You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
C-arm-free MIS techniques can offer significantly reduced rates of postoperative complications such as inadequate decompression, blood loss, and instrumentation misplacement. Another advantageous long-term aspect is the notably diminished exposure to radiation, which is known to cause malignant changes.
- C-arm free
- minimally invasive spine surgery
- adult spinal deformity
- lateral access spine surgery
- oblique lumbar interbody fusion
1. Introduction
The basic principles of spine treatment can be traced back to ancient Greece, where Hippocrates outlined the importance of spine function. Many centuries afterward, along with medical technology evolution, several attempts were made to increase perioperative accuracy [1]. The first intraoperative guidance pertaining to screw placement consisted of plain radiographs, but that method was abandoned due to low accuracy [2]. In 2013, it was estimated that more than 78% of spine surgeons were successfully using fluoroscopy navigation for intraoperative guidance [3]. Nowadays, C-arm-free navigated MIS techniques offer a variety of advantages, such as minimal soft tissue and muscle detachment, smaller incisions that can reduce pain and infection rates, high precision, and reduced hospitalization [4]. Additionally, as the elderly population is gradually increasing and severe degenerative spine pathology is often associated with opioid overuse and poor conservative results, which overall is a socioeconomic burden to the medical system, the application of those techniques can provide a reliable surgical treatment [5]. On the other hand, a learning curve is expected to be reached in order to obtain an optimal result [6]. The O-arm scan and the navigation registration can be time-consuming and since the equipment and those procedures require familiarization, it can lead to prolonged surgical time. Moreover, the accuracy is not out of the margin of error. If the reference frame is moved, it can significantly compromise the precision, so a second O-arm scan is necessary.
Radiation hazard is also an uprising phenomenon related to fluoroscopy-dependent techniques [7]. Often, prolonged exposure to radiation after years of work can lead to perilous consequences such as malignancy [8]. C-arm-free-navigated techniques can offer zero-level radiation exposure to the operating room staff by performing an O-arm scan. Furthermore, implant misplacement can lead to vessel injury, neurological deterioration, secondary pain, and high revision rates [4]. High-precision fixation is obtained using navigation systems that provide a 3D live image reconstruction during operation [9].
2. Occiput–Cervical Spine
2.1. Anterior Application
OPLL Resection
With the anesthetized patient on the Jackson frame, and the neck in adequate extension. Initially, a classic Smith–Robinson approach is performed to the appropriate cervical levels. After the Caspar pin retractor is placed and distraction is achieved, a special adaptor is used for the placement of the reference frame on the retractor, following an O-arm scan, which offers 3D reconstructed images (Figure 1B). The accuracy can be confirmed by checking at least three different anatomical surfaces. In any doubt, a second scan is strongly recommended. During the next surgical step, discectomies are performed at the superior and inferior disc segments. A navigated high-speed burr is used for bone spur resection in anterior cervical discectomy and fusion (ACDF) or anterior cervical corpectomy and fusion (ACCF). It starts medially to the uncovertebral joints bilaterally in width and to the posterior cortex in-depth (Figure 1C-D).
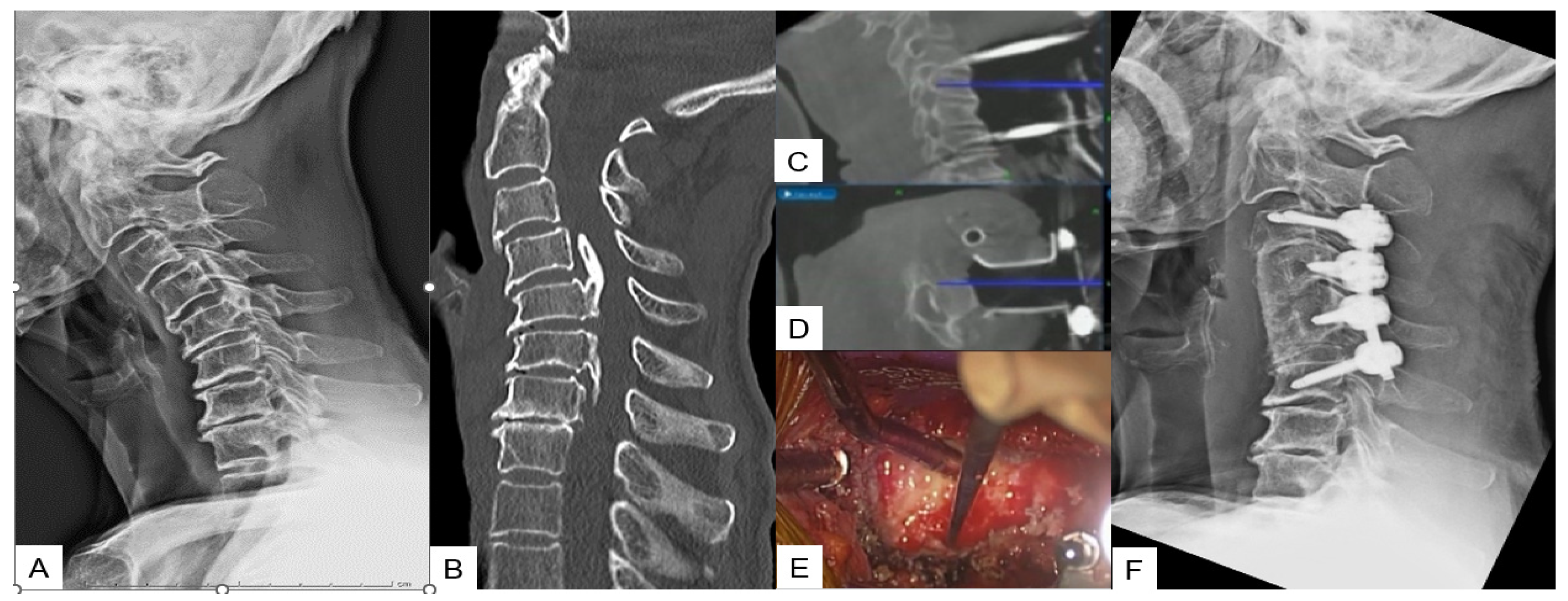
Figure 1. Case 1: 82-year-old male; cervical OPLL, anterior cervical corpectomy and fusion. (A) Preoperative cervical lateral radiogram. (B) Preoperative CT sagittal reconstruction image. (C) Intraoperative sagittal navigation image. (D) Intraoperative axial navigation image. (E) Intraoperative image. (F) Postoperative cervical lateral radiogram.
2.2. Posterior Application
2.2.1. Posterior Fossa Decompression for Chiari Malformation
The surgical technique starts with an approximate skin incision of 7 cm from the greater occipital protuberance down to the C2 spinous process, followed by a subperiosteal detachment of dorsal cervical muscles from the linea alba, spinous process, and C2 lamina to avoid excessive bleeding. The most convenient point used for reference frame attachment in O-arm scanning is the C2 spinous process (Figure 2A). Next, using a high-speed burr the C1 posterior arch is resected (Figure 2B,C). In some cases, when severe tonsillar herniation exists, the researchers advise performing C2 lamina resection. Excellent knowledge of anatomy is needed to avoid vertebral artery injury. Preoperatively, anatomical variations should also be considered. Thirdly, navigation-assisted craniotomy is performed. Usually, a 3 cm peripheric to foramen magnum is considered adequate. The merit of navigation is the ability to verify the exact bone width and location of the foramen magnum to avoid intraoperative complications. During the last step, duraplasty following ultrasonic monitor verification is performed. In case of inadequate decompression, the researchers recommend the extension of bone resection or duraplasty with the use of artificial dura.
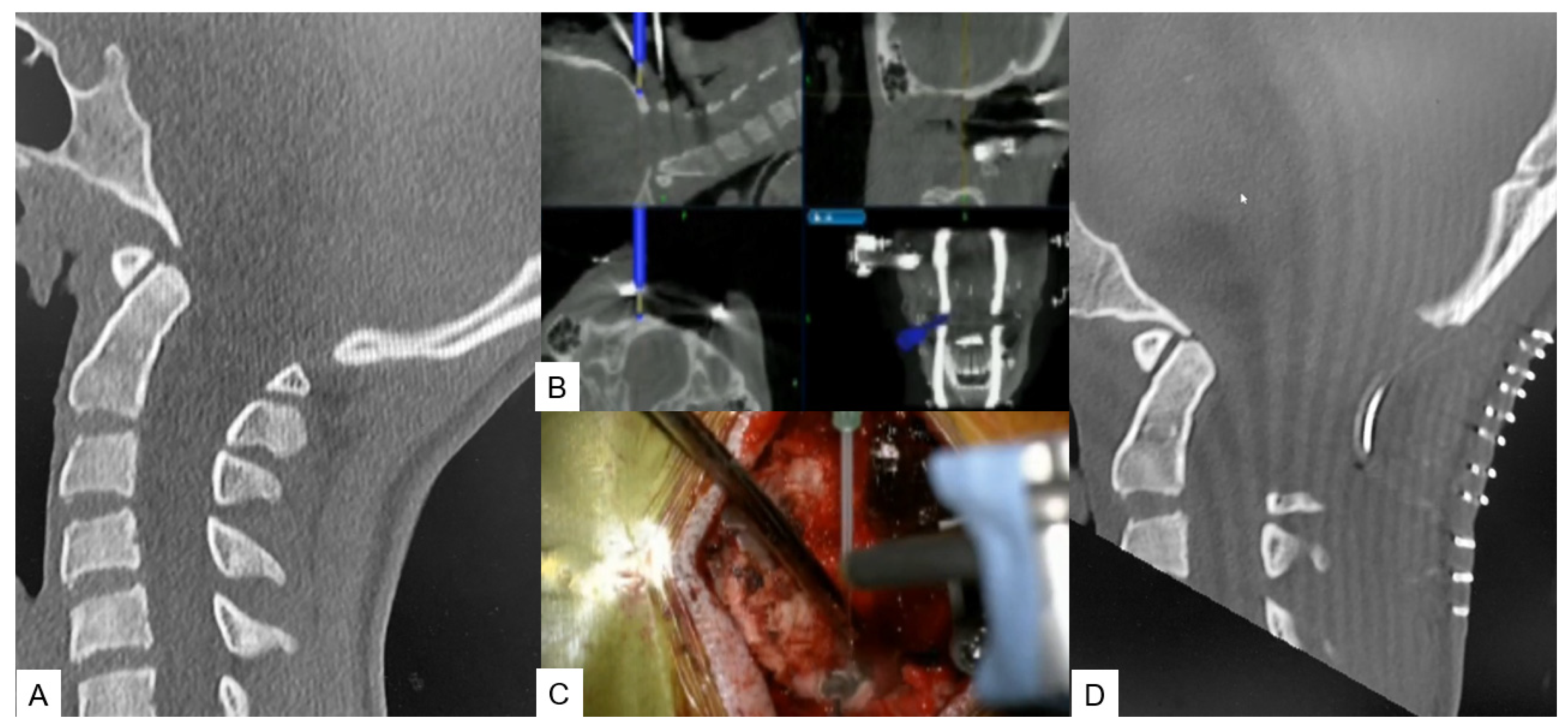
Figure 2. Case 2: 14-year-old female; Chiari malformation, foramen magnum decompression. (A) Preoperative occipitocervical CT sagittal reconstruction. (B) Intraoperative navigation image. (C) Intraoperative image. (D) Postoperative occipitocervical CT sagittal reconstruction.
2.2.2. Modified Goel Technique
Stabilization of the C1–C2 level can prove to be challenging due to lower fusion rates compared with the other cervical levels, as the motion in this particular segment is significantly higher. For this approach, the researchers propose C1 LMS with C2 pedicle screw insertion. A careful posterior approach, as described in the midpoint technique, should be made to avoid vessel injury. After that, the ideal entry point should be marked with a high-speed burr without applying strong downward forces at the C2 vertebra. Finally, the trajectory is made using a navigated pedicle probe with appropriate tapping (Figure 3).
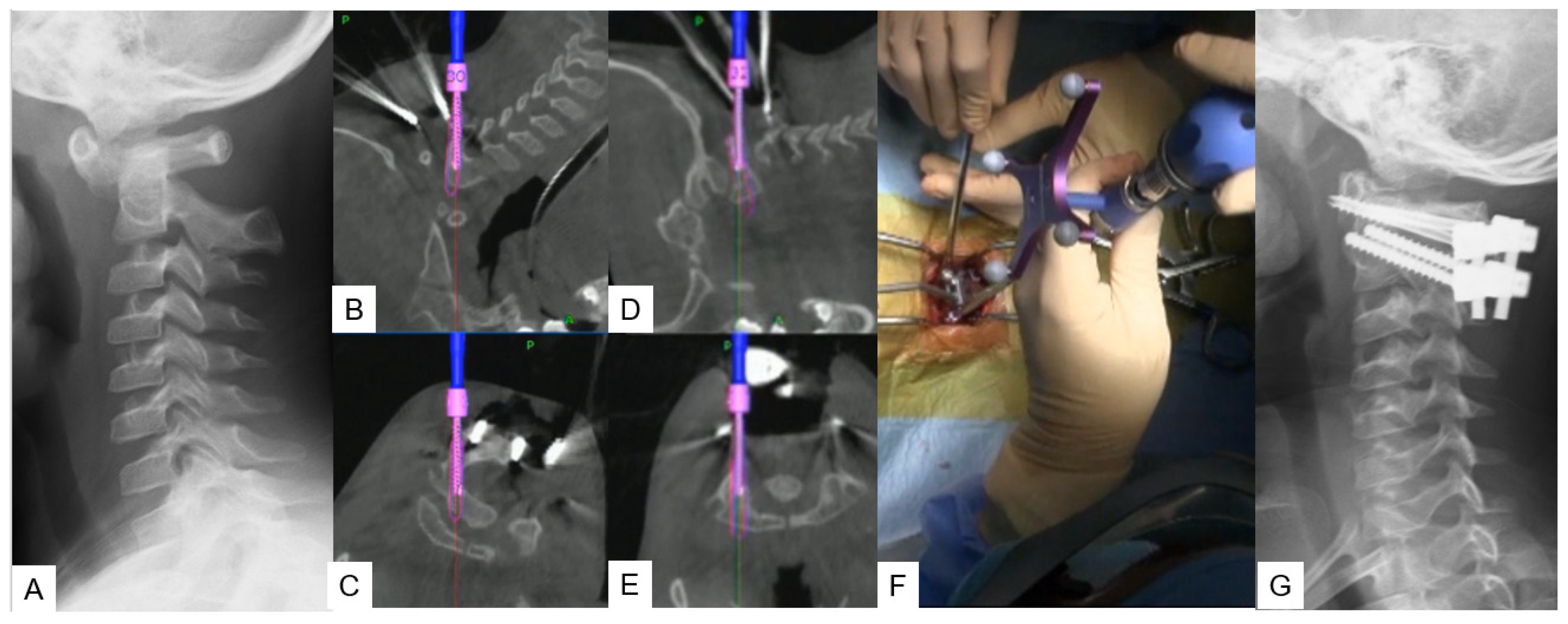
Figure 3. Case 3: 5-year-old male; Down syndrome, anterior atlantoaxial subluxation, modified Goel technique. (A) Preoperative lateral cervical radiogram. (B,C) Intraoperative navigation image of C2 pedicle screw insertion. (D,E) Intraoperative navigation image of C1 lateral mass screw insertion. (F) Intraoperative image. (G) Postoperative lateral cervical radiogram.
2.2.3. Midpoint Technique for C1 Lateral Mass Screw (LMS) Placement
A careful and precise posterior surgical exposure is required for this technique. Following this, retraction of the posterior atlantoaxial venous plexus inferiorly is necessary using a Penfield retractor to separate the posterior arch from the inferior aspect. Firstly, the entry point for this screw is 8 mm anterior from the posterior arch and caudal aspect (midpoint). A navigated high-speed burr with a 2 mm tip is used for precision and safety. Secondly, using a navigated pedicle probe, penetration down to the anterior aspect of the cortex of the C1 anterior arch must be made. At the end, bicortical placement of C1 LMS is performed (Figure 4).
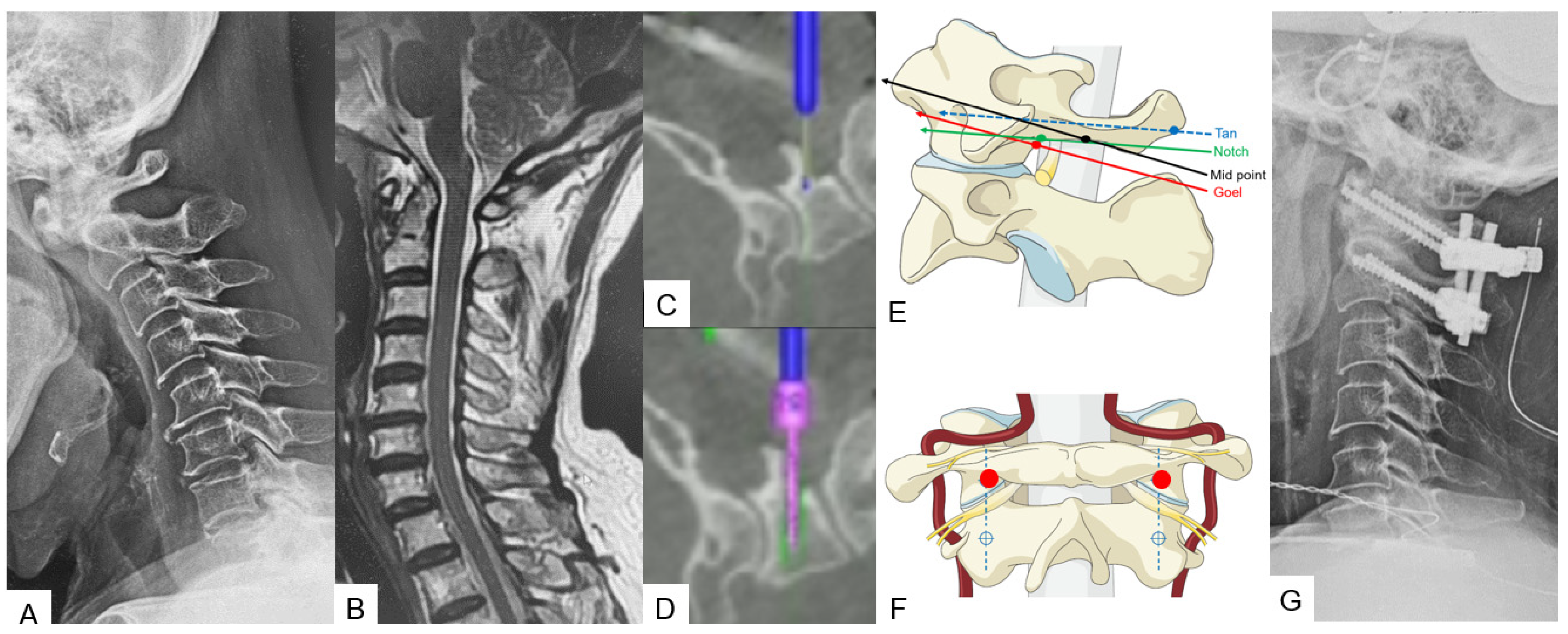
Figure 4. Case 4: 68-year-old male; anterior atlantoaxial subluxation, modified Goel fixation. (A) Preoperative cervical lateral radiogram. (B) Preoperative midsagittal T2 weighted MR imaging. (C) Intraoperative navigated high-speed burr. (D) Intraoperative lateral mass screwing. (E) Various C1 lateral mass entry points in lateral. (F) Insertion point of mid-point technique (red circle). (G) Postoperative cervical lateral radiogram.
2.2.4. Minimally Invasive Cervical Pedicle Screw Fixation (MICEPS)
During this technique, the patient’s positioning is very important. Thus, a prone position is recommended with a carbon Mayfield cranial support on a Jackson frame to provide additional stability. Initially, a small incision at the prominent C7 spinous process for reference frame attachment is advised, followed by an O-arm scan. Preoperatively, a CT scan verification for pedicle anatomy should be performed and studied. Usually, bilateral 4 cm skin incisions after navigation verification are enough for C3–C6 fixation. At the end, a new O-arm scan should be performed to verify anatomical pedicle screw placement. During open posterior cervical screw fixation, complications such as high infection rates, postoperative pain, and kyphotic deformity are frequently met. Thus, with this lateral minimal technique, it is feasible to reduce the complication rate due to minimal surgical exposure (Figure 5).
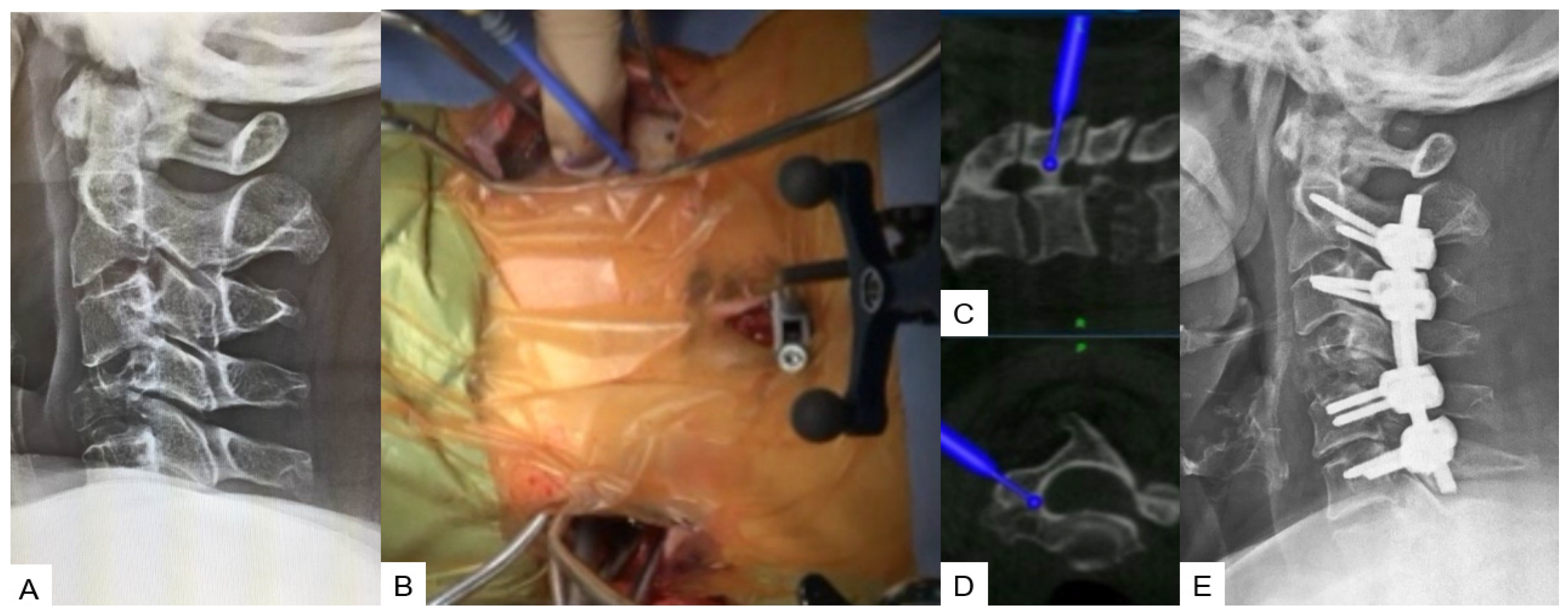
Figure 5. Case 5: 62-year-old female; Brest cancer, C4 metastasis, C2–C6 posterior fusion. (A) Preoperative cervical lateral radiogram. (B) Intraoperative image. (C) Intraoperative sagittal navigation image. (D) Intraoperative axial navigation image. (E) Postoperative cervical lateral radiogram.
3. Thoracic Spine
3.1. Anterior Application
Anterior Correction for Lenke Type 5
A lateral right decubitus position is appropriate for this approach. A left oblique skin incision of approximately 20 cm is made along the 10th or 11th rib. After superficial dissection through abdominal fat, external oblique, internal oblique, and transverse abdominal muscles are split in the mentioned order. Then, a circumferential dissection of the diaphragm is necessary for further exposure. Most of the desired correction can be obtained during the first step by placing the anterior rod and rotating it 90 degrees to create lordosis. Following this, the only way to achieve further correction is with in situ benders. At the end of the correction, an X-ray verification should always be made (Figure 6).
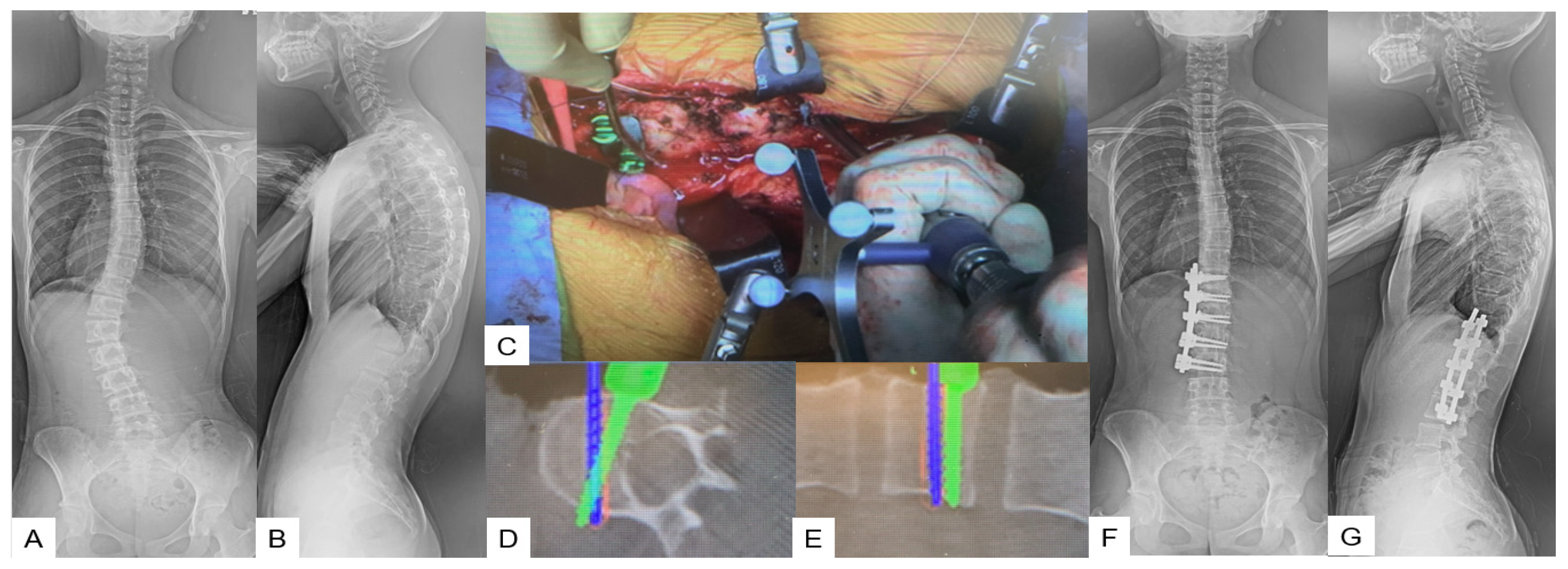
Figure 6. Case 6: 15-year-old female; adolescent idiopathic scoliosis LT11-L3enke Type 5C, T12-L3 anterior fusion. Preoperative 46 degrees of Cobb angle (T12-L3) became 1 degree. (A) Preoperative posteroanterior spine radiogram. (B) Preoperative lateral spine radiogram. (C) Intraoperative image. (D) Intraoperative axial navigation image. (E) Intraoperative coronal navigation image, (F) Postoperative posteroanterior spine radiogram. (G) Postoperative lateral spine radiogram.
3.2. Posterior Application
Transdiscal Screw for DISH (Diffuse Idiopathic Skeletal Hyperostosis) Fracture
The patient can be positioned in a prone or lateral decubitus position, though sometimes, the prone position can widen the fracture window, especially in DISH cases. A 1.5 cm incision in the middle line above the most cranial fused spinous process level is recommended for reference frame placement, followed by an O-arm scan. After marking the appropriate pedicle entry point, the probe for the transdiscal screw trajectory should be aimed through the pedicle to the upper endplate and the anterior 1/3 of the inferior endplate of the upper vertebra without reaching the anterior vertebral body wall. The desired angulation should be no more than 25–30 degrees; otherwise, the rod cannot be held firmly well in the tulip. Postoperatively, X-rays should always be taken for screw placement and alignment verification (Figure 7).
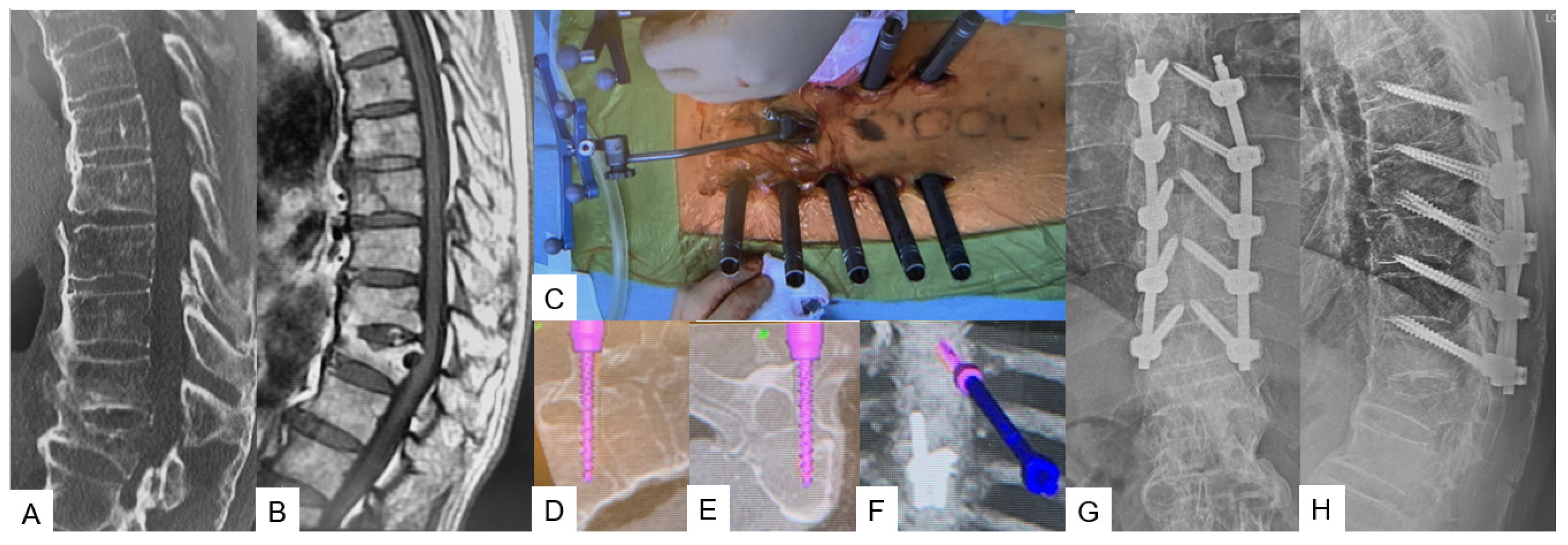
Figure 7. Case 8: 85-year-old male; T8 fracture of diffuse idiopathic skeletal hyperostosis (DISH) patient. (A) Preoperative sagittal reconstruction CT. (B) Preoperative sagittal T1 weighted MR imaging. (C) Intraoperative image. (D) Intraoperative sagittal navigation image. (E) Intraoperative axial navigation image. (F) Intraoperative 3D navigation image. (G) Postoperative anteroposterior radiogram. (H) Postoperative lateral radiogram.
This entry is adapted from the peer-reviewed paper 10.3390/medicina59101779
References
- Momin, A.A.; Steinmetz, M.P. Evolution of Minimally Invasive Lumbar Spine Surgery. World Neurosurg. 2020, 140, 622–626.
- Patel, P.D.; Canseco, J.A.; Houlihan, N.; Gabay, A.; Grasso, G.; Vaccaro, A.R. Overview of Minimally Invasive Spine Surgery. World Neurosurg. 2020, 142, 43–56.
- Härtl, R.; Lam, K.S.; Wang, J.; Korge, A.; Kandziora, F.; Audigé, L. Worldwide survey on the use of navigation in spine surgery. World Neurosurg. 2013, 79, 162–172.
- Willson, M.C.; Ross, J.S. Postoperative spine complications. Neuroimaging Clin. N. Am. 2014, 24, 305–326.
- Walsh, E.K.; Hansen, C.R.; Sahm, L.J.; Kearney, P.M.; Doherty, E.; Bradley, C.P. Economic impact of medication error: A systematic review. Pharmacoepidemiol. Drug Saf. 2017, 26, 481–497.
- Sharif, S.; Afsar, A. Learning Curve and Minimally Invasive Spine Surgery. World Neurosurg. 2018, 119, 472–478.
- Simony, A.; Hansen, E.J.; Christensen, S.B.; Carreon, L.Y.; Andersen, M.O. Incidence of cancer in adolescent idiopathic scoliosis patients treated 25 years previously. Eur. Spine J. 2016, 25, 3366–3370.
- Cristante, A.F.; Barbieri, F.; da Silva, A.A.R.; Dellamano, J.C. Radiation Exposure during Spine Surgery Using C-Arm Fluoroscopy. Acta Ortop. Bras. 2019, 27, 46–49.
- Croci, D.M.; Nguyen, S.; Streitmatter, S.W.; Sherrod, B.A.; Hardy, J.; Cole, K.L.; Gamblin, A.S.; Bisson, E.F.; Mazur, M.D.; Dailey, A.T. O-Arm Accuracy and Radiation Exposure in Adult Deformity Surgery. World Neurosurg. 2023, 171, e440–e446.
This entry is offline, you can click here to edit this entry!
