puberty
CPP accounts for about 80% of all forms of PP and is caused by early activation of the HPG axis with increased GnRH secretion and gonadal activation [3,18]. Although rarer, CPP in males is more often related to underlying hypothalamic-pituitary organic lesions [7]. Although it is often idiopathic, numerous genetic mutations related to CPP have been identified, among these, the loss of function mutation of the MKRN3 gene is one of the most involved [12]. The loss of function of the MKRN3 gene, located within the Prader-Willi syndrome region on chromosome 15q11.2, is responsible for a stimulatory action on GnRH secretion [19,20].
CPP could have a familial form in almost one quarter of the children [21]. The detection of this inherited condition increased after the discovery of autosomal dominant CPP with paternal transmission due to mutations also in the DLK1 gene [21]. Indeed, it has been shown that the incidence of familial CPP was disclosed at 22%, with a comparable frequency of paternal and maternal transmission [21]. Lineage analyses of families with maternal transmission indicated an autosomal dominant inheritance.
Another peptide involved would appear to be ghrelin, a peptide with orexigenic action produced in the stomach, which instead has an inhibitory action on the production of GnRH by decreasing the responsiveness of LH to its release factor [10] and simultaneously increasing pituitary growth hormone (GH) secretion, thus acting at the intersection of gonadotropic and somatotropic axes [23].
1.3. Peripherical Precocious Puberty
PPP is characterized by an increase in adrenal and gonadal sex steroids in the absence of HPG axis activation; the pubertal characteristics may be valid for the child’s sex (isosexual) or inappropriate, with virilization of girls and feminization of boys (contrasexual) [29]. It can be congenital, with the most frequent forms represented by congenital adrenal hyperplasia and McCune–Albright syndrome, or acquired, mainly related to hormone-secreting endocrine tumors [2,11,30].
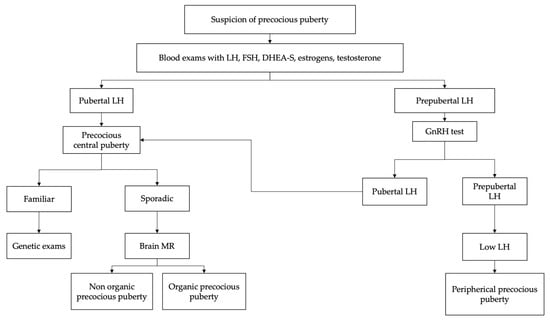
2. Diagnosis
Regardless of the cause, PP must be promptly recognized since it is associated with accelerated stature growth and skeletal maturation if untreated, inducing an early growth arrest with premature epiphyseal fusion due to excess sex steroids, which sometimes results in short adult height [2]. The key features suggesting PP are the progressive development of breasts in girls and testicular volume in boys over a short period of observation (3–6 months) associated with rapid height growth (height velocity >6–7 cm/year) especially in girls aged between 6 and 8 years, when this condition occurs more frequently [5,31,32].
In the suspicion of early sexual maturation, in kids presenting signs of secondary sexual development before the age of eight (females) or nin (males), the evaluation should begin with an accurate medical and familial history as shown in Figure 1 [22,34]. It is important to collect information regarding age of onset, rate of maturation of secondary sexual characteristics, exposure to exogenous sex steroids and the presence of neurological symptoms [10,14].

The physical examination, in addition to the evaluation of secondary sexual characteristics, must also include the evaluation of auxological parameters, such as weight, height, body mass index and height velocity (cm/year) [12,34]. Growth spurt is an important feature of pubertal development. In fact, growth acceleration with growth centile change supports the diagnosis of pubertal activation and therefore of PP [5,26].
Evaluation of bone age should be performed because children with PP frequently show advanced bone age, greater than two standard deviations beyond chronological age [35,36,37]. However, an advanced bone age does not rule out a benign pubertal variant, since up to 30% of kids with benign premature adrenarche have bone ages ≥2 years in advance of their chronologic age [25,38,39].
The physical analysis should include the assessment of visual fields (given the possibility of a central nervous system (CNS) lesion) and the examination for café-au-lait spots (suggestive of McCune–Albright syndrome or neurofibromatosis) [29,40].
Premature adrenarche is also a benign condition, characterized by the appearance of acne, axillary hair and pubic hair that occurs in girls under 8 years of age and in boys under 9 years of age, in the absence of other signs of pubertal development and is caused by adrenal androgen secretion in the absence of activation of the HPG axis [22].
Initial laboratory investigations should include serum gonadotropin (LH and FSH) levels and sex steroids, estradiol in girls and testosterone in boys [44,45,46]. Baseline LH > 0.3 mIU/mL is considered diagnostic for central precocious puberty; however, values below this limit do not exclude the diagnosis and require further diagnostic investigations. Measurable estradiol values or testosterone values >30 ng/dL suggest but do not confirm the diagnosis [47,48]. Therefore, children with clinical signs of early pubertal development and baseline LH values <0.3 mIU/mL are candidates for GnRH stimulation testing to identify HPG axis activation.
In contrast, suppressed FSH and LH values associated with increased sex steroids suggest the diagnosis of PPP. In these cases, it is essential to complete the diagnostic procedure with the measurement of tumor markers (alpha 1-fetoprotein, beta-HCG, CEA and CA125), in order to exclude hypersecretion of sex steroids of a neoplastic or paraneoplastic nature, and the measurement of serum dehydroepiandrosterone sulfate (DHEA-S) and 17-hydroxyprogesterone levels is advisable, which may be increased in adrenal tumors or congenital adrenal hyperplasia due to 21-hydroxylase deficiency [11,49].
In the diagnostic process of suspected precocious puberty, it is important to exclude an unknown and therefore untreated hypothyroidism condition, mainly if there is slow instead of rapid growth and clear hypothyroid signs and symptoms [50]. Initial evaluation of the child with suspected precocious puberty should include the assessment of bone age, because children with precocious puberty frequently have advanced bone age, greater than two SDs of chronological age [35,36,37].
Furthermore, pelvic ultrasound, a quick, non-invasive and low-cost examination, is a useful support for diagnosis by evaluating uterine development and ovarian volume and investigating the presence of ovarian cysts or tumors [7,51]. During infancy, the ovarian volume is stable, the fundus of the uterus and the cervix have a similar width and assume a tubular configuration, while in the pubertal phase, the uterus increases in volume and the cervix assumes the typical pear shape of adulthood [11,52].
The following ultrasound criteria aid in correctly identifying PP:
The presence of an endometrial stripe on pelvic ultrasound is also indicative of precocious puberty [56,57]. Overall, the ovarian volume represents the best indicator of PP, whereas uterine length is more capable of differentiating isolated premature thelarche from premature puberty [55]. A uterine fundal/cervical ratio ≥1 is generally inaccurate and no longer used [55].
Ultrasound examination also aids in the evaluation of boys with suspected PP, in which testicular enlargement represents the first sign of HPG axis activation. Specifically, testicular ultrasound represents the gold standard technique for the assessment of testicular volume [58], with a cut-off of 2.7 mL (calculated employing Lambert’s formula) corresponding to the traditional criteria of 4 mL defining Tanner stage II [59].
3. Treatment
Treatment of PP aims at preserving growth potential, synchronizing pubertal development with peers and improving psychological distress [11,12]. The main clinical criterion for initiation of therapy is the finding of pubertal progression, in children under the age of eight (females) or nine (males), with growth acceleration confirmed in a 3–6 month follow-up period [66,67,68]. This observation period may not be necessary if the bone age is markedly advanced or if the girl or boy presents with Tanner stage III [41,43]. The treatment is also indicated if PP is responsible for psychological and psychosocial disorders that can compromise the quality of life of patients and cause emotional and behavioral disorders that can also be detected at later ages [1,69].
The gold standard in the CPP treatment is represented by GnRH analogs (GnRHa) [70]. Their rationale for use is based on the recognition that, after an initial transient stimulation of gonadotropin secretion from the pituitary (termed “flare up”), high concentrations of GnRH eventually cause a complete, but reversible, suppression of the HPG axis by down-regulating the GnRH receptor, consequently inhibiting the secretion of gonadotropins [10,12]. GnRHa is available in different formulations: although slow-release formulations administered monthly were previously the most frequently used, formulations administered every 3 or 6 months (leuprolide and triptorelin) have been introduced in recent years, as well as subcutaneous implants of histrelin capable of inducing suppression of the hypothalamic-pituitary-gonadal axis for a period of 12–24 months [1,66,71,72]. As regards the therapeutic dosage required for the suppression of the HPG axis, there is no univocal opinion; in the USA, higher dosages are used (7.5 mg/month) while, in Europe, the monthly dosage used is 3.75 mg every 28 days [72,73].
Major factors affecting height prognosis include timely initiation of treatment, age at onset of puberty, bone age and height at diagnosis and target height [33,43]. Girls who start treatment before the age of 6 have better outcomes than patients who start treatment between 6 and 8 years while starting therapy after 8 years of age does not appear to be associated with an increase in height in adulthood [1,11,71]. GnRHa therapy is generally well tolerated in childhood, although the most frequently described adverse events are headache, injection site reactions and hot flushes, which in most cases occur early and are resolved by subsequent GnRHa administrations [11,78,79,80].
4. Conclusions
Puberty is a multifaceted process of transition from childhood to adulthood and its mechanisms are still not well known. PP is more common in females and more frequently it concerns idiopathic forms of CPP, but the recent characterization of genes involved in pubertal development underlines the important role of these factors in determining pubertal timing. It is important to identify the child with pathological pubertal development in order to undertake an accurate diagnostic and therapeutic procedure. The main goals of the treatment of precocious puberty are the preservation of growth potential, the synchronization of pubertal development with peers and the improvement of psychological distress. PPP is an extremely heterogeneous condition and can represent a manifestation of numerous pathologies, therefore an accurate etiological diagnosis is essential for correct management.
This entry is adapted from the peer-reviewed paper 10.3390/children10101672
This entry is offline, you can click here to edit this entry!
