Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
The Mediterranean diet (MD), a plant-based diet, which is considered one of the healthiest nutritional models, has potential to help in the postdiagnosis phase of breast cancer (BC), due in part to the presence of several nutraceuticals: bioactive compounds and food components that, in addition to nutritional properties, have well-established antioxidant, anti-inflammatory, and anticancer effects.
- breast cancer
- functional nutrition
- Mediterranean diet
- antioxidants
- obesity
1. Introduction
Providing a unique definition of MD is not easy [68]. The MD should be understood as the typical dietary style of countries bordering the Mediterranean Sea, characterized by the consumption of certain foods, with attention to their origin and cooking methods, combined with regular physical activity, adequate rest, and proper conviviality [33].
Although the MD includes different eating styles based on the geography, culture, and local traditions of the various Mediterranean countries, there are some common features, such as the daily consumption of cereals, especially whole grain cereals; other fiber-rich unrefined grains; and seasonal fresh fruits and vegetables of several colors and textures, rich in micronutrients, fiber, and phytochemicals. Olive oil is the main source of dietary fat; olives, nuts, and seeds are also included. Protein intake is preferably represented by legumes, fish, or seafood, as well as a moderate consumption of eggs and white meat, while red or processed meat could be included 1–2 times per month in small portions. Calcium intake is provided by the daily consumption of dairy products, especially low-fat yoghurt or, less frequently, small portions of cheese. Good hydration is ensured by a daily intake of at least 1.5/2.0 L of water, and it is also contemplated a moderate intake of wine, preferably red, and always with meals [67,69,70].
Based on current evidence, MD is a recommended dietary approach in the prevention of various noncommunicable diseases (NCDs), including cardiovascular disease (CVD), type 2 diabetes, and some cancers [33].
With regard to BC, a high adherence to a Mediterranean dietary pattern has been shown to be beneficial both in the prevention and in the reduction of mortality in BCS [71]. On the contrary, Western diets (WD), abundant in total meat, red and processed meat, or high-glycemic-index (GI) foods, appear to be associated with a greater risk of BC [72].
Moreover, MD has been shown to be negatively associated with weight gain [73,74], particularly with abdominal and visceral adiposity [75,76], and a higher adherence to this nutritional model is likely associated to weight maintenance [77,78]. Therefore, due to the role of excess weight and visceral fat in the development and in clinical outcomes of BC, the MD could be an optimal dietary choice. The increased likelihood of maintaining weight, which is a crucial issue in the management of overweight and obesity [79], should be considered an additional important reason to recommend this diet in BCS. Indeed, there are limited data regarding long-term weight maintenance among these patients [80].
The favorable effects of the MD have been attributed to its characteristic ingredients, which are known for their lipid-lowering, insulin-sensitizing, antioxidant, anti-inflammatory, and antithrombotic properties [81]. The MD is rich in beneficial substances, such as minerals, vitamins, lecithin, sulfides, salicylates, phytoestrogens, phytosterols, polyphenols, and glucosinolates, found in fruits and vegetables. These compounds provide a protective effect by acting as antioxidant, deactivating carcinogens, preventing spontaneous mutations and oxidative DNA damage related to metabolic processes [82].
Classic examples are the phenolic compounds present in EVOO (extra virgin olive oil), which are useful in reducing LDL and its oxidation, oxidative DNA damage, and the production of proinflammatory cytokines; increasing insulin sensitivity through cell membrane modification; and improving endothelial function through increased bioavailability of vasodilating agents. Additional benefits of the MD are attributable to fiber, phytosterols, and polyphenols such as resveratrol, MUFAs (monounsaturated fatty acids), and PUFAs (polyunsaturated fatty acids), vitamins, and minerals [34,71].
Foods rich in micronutrients can exhibit nutraceutical properties. Nutraceuticals are indicated as “any substance that may be considered a food or part of a food, and provides medical or health benefits, including the prevention and treatment of disease” [83] (Figure 4). The MD is a food model particularly rich in nutraceutical foods. A cornerstone of this dietary pattern is the aforementioned EVOO, with a high content of MUFAs. Other compounds, such as squalene, triterpenes, pigments, tocopherols, or phenolic compounds, although present in small amounts, possess antioxidant, anti-inflammatory, and anticancer properties, or act as regulators of the GM [31,84]. The MD is also rich in senolytic molecules, such as oleuropein and (-)-epigallocatechin gallate (EGCG), which are natural or artificial compounds able to act against senescent cells. These cells are known for their disrupting action towards metabolism and are associated with a higher risk of cancer [85].
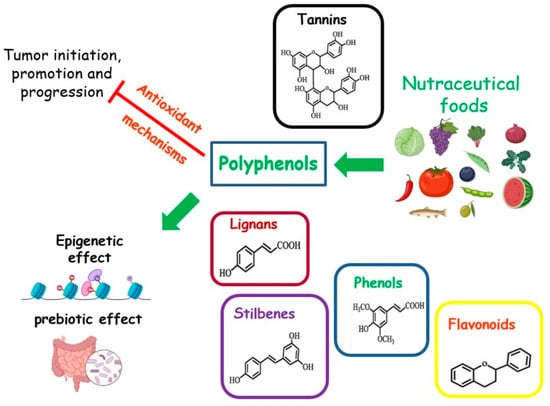
Figure 4. Effects of nutraceutical foods on tumor dynamics.
2. Phenols
In recent decades, several studies have indicated the chemosensitizing effects of food phenols in patients with BC. However, these compounds have a scarce bioavailability in their native form because after ingestion, phenolic compounds may undergo modification by the GM or enterocytes to generate different molecules. These molecules can be found in circulation for several days and can mediate the biological effects of their parental compound [86]. Transformation of dietary polyphenols may affect their biological activity [87,88,89]. For instance, some metabolites of the GM, derived from ellagic acid and ellagitannins, have shown antiproliferative action on cancer cells [90].
Cell-based experiments have shown that curcumin treatment was able to reduce the levels of the antiapoptotic factor Bcl-2 in cultures of BC cells [91]. Another study has shown that treatment with green tea polyphenols and sulforaphane results in chromatin changes and induces activation of tumor suppressor genes such as p21(CIP1/WAF1) and Klotho [92]. In addition, mammary tumors carrying BRCA1 mutations display low levels of SIRT1 and increased levels of Survivin. BRCA1 has been shown to increase SIRT1 expression, which in turn inhibits Survivin, whose function is expected to favor tumor growth. Increased activity of SIRT1 by resveratrol reduces Survivin expression, resulting in inhibitory effects on BRCA1 mutant cancer cell expansion. These data suggest that resveratrol represents a promising therapy against breast tumors associated with BRCA1 mutations [93]. Antineoplastic properties have also been shown by ferulic acid, a polyphenol isolated from Ferula foetida, which is able to reduce the viability of BC cells MDA-MB-231, while also reducing the metastatic potential by counteracting the epithelial–mesenchymal transition [94].
Evidence for the efficacy of polyphenols in in vivo models of breast tumor has been provided by Luo et al. [95] who inoculated 4T1 BC cells in Balb/c mice cotreated with the antitumor Paclitaxel and the green tea polyphenol EGCG. The study showed that EGCG promoted apoptosis of cancer cells and inhibited tumor growth. The antitumor effects of EGCG were also confirmed by Thangapzham et al. [96] in nude mice inoculated with MDA-MB-231 cells. Treatment of these mice with EGCG resulted in inhibition of tumor proliferation via induction of apoptosis in cancer cells. Decreased expansion of MDA-MB-231 tumors, through increased apoptosis and reduced angiogenesis, was also observed in nude mice upon treatment with resveratrol, confirming the potential anticancer properties of this polyphenol, also in vivo [97].
In general, diets rich in phenols, (flavonols, anthocyanins, flavanones, lignans, flavan-3-ols, resveratrol, curcumin, and hydrolyzable tannins), are associated with reduced cancer growth, incidence, and metastasis, as well as a longer period of cancer latency, and linked to a higher survival rate [86].
As regards isoflavones, which are contained in a wide range of food sources, such as soy, asparagus, and borlotti beans, different studies have shown inhibitory or proliferative effects on BC cells, depending on experimental models and conditions. Interestingly, the phase of life in which consumption of isoflavones begins influences the risk of BC, which may be lower when soy is consumed in the diet from the early years of life. This could explain the greater chemoprotective effect of soy-based products in Asian women, who consume such foods throughout life, while Western women often introduce soy-based foods only in adulthood. Notably, modulation of the risk of cancer recurrence by isoflavones might be also affected by genetic polymorphisms, in particular in genes involved in this disease, i.e., cytochrome P450 1B1, the oncogene MDM2, and p53 [98,99].
Besides the more specific antineoplastic effects described above, some evidence supports a beneficial effect of dietary polyphenols in reducing excess body fat. Several mechanisms have been proposed to explain their benefits, such as the inhibition of digestive enzymes promoting obesity, the modulation of neuropeptides involved in eating behavior, the stimulation of energy expenditure or mitochondrial biogenesis, and favorable changes in gut microbiota profile, suggesting a potential role of these compounds in the treatment of obesity [100]. Given the commonly observed weight gain in BCS, and the strong impact of obesity on the clinical outcomes of BC, a diet rich in polyphenols, such as the MD, could provide a valid support for these patients, also due to its antiobesity action. However, defining the exact level of a bioactive substance in food is difficult, and further research is needed to establish the optimal intake of dietary polyphenols.
2.1. Polyphenols in Extra Virgin Olive Oil
Extra virgin olive oil (EVOO) is mainly composed of monounsaturated acids (oleic acid) and, to a lesser extent, of acids with eighteen carbon atoms omega 6 and 3, linoleic acid (LA), and α-linolenic acid (ALA), and a low content of saturated fatty acids. Tocopherols are also present, as well as phenolic and alkaline acids, flavonoids, secoiridoids, lignans, and hydroxysichromans. The phenolic portion exhibits an antioxidant action, which is very useful for the long shelf life of EVOO. The consumption of EVOO is associated with a low incidence of atherosclerosis, skin diseases, inflammatory diseases, autoimmune diseases, diabetes, ageing, and tumors. Oleocanthal polyphenol, which is responsible for the pungent taste sensation of EVOO, has inhibitory actions on inflammatory diseases similar to non-steroid anti-inflammatory drugs (NSAID) [101,102]. In vivo and in vitro studies show that EVOO and/or its compounds can influence the promotion, initiation, and progression of carcinogenic processes through multiple and various direct or indirect mechanisms, which are able to influence different signaling pathways. They are, in fact, capable of inhibiting proliferation, inducing apoptosis, arresting the cell cycle, and reducing inflammation, immune evasion, migration, and angiogenesis [103]. Finally, polyphenols in olive oil appear to have lipid-lowering, insulin-sensitizing, and prebiotic effects, thus contributing to metabolic health [104,105].
2.2. Quercetin
Quercetin is a flavanol mainly present in some vegetables such as red onion, capers, lettuce, cruciferous vegetables, celery, tomatoes, asparagus, and shallots [85]. It is also present in some fruits such as berries, green tea, black grapes, pomegranates, citrus fruits, apples, pistachios, nuts, and animal products such as propolis. Overall, the quercetin content in various foods ranges from 1.8 mg/100 g in foods like red onions or cruciferous vegetables to 3.5 mg/100 g in berries and 4.7 mg/100 g in apples [106].
The biosynthesis of quercetin occurs due to the action of the sun, after which it is stored in the peel of fruit and in the leaves [107].
The antioxidant activity of quercetin is related to the presence of a phenolic group in its chemical formula. It also expresses anti-inflammatory, antimicrobial and antidiabetic effects [85].
In the field of oncology, it has been observed that quercetin, even at low doses, can exhibit chemopreventive effects due to its antioxidant action, while at high doses, it exerts chemotherapeutic effects, acting as a pro-oxidant. In mouse models, 1 mg/Kg for 15/30 days reduces breast tumor volume. Six doses of 1 mg/Kg given orally every three days reduce the tumor volume of breast adenocarcinoma in mice. Meanwhile, 5 mg/Kg administered intraperitoneally twice daily for a month reduces the tumor size of breast cancer in mouse models [108].
Anticancer effects of quercetin are based on its ability to reduce the proliferation of cancer cells, inducing apoptosis, arresting the cellular cycle and inhibiting mitotic processes. In particular, in cultures of MCF-7 human BC cells, quercetin has been shown to induce apoptosis and necroptosis by modulation of Bcl-2 and Bax expression levels [109]. Another study has investigated the effects of quercetin in MCF-7 BC cells treated with the chemotherapeutic drug 5-fluorouracil (5-FU) [110]. Quercetin has been shown to enhance the anticancer properties of 5-FU by inducing apoptosis through increased levels of Bax, p53, and caspase-9 and, in parallel, reducing Bcl2 expression. These data suggest that quercetin is able to enhance the sensitivity of BC cells to 5-FU [110].
Other studies have shown that quercetin can be used as adjuvant treatment in combination with the antimitotic compound docetaxel [111]. In fact, at the molecular level, upregulation of the transcription factor Lef1 leads tumor cells to become drug-resistant. Cell culture experiments displayed that the reduced expression of Lef1 by treatment with quercetin enhances the sensitivity of BC cells to docetaxel [111].
In human MDA-MB-231 BC cell cultures, quercetin treatment was able to counteract the metastatic phenotype and epithelial–mesenchymal transition by reducing the levels of the transcription factors Snail and Slug and decreasing IGF-1 production. In order to investigate the impact of quercetin on IGF1-induced invasion and metastasis in vivo, MDA-MB-231 cells were implanted into SCID mice treated with this flavanol, which was able to reduce the pulmonary nodules and lung metastases formation in these animals. In tumor tissues, quercetin induced a reduced expression of mesenchymal markers (i.e., Snail, Slug, fibronectin), in parallel, increasing the levels of the epithelial markers keratin 18 and keratin 19 [112].
Additional studies on the effects of quercetin on cancer stem cells (CSCs) have shown that this flavonoid represses cell viability and clone formation of CD44-/CD24- BC cells, while reducing metastasis formation in nude mice, which were effects mediated by decreased levels of cyclinD1 and Bcl-2 and by the downregulation of the phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR pathway [113].
The antineoplastic effects of quercetin (corresponding dose of 1311 mg/g per 16 weeks for a man of 60 Kg of BW [114]) have also been described in another mouse model of BC, i.e., in C3(1)/SV40 Tag mice, which displayed a quercetin-induced reduction in tumor volume, as well as revealing a decreased expression of transmembrane protease serine 4, a known marker of poor prognosis for BC [114,115].
At metabolic level, quercetin has demonstrated, in cell and animal models, insulin-sensitizing, antioxidant, and anti-inflammatory effects [116]. However, a clear antiobesity effect has not been observed with quercetin supplementation [117].
2.3. Fisetin
Fisetin belongs to the category of flavonols. It is found in various plant products, including apples (26 µg/g), persimmons (10.5 µg/g), grapes (3.9 µg/g), strawberries (160 µg/g), onions (4.8 µg/g), and cucumbers (0.1 µg/g) [118].
Fisetin is known for its senolytic effects [85]. It has been demonstrated that fisetin delays the growth of various cancer cells. Its targets of action are the signaling pathways that regulate tumor cell survival, apoptosis, metastatic, and angiogenic processes. Scientific evidence supports the hypothesis that fisetin could potentially act in cancer treatment and prevention through its epigenetic effects [118]. Some proapoptotic effects are also linked to actions on the cellular membranes [119]. Moreover, fisetin can sensibilize cancer cells to radiotherapy interfering with protection of DNA [120]. In more detail, fisetin (10, 25, and 50 μM) has been shown to induce the apoptosis of breast MDA-MB-453 cancer cells through proteasomal degradation and modulation of the PI3K/Akt pathway [121]. The inhibition of metastasis by fisetin (5 µM) has been suggested through the reduced expression of MMP-2 and MMP-9 enzymes, as observed in BC cell cultures (4T1 and JC cells) treated with this flavonol [122]. In experiments with different models of BC cells, fisetin has shown to be able to inhibit the growth of MCF-7, MDA-MB-468, and MDA-MB-231 triple-negative BC cells, as well as SK-BR-3 human cells overexpressing epidermal growth factor receptor 2 [123]. At the molecular level, fisetin could exert these effects also through apoptosis induction by the inhibition of Aurora B kinase activity. Of note, fisetin has been shown to enhance the cytotoxic effects of cisplatin, 4-hydroxycyclophosphamide, or 5-FU on MDA-MB-468 cells [123]. In accordance with cell-based experiments, in vivo experiments have confirmed that fisetin (20, 40, and 80 μM) is able to counteract tumor growth in a 4T1 orthotopic mammary tumor model by reducing the activation of PI3K/Akt/mTOR signaling pathway [124].
Concerning its role against obesity, fisetin has shown antiadipogenic effects by inhibiting mTORC1 signaling in preadipocytes, and combined with other antiadipogenic phytonutrients, it has proven to be effective in reducing total and visceral fat, lipid levels, and inflammation in murine models [125].
2.4. Anthocyanins
Anthocyanins are among the most important groups of purple pigments found in vegetables, but also in fruits. High amounts of anthocyanins, ranging from 94–120 mg/100 g to 442 mg/100 g, can be found in eggplants, black grapes, pomegranates, beets, cherries, mallows, apples, cruciferous vegetables, citrus fruits, berries, and black rice. The more intense their color (reddish or bluish), the higher their anthocyanin content [126]. Growing evidence, although it should be improved, indicates that the major mechanism of their antitumoral effects involves the inhibition of cellular growth and metastasis, including the block of the VEGF signal and pathway, and the degradation of the extracellular matrix. Anthocyanins (500 µg/mL in murine cells) may reverse the multidrug resistance of cancer cells, improving their chemotherapy sensitivity [127].
In particular, black rice anthocyanins have been shown to repress migration and invasion of MDA-MB-453 cells expressing HER2 by downregulating the RAS/RAF/MAPK signaling pathway [128]. In the same BC model, treatment with anthocyanins of blue corn resulted in reduced cell viability and induced apoptosis of BC cells. Of note, the induction of apoptosis was further stimulated by the cotreatment of anthocyanins with the antitumor drug nocodazole [129]. In MCF-7 BC cells displaying high resistance to cisplatin, anthocyanins isolated from Vitis coignetiae Pulliat stimulate the sensitivity to this antitumor drug by reducing Akt and NF-kB activation [130]. Antineoplastic effects of black rice anthocyanins (150 mg/Kg/day for three weeks) were observed in vivo, in a murine xenograft model bearing BC cells MDA-MB-453 expressing HER2, whose high levels represent a metastasis risk factor [131]. This study suggested that black rice anthocyanins could repress tumor growth by inhibiting tumor cell motility and invasion, which are two key steps in metastasis [131]. In addition, another in vivo study has shown that treatment with the anthocyanin cyanidin-3-glucoside (6 mg/mL twice a week for 25 days), in a xenograft model bearing BT-474 BC cells (expressing HER2), was able to potentiate the antineoplastic effects of trastuzumab [132].
Regarding metabolic effects, several clinical studies have observed improvements in dyslipidemia or glucometabolic status, as well as a reduction in body weight following supplementation with anthocyanins. Although there is still limited evidence, particularly in humans, the regular dietary consumption of foods containing anthocyanins could be encouraged [133].
2.5. Resveratrol
Resveratrol (RE) belongs to the polyphenols and is one of the phytoalexins naturally produced by many plants. Indeed, it is found in grapes, wine, some berries, and oilseeds, tea, and peas. The highest amount is found in grapes (50–100 g/kg net weight), in which it is present only in the skin, while its presence in wine (approximately 0.27 mg/100 g) depends on the cultivar, the geographical origin of cultivation, and the fermentation period.
Besides the defensive function against pathogens such as fungi and bacteria, this non-flavonoid polyphenol is able to exert a powerful antioxidant, antiglycation, anti-inflammatory, neuroprotective and antiaging action [134]. In 4T1cells, RE has been shown to block the cell cycle and induce apoptosis [135]. In experiments with MDA-MB-231 human BC cells, this polyphenol was found to inhibit cell migration and the secretion of matrix metalloproteinase MMP-2 and MMP-9. Importantly, RE was able to reverse the epithelial–mesenchymal transition induced by TGF-β1, which represents a key step for tumor migration [136]. In a murine model bearing MDA-MB-231 human BC cells, treatment with RE reduced the formation of lung metastatic nodules, confirming the antineoplastic properties displayed in in vitro experiments [136]. In MCF-7 BC cells, RE was shown to increase chemosensitivity to the chemotherapy drug Adriamycin, promoting apoptosis by reducing the expression of cyclin-dependent kinases and Bcl-2. Interestingly, modulation of these proteins was suggested to be mediated by resveratrol-induced upregulation of miR-122-5p [137]. In addition, another study observed that RE increases the chemosensitivity of BC cells to the poly (ADP-ribose) polymerase inhibitor talazoparib, leading to reduced proliferation in vitro, as well as decreased tumor growth in SCID-mice [138]. Several studies have shown that RE modulates several genes involved in cancer through DNA methylation. In MDA-MB-231 BC cells, DNA methylation changes were associated with the modulation of transcript levels of tumor-related genes [139]. Other preclinical studies have observed that RE is able to reduce the time of tumor formation and its incidence by reducing angiogenesis and revealing agonist and antagonist actions on estrogen receptors [140].
It is estimated that it is not possible achieve a therapeutic amount of RE with foods [141]. Actually, there is a paucity of data from human trials; however, RE (1 mg/day for 12 weeks) can act on tumor growth by modulating sex hormone metabolism and blocking cell proliferation [142].
It has been observed that RE is able to strongly stimulate mitochondrial activity and oxidative type-I muscle fibers, and regulate the energy balance [143]. However, the benefits observed in preclinical studies have been obtained with much higher doses of RE than those achieved with the usual diet [144]. Moreover, with respect to the antiobesity effects of low-to-moderate wine consumption, human studies have revealed conflicting results [145]. Furthermore, wine contains alcohol, and it has been reported that even very low alcohol consumption is associated with an increased risk of BC recurrence, while the association with a second primary BC or BC-specific mortality is less clear [146,147], and overall, data from the existing studies show a marked heterogeneity.
2.6. Curcuma Longa
Although turmeric is not a typical component of the MD, it has been included here because it is a spice widely used and studied. Curcuminoids are among the main constituents of turmeric. Curcumin is a polyphenol with anti-inflammatory, antilipidemic, and antioxidant actions. It is able to implement epigenetic modulation and regulation mechanisms by which it could affect various pathologies [148]. Curcumin, even at a dosage of 6 g/day for seven consecutive days every three weeks, is claimed to have anticancer and chemopreventive activity against BC, including antiproliferative effects, antimetastatic effects, the stimulation of apoptosis, blockage of the start of the cell cycle, and the enhancement of several chemotherapeutic effects. These effects may be achieved through various specific mechanisms of action, as well as a complex network of molecular signals that involve the mechanisms underlying cell proliferation. The mechanisms of action include the inhibition of the proliferative and metastatic effects of growth hormone, and the beta-catenin pathway, by triggering onco-suppressor miRNAs and deactivating the oncogenic ones [149]. Unfortunately, curcumin is not highly bioavailable because of its low intestinal absorption, rapid metabolism, and low water solubility. The association of some chemical groups in the molecule can increase bioavailability and efficacy [150]. This may occur with the addition of piperine, which implements liposomal delivery systems, phospholipid complexes, or emulsions with polysorbate [151]. The complexation with proteins and other natural compounds such as quercetin and resveratrol may also improve bioavailability [152].
Regarding the metabolic effects of curcumin, some studies on cell or animal models have shown a beneficial effect on body weight gain, insulin resistance, and lipogenesis [153]. Moreover, some human clinical studies have observed significant weight loss in overweight or obese subjects after supplementation with curcumin [154]. However, further studies are needed to investigate the effects of the dietary use of this compound.
2.7. Epigallocatechin-3-Gallate
EGCG is a catechin (flavonol) typical of green tea (27.16 mg/100 mL). It is a polyphenol with anti-inflammatory and antioxidant activity, which is also found in foods such as plums, apples, raspberries, blackberries, walnuts, pistachios, peaches, and avocados. It exerts an anticancer effect by inhibiting some key enzymes involved in the glycolytic pathway and by suppressing glucose metabolism. Some models show EGCG can act as adjuvant of therapies and boost their action against cancer cells. EGCG is a potent antiangiogenic, antioxidant, and anticancer agent, through a modulating action on tumor cells. Preclinical evidence shows that EGCG activity modulates differentiation, proliferation, apoptosis, angiogenesis (40 mg/L of green extract tea), inflammation (2 mg/Kg), and metastasis spreading [155].
In addition, EGCG exerts antilipogenic effects by inhibiting lipid biosynthesis and by stimulating lipolysis [100]. Furthermore, both observational studies and clinical trials have reported a significant association between the regular consumption of green tea or supplementation with green tea catechin and weight loss [145].
3. Carotenoids
Retinoids are liposoluble compounds related to vitamin A that are present in red and yellow vegetable foods (from 22 to 4000 µg/100 g), eggs, meat, offal, milk, and some fish and shellfish. They are characterized by an unsaturated isoprenoid chain structure. Vegetables are typically a source of carotenoids, some of which are precursors of vitamin A, while animal foods contain bioactive retinoids, synthesized from carotenoids [156,157]. Their bioavailability varies depending on cooking and processing methods and on the presence of fiber and lipids. They are important for visual functions, defense against infections, the maintenance of epithelial cells, reproductive functions, and as an antioxidant, but only if not overdosed. In this case, they appear to have the opposite effect. The daily dietary requirement (indicated as RAEs, that is, retinol activity equivalents) is 12 µg for β-carotene and 24 µg for α-carotene [158]. Carotenoids improve communication at the level of intercellular junctions, which are involved in mechanisms that regulate tumor growth, playing a role in the regulation of cell growth, apoptosis, and differentiation. In fact, gap junction communication is deficient in many forms of cancer, and its restoration leads to a reduction in cell multiplication. They also have anti-inflammatory and immunomodulatory effects. However, further studies are necessary to confirm and define these effects on cancer [159].
A recent study has shown that carotenoids extracted from Bixa orellana L. exert antiproliferative effects on MCF-7 cells [160]. Several preclinical studies have observed that the carotenoid fucoxanthin, found in seaweeds and diatoms, acts as a very efficient inhibitor of tumor growth, by modulating signaling pathways involving NF-κB, MAPK, MMP2/9 [161]. Interestingly, in vitro experiments have shown that lutein is able to induce apoptosis in SV40 transformed, but not in normal mammary cells [162]. It also displays antiproliferative effects on MCF-7 and MDA-MB-468 cells [163], thus suggesting promising chemotherapeutic applications. In BC BT474 and SKBR3 cells, cotreatment with all-trans retinoic acid (ATRA) and the antitumor agents tamoxifen or trastuzumab led to apoptosis. Interestingly, in the BT474 cell line, the single treatment with only retinoids such as ATRA, 13-cis-retinoic acid, or N-(4-hydroxyphenyl) retinamide was able to induce apoptosis without the requirement of antiproliferative agents [164]. In another study, cotreatment of the PPARγ ligand rosiglitazone and 9-cis-retinoic acid exerted antiproliferative effects in several BC cell lines such as MCF-7, MCF-7TR1, and SKBR-3. Of note, such effects were not observed in non-cancer breast epithelial cells, indicating that such a multidrug strategy may be a successful approach to selectively inhibit BC cell proliferation [165]. On the other hand, treatment with 9-cis retinoic acid has been shown to stimulate the expression of angiogenesis-related genes, including vascular endothelial cadherin, suggesting potential proangiogenic detrimental effects on tumor growth [166]. Interestingly, retinoids have also been proposed to exert antineoplastic effects by stimulating hepatic detoxification pathways of Bisphenol A, a plastic toxic compound detectable in the food chain, which can elicit BC in various ways, notably activating estrogen receptors and promoting cancer cell proliferation [167,168].
Several clinical studies report low plasmatic concentrations of carotenoids in obesity or related metabolic alterations, while animal studies have found beneficial effects of carotenoid consumption against obesity, insulin resistance, hepatic steatosis, and chronic inflammation. Although these compounds appear to target the adipose tissue, regulating the energy homeostasis indirectly, some studies have hypothesized a direct effect at the brain level [169].
Lycopene
Lycopene is a natural antioxidant of the carotenoid family, found in tomatoes, watermelon, pink grapefruit, apricots, grapes, papaya, carrots, and pumpkins. The highest concentrations are found in ripe tomatoes, and their bioavailability and adsorption can increase when ripe tomatoes are cooked or heated. Among carotenoids, lycopene is the most powerful and exerts an anticancer effect, particularly in ER-negative BC. This action is exerted through a cytotoxic and antiestrogenic effect. Moreover, it reduces cell proliferation and blocks the tumor cell cycle. These effects are not observed in healthy subjects [70]. Lycopene is a carotenoid with two unconjugated double bonds that provide a powerful antioxidant property, even at near-physiological levels (0.52 µM), by inhibiting cell proliferation, inducing apoptosis, and suppressing metastasis, but also by potentiating the effect of anticancer drugs (paclitaxel, docetaxel, cisplatin, and adriamycin). The treatment that tomatoes undergo for food use (crushing and/or heat treatment) results in cis-isomerization, which increases their bioavailability compared to the fresh product; an enhancing effect is also due to the synergy between lycopene and other dietary nutrients (lipids, proteins, and polysaccharides, quercetin, curcumin, and genistein) [170]. The lycopene content in ripe tomatoes ranges from 0.88 to 7.74 mg/100 g, while the plasma concentration (after a daily intake of 10 mg of lycopene) is around 0.52/0.6 µM. Lycopene inhibits free radicals by reducing oxidative damage and inflammatory response; counteracting lipid peroxidation; protecting lipids, RNA, and DNA from oxidation by preventing mutations; and inhibiting the growth of cancer cells and unhealthy cells [170,171]. High levels of lycopene as well as serum α-carotene, β-carotene, and lutein/zeaxanthin were associated with a lower risk of BC among all ER- or progesterone receptor-positive subtypes [172].
An inverse association has been shown between lycopene intake and BC risk, which seems to be greater in lean women than in overweight and obese women. This finding is probably due to the fact that lycopene is fat-soluble and easily stored in adipose tissue, but less available at the plasma level. In that study, the subjects showed an association between the dietary intake of carotenoids and reduced risk of BC, even for the aggressive and lethal forms [173]. Sisti et al. demonstrated the inverse association only in postmenopausal women, and attributed this result to the interaction with hormonal factors [174]. It is known that a high BMI in postmenopausal women increases BC risk. Llanos et al. observed that the consumption of lycopene in postmenopausal obese women determined an increase in circulating levels of adiponectin, which regulates glucose homeostasis and fatty acid metabolism. Thus, it can be hypothesized that lycopene can improve insulin sensitivity and the lipid profile, with potential protective effects on BC [175].
Fukuschi et al. observed that a six-month administration of tomato juice at a dose of 160 g/day produced an early increase, during the first month, of serum lycopene content, which reached its highest values at 3 and 6 months and returned to baseline levels at 12 months. This long-term intake of tomato juice contributed to the recovery of early and late skin adverse events caused by radiotherapy that women underwent after breast-conserving surgery [176].
Although the main effect of lycopene, in preventing carcinogenesis, can be attributed to its high antioxidant property, through which it can suppress the tumoral promotion and progression, other mechanisms have been proposed. Among these, there are the regulation of growth factor signaling, the induction of apoptosis, cell cycle arrest, the reduction of metastasis, angiogenesis, and cell invasion. Lycopene treatment has been shown to reduce proliferation and increase the rate of apoptosis of MCF-7 human BC cells by upregulating levels of p53 and Bax transcript [177]. In accordance with these data, in MDA-MB-468 cells, lycopene administration results in the reduced activation of Akt and mTOR, involved in cell proliferation, and increased levels of the proapoptotic protein Bax [178]. In addition, lycopene may exert antitumor effects by down-regulating the NF-κB signaling pathway [179]. These effects are certainly demonstrated by preclinical evidence but need further confirmation in the clinical field, which, however, appears to be promising [178].
To summarize, lycopene exerts antioxidant, anti-inflammatory, and anticancer effects. In addition, it appears to have a protective role against obesity and diabetes, although prospective studies are needed to establish the possible therapeutic effect against metabolic disorders [180].
4. Folates
Folate is abundant in some vegetables and fruits, in particular leafy vegetables. In the body, it has the function of donating a one-carbon group in DNA methylation and synthesis [181]. An intake of 100 micrograms per day (found in about 160 g of spinach, including cooked spinach) has been associated with a 10 percent decrease in BC risk among women who drink moderate amounts of alcohol (a risk factor for breast cancer). [182]. However, although some studies suggest a reduction in cancer risk [183,184], other studies reported an increase. The influence of folate intake on BC risk may also be linked to menopausal status. Indeed, the study by Ren et al. showed a linear negative correlation between folate intake and reduced breast cancer risk, that is, each 100 μg/day increase in folate reduces BC risk by 2%, only in premenopausal women [185]. Moreover, Frederick et al. found increased folate concentration in the breast tissue of premenopausal women, while in the same study, obesity was associated with low serum folate levels. The authors believe that this finding may explain the discrepancies reported in the literature as regards the effects of folate on breast cancer. In fact, these authors hypothesize that folate may have a preventive effect in premenopausal women through an epigenetic mechanism, and a carcinogenesis-supporting effect in postmenopausal women by promoting tumor growth through DNA synthesis [186].
Epigenetic changes (such as DNA hypomethylation and hypermethylation) have been observed in some cancers, including BC, and several studies have shown an overexpression of folate receptors (FR) in different tumor surface types, including BC. This overexpression tends to increase as the disease progresses, which is considered a negative prognostic factor. Therefore, such receptors have been proposed as a useful target in personalized therapy against BC [187,188]. This target is particularly useful in triple-negative BC (TNBC), in which reduced folate intake or the use of antifolate drugs result in mitochondrial dysfunction, which reduces metabolic plasticity [189].
A very strong inverse association between folate intake and survival was also demonstrated in the prospective study by Harris et al. among patients with ER-negative BC. This effect could be attributed to the fact that insufficient folate intake can result in an improper methylation of DNA and RNA, thereby altering their integrity and repair, which are critical factors in cancer survival [190].
Moreover, a U-shaped relationship has been observed, indicating that increased folate intake before the development of preneoplastic lesions may prevent tumor formation, but the supplementation with folate after the lesions are present may promote tumor progression [185].
Furthermore, folate supplementation seems to be able to reduce chemotherapy drug toxicity, but an excessive supplementation increases drug resistance [191]. Such a window would need to be better defined, but it is likely that an adequate folate intake could fall within the ideal dose window to better balance the two effects.
Notably, McEligot observed that a plasma folate concentration of 25.2 nmol reduces the risk of death after BC diagnosis by about 30 percent, while 40.6 nmol of folate reduces the risk of death after BC diagnosis by more than 50 percent. However, a dose of 1000 μg appears to lose this effect, and it may also increase the risk [192].
5. Indole-3-Carbinolo (I3C), Di-Indoylmethane (DIM) E Sulforaphane
DIM and its precursor, I3C, are active compounds present in Brassicaceae or vegetables derived from them: broccoli, cabbages, Brussels sprouts, and savoy cabbage. Anticancer properties associated with cruciferous vegetables are mainly due to glucosinolates, a family of secondary metabolites, which are typical only of this family and play a dominant role in defending plants against parasites. Glucosinolates are beneficial for general health due to their ability to arrest the initial stages of carcinogenesis. The effects have been shown by the suppression of the proliferation of several cancerogenic cell lines; the induction of apoptosis, but not in healthy cells; and the modulation of estrogen metabolism, thereby reducing cancer risk in hormone-sensitive cells [193].
It is unlikely that the quantities present in the diet are sufficient to have therapeutic effects; thus, a supplementation can be considered [194]. Supplementation with red cabbage extracts containing isothiocyanates has shown interesting results in modulating gut microbiota. In particular, an increased production of butyric acid has been observed, and due to the possible regulation of food intake mediated by short-chain fatty acids, this finding could pave the way for a promising treatment for overweight or obesity [195].
6. Capsaicin
Capsaicin is a chemical compound found in varying concentrations in plants of the genus Capsicum such as hot pepper, which gives them their characteristic irritant power. Chili peppers are the main source of capsaicinoids in nature. Capsaicin content has been found to range from 2.19 to 19.73 mg/g dry weight of Capsicum fruits [196]. It could be used clinically to relieve pain, prevent cancer, and promote weight loss due to its antioxidant and anti-inflammatory properties. Capsaicin manifests anticancer activity through delicate epigenetic molecular mechanisms. On one hand, it inhibits the expression of vascular-endothelial growth factor) (VEGF), which is responsible for tumor vascularization and the angiogenic process that fuels the cell growth and the development of metastasis [197]. On the other hand, it facilitates apoptosis and induces antiproliferative effects through the reduction of FBI-1 protein expression. These effects were observed after intraperitoneal administration of 10 mg/Kg every three days for a total of 21 days [198].
7. Dietary Fiber and Whole Grains
Consumption of whole grains has been shown to be able to reduce the risk of obesity, diabetes, and cardiovascular diseases, as well as prevent cancer. Whole grains could influence obesity by promoting the sense of satiety, reducing calorie intake, and managing body fatness due to its high content of fiber, which can reduce energy density [199].
The high fiber content in whole grains could significantly control insulin resistance and the expression of insulin-like growth factors [200], and enhance metabolic flexibility [201]. In addition, decreasing body adiposity and insulin resistance may contribute to reducing cancer risk.
Whole grains, as well as fruit and vegetables, are rich in phytochemical substances such as phenolic acids [202], carotenoids, alkylresorcinols, phytosterols, lignans, anthocyanins, vitamin E, and polysaccharides [203].
Several experimental studies have shown that the bioactive compounds of whole grains exert anticancer activity through the inhibition of cellular multiplication, the modulation of the immune system, and the inhibition of tumor metastasis. Whole foods are sources of isoflavones like phytoestrogens, which may influence hormone levels and activity. Phytoestrogens combined with dietary fiber may decrease internal estrogen concentration, inhibit cancer development, and weaken the early expression of markers of BC risk, although possible negative effects should be carefully evaluated [204].
As for fiber intake, a protective association between fiber intake and BC risk is around a 12% reduction. In addition, an increase of every 10 g/d of dietary fiber is associated with a 4% risk reduction [205,206].
However, regarding the association between whole grain consumption and BC risk, discordant and inconsistent results are reported, possibly due to the different study design and the lack of adjustment for risk factors. Moreover, epidemiological studies have considered “whole grains” as a general parameter of consumption without specifying the different grain species and growing regions, and without accounting for the varying concentrations of bioactive phytochemicals that are associated with these factors.
There are not many studies investigating the effect of fiber intake in the postdiagnosis period of BC. In a randomized controlled trial, BC survivors who increased vegetable consumption by 65%, fruit consumption by 25%, and fiber consumption by 30% did not have reduced mortality and recurrences after a follow-up of 7.3 years [30]. Another study, despite the weak and not significant inverse association between fiber intake and BC mortality and recurrence, suggested that the positive trend should be considered because fiber intake reduces estrogen levels, modulates insulin resistance, and reduces levels of inflammatory cytokines such as IL-6, TNF-α-R2, and C-reactive protein [207]. The study by Villasenor et al. supports this hypothesis, as patients who had consumed more than 15 g/day of insoluble fiber, 24 months after diagnosis, had a 49% reduction in the likelihood of having elevated PCR concentrations compared to patients who consumed amounts of less than 5 g/day [208]. More recently, Zengul et al. did not find any correlation between dietary fiber and estrone or estradiol levels, but they found an inverse association between soluble fiber and estradiol levels. High fiber consumption was correlated with low levels of Clostridium hathewayi sp., Clostridium (Erysipelotrichaceae family), and increased Bacteroides uniformis. All of these bacteria are associated with increased ß-glucuronidase activity, which is important for luminal hormone metabolism [209].
In conclusion, there are no conclusive data regarding the beneficial effects of fiber intake on the prognosis of BC; however, the observed positive trend would merit further investigation.
8. N-3 Fatty Acids (or Omega-3)
The most abundant n-3 PUFA in the diet is alpha-linolenic acid (ALA), usually found in plants, while the n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are widely found in cold-water fatty fish such as salmon (1.04 g/100 g), sardines (992 mg/100 g), and mackerel (3 g/100 g). EPA and DHA can also be synthesized within the body from ALA through the activity of desaturase and elongase, which have a higher affinity for ALA than n-6 linoleic acid (LA), typical of some plant oils. However, modern dietary habits lead to a much higher intake of LA, with a consequent prevalence of n-6 arachidonic acid synthesis compared with EPA and DHA. The synthesis of long-chain PUFA depends on the activity of elongases and desaturases, which shows considerable variations among individuals [210,211,212,213].
The suggested intake of EPA+DHA is 500 mg/day [84] or from 0.5 g at 6 months old to 1.1/1.6 g for an adult person [209].
Large amounts of LA are found in vegetable oils, seeds, and nuts, while ALA is found mainly in leafy vegetables, chia seeds, nuts, seed oils, flaxseeds, and soybean oil, with varying amounts (from 0.5 g/100 g in soybeans oil to 10 g/100 g in nuts, and 50 g/100 g in flaxseeds). As for n-3 PUFAs, they can be conjugated with various amines, including dopamine and serotonin, and be further metabolized through enzymatic and nonenzymatic pathways [214]. Enzymatic mechanisms, through the action of cyclooxygenases (COX), lipoxygenases, and cytochrome P450, lead to the formation of oxygenated derivative compounds. All these products have been shown to be effective in inhibiting tumor growth, both in vivo and in vitro. Additionally, they exhibit immunomodulatory effects by attacking tumor cells while preserving healthy cells [214]. The peculiarity of unsaturated fatty acids such as n-3 PUFAs is that they are peroxidable and easily incorporated into membrane phospholipids, especially in BC cells with compromised membrane integrity, by breaking down or sequestering membrane proteins. Furthermore, they neutralize reactive oxygen species (ROS) produced by cancer cells [215]. Regarding the other compounds, n-3 PUFAs can modulate the expression of genes involved in cell death and lipid metabolism, while EPA and DHA also manage to produce metabolites that reduce inflammation. In particular, EPA, due to the action of COX and LOX, gives rise to prostaglandins, thromboxanes, and leukotrienes with anticancer and anti-inflammatory properties, thus inhibiting the growth and invasion of tumor cells [216]. The enrichment of the membranes with EPA and DHA induces an increase in resolvins and protectins, which are important for healthy cells by protecting them from toxic chemicals such as chemotherapy drugs [215,216,217]. The only supplement recommended by ESPEN for cancer cachexia is n-3 PUFAs [218], which can be of particular interest in TNBC [219]. Interestingly, omega-3 free fatty acids have been suggested to modulate the insulin signaling pathway and affect BC cell growth. Increased levels of insulin represent a risk factor for BC, and in vitro studies have shown that omega-3 free fatty acids are able to dampen the activating phosphorylation of protein AKT, which is a downstream target of the insulin receptor, and to inhibit the proliferation of MCF-7 cells [220]. DHA and EPA, by acting as ligands of PPARγ, are able to modulate PPARγ transcriptional activity with subsequent gene expression modulation, which inhibits mTOR signaling and stimulates autophagy [221]. Of note, DHA-dopamine and EPA-dopamine conjugates have been shown to reduce the in vitro viability of BC cell lines such as MCF-7 and MDA-MB-231, with no effects on breast non-cancerous epithelial cells. Both compounds have been found to stimulate the transcription of the autophagy regulator Beclin-1 by PPARγ, with potential antineoplastic effects [222].
This entry is adapted from the peer-reviewed paper 10.3390/antiox12101845
This entry is offline, you can click here to edit this entry!
