Anthocyanins (ACNs) have attracted considerable attention for their potential to modulate the immune system. Research has revealed their antioxidant and anti-inflammatory properties, which play a crucial role in immune regulation by influencing key immune cells, such as lymphocytes, macrophages, and dendritic cells. Moreover, ACNs contribute towards maintaining a balance between proinflammatory and anti-inflammatory cytokines, thus promoting immune health. Beyond their direct effects on immune cells, ACNs significantly impact gut health and the microbiota, essential factors in immune regulation. Emerging evidence suggests that they positively influence the composition of the gut microbiome, enhancing their immunomodulatory effects. Furthermore, these compounds synergize with other bioactive substances, such as vitamins and minerals, further enhancing their potential as immune-supporting dietary supplements.
- immune cells
- gut microbiota
- anthocyanins
1. Introduction
2. Chemistry and Natural Sources of Anthocyanins
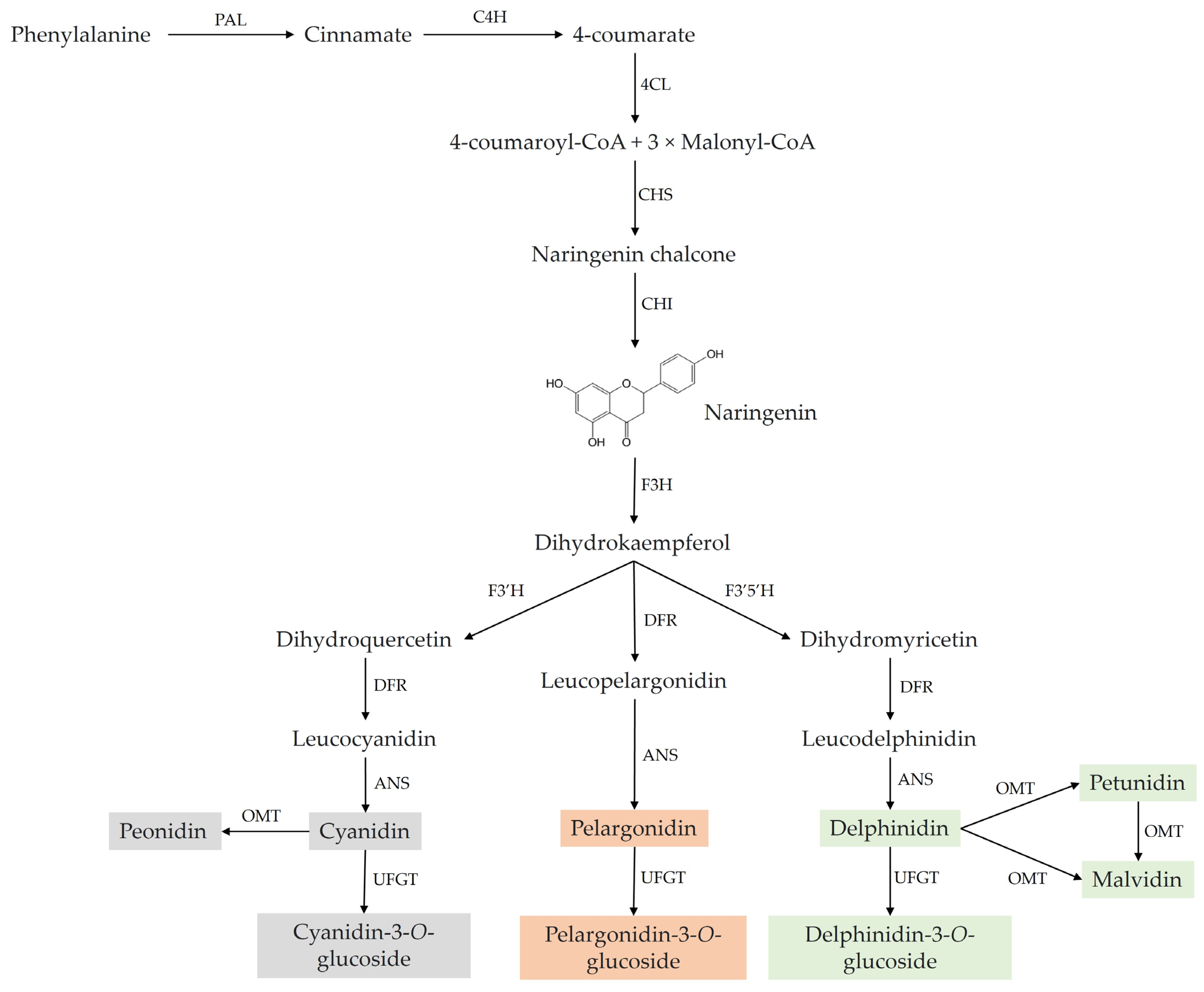
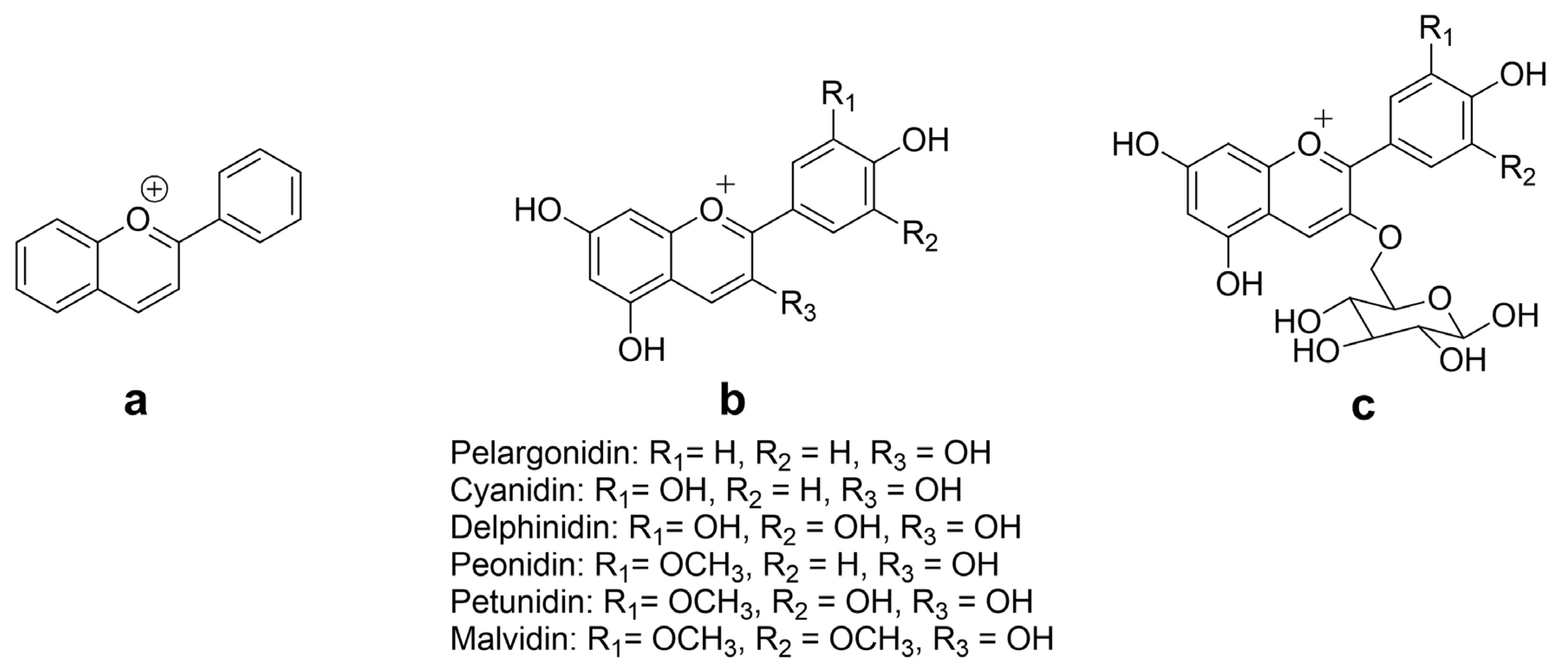
3. Immunomodulatory Potential of Anthocyanins
-
Inflammation disrupts immune homeostasis, leading to diverse diseases or disease conditions. However, ACNs have been proven to play a crucial role in regulating inflammatory pathways by inhibiting the production of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and proinflammatory mediators (COX, LOX, MPO, and PGE2), thereby offering protection against the development of various inflammatory conditions [26][29][47];
-
ACNs influence immune cell activation and proliferation by modulating various pathways. Moreover, they have a significant impact on gene expression within immune cells, leading to the heightened expression of genes responsible for antioxidant defense, anti-inflammatory pathways, and immune cell activation [26][48][49];
-
Recent research indicates that ACNs can influence gut microbiota composition and functionality. Given the pivotal role of the gut microbiota in regulating the immune system, this interaction has the potential to contribute significantly to the immunomodulatory effects exhibited by ACNs [50][51][52][53][54].
| Anthocyanin and Its Aglycons | Dose | Methodology | Major Findings | Reference |
|---|---|---|---|---|
| Cyanidin-3-glucoside | 100 μM | In Vitro Osteoclasts from C57BL/6J mice cultured with M-CSF (30 ng/mL) and the receptor activator of a nuclear factor kappa B ligand (RANKL) (100 ng/mL) and supplemented with the test sample. |
Inhibited osteoclast differentiation and formation induced by RANKL. | [57] |
| Protocatechuic acid (anthocyanin metabolite) |
0–25 μM | In Vitro Osteoclasts from ICR mice were cultured in the presence of M-CSF (30 ng/mL) and RANKL (50 ng/mL) and treated with the test sample. |
Inhibited RANKL-induced osteoclast differentiation and suppressed the bone-resorbing activity of mature osteoclasts. | [58] |
| 25 mg/kg/d | In Vivo Nine days of administration in LPS (5 mg/kg)-induced bone destruction in male ICR mice. |
Recovered the bone volume per tissue volume, trabecular separation, and trabecular number. | ||
| Delphinidin | 50 μM | In Vitro Jurkat E6-1 cells were preincubated with gadolinium and N-(4-[3,5-bis(trifluoromethyl)-1H-pyrazol-1-yl]phenyl)-4-methyl-1,2,3-thiadiazole-5-carboxamide for 15 min. |
Induced Ca2+ and Sr2+ influx through the CRAC channel and induced NFAT pathway activation; thereby, the production of IL-2 occurs. | [59] |
| Anthocyanidins | 5–20 μM | In Vitro TPA (20 ng/mL)-induced cell transformation was investigated in the parental JB6 Cl41 cells or AP-1-luciferase stable transfectant JB6 cells. |
Ortho-dihydroxyphenyl structure (delphinidin) on the B-ring of aglycon inhibited cell transformation and activator protein-1 transactivation. | [60] |
| Delphinidin | 25–100 μM | In Vitro RAW264 cells were treated with LPS (40 ng/mL) for 6 h. |
Inhibited MAPK-mediated COX-2 expression. | [61] |
| Cyanidin-3-O-galactoside and cyaniding-3-sambubioside rich extract of Sambucus ebulus | 200 mL infusion | Interventional study Fifty-three volunteers enrolled in a 4-week intervention involving the consumption of infusion. |
Decreased pro-inflammatory status and complemented activity markers in healthy volunteers. | [62] |
| Cyanidin-3-O-glucoside and peonidin-3-O-glucoside rich fraction of black rice germ and bran. | 0–200 μg/mL | In Vitro A549 cells and THP-1 macrophages exposed to SARS-CoV-2 spike glycoprotein S1 (100 ng/mL). |
Inhibited NF-κB activity and the NLRP3 inflammasome pathway, leading to the inhibition of inflammatory genes and cytokine secretions. | [63] |
| Cyanidin-3-O-glucoside | 0–20 μg/mL | |||
| Peonidin-3-O-glucoside | 0–20 μg/mL | |||
| Cyanidin-3-O-glucoside delivered by enteric sodium alginate | 25 mg/kg/d | In Vivo Male BALB/c mice received two sensitization doses of 100 µg ovalbumin and 2.0 mg alum, administered in 200 µL of phosphate-buffered saline via intraperitoneal injection on day 0 and day 14. |
Enhanced the intestinal epithelial barrier by up-regulating the tight junction protein expression and promoting secretory IgA and β-defensin secretion and regulated Th1/Th2 immune balance in the intestinal mucosa. | [64] |
| Anthocyanin-rich roselle extract | 0–400 mg/kg | In Vivo 1-day-old Ross 308 broiler chicks were fed on basal diets supplemented with extract for 35 days. |
Improved metabolic functions, blood biochemistry, intestinal morphology, antioxidant activity, immune status, and higher ω-3 content in the breast muscle. | [65] |
| Cyanidin-3-glucoside-enriched diet. | 26 mg/kg/d | Ex Vivo Myocardial ischemia/reperfusion (I/R) injury in mice. Pretreated with the sample for a month. |
Diet changed the microbiome and the transplantation of the fecal microbiota transferred the cardioprotection. | [66] |
| Total anthocyanin from boysenberry and apple juice concentrate | 2.5 mg/kg | In Vivo Ovalbumin-induced airway inflammation in male C57BL/6J mice. |
Reduced immune cell infiltration and tissue damage. | [67] |
| Cyanidin 3-O-glucoside | 10 mg/kg | In Vivo Five-week-old male spontaneously hypertensive and Wistar-Kyoto rats were treated daily for a period of 15 weeks. |
Treatment normalized the splenic production of TNFα and IFNγ and the proportion of CD62Llo and CD62L− helper T-cells. | [68] |
| Cyanidin-3-O-glucoside | 25 mg/kg | In Vivo Treated twice per week for six consecutive administrations in Sprague Dawley with bovine type II collagen-induced arthritis. |
The study suggests that CD38+ NK cells inhibiting Treg cell differentiation may contribute to the immune imbalance seen in both rheumatoid arthritis and collagen-induced arthritis. | [69] |
| 25–100 μM | In Vitro RA synovial fibroblasts collected from arthritic patients. |
|||
| 50 μM | In Vitro Mononuclear cells were collected from arthritic patients. |
|||
| 50 μM | In Vitro Mononuclear cells were cocultured with CD38+ NK cells. |
|||
| Delphinidin-3-O-glucoside and cyanidin-3-O-glucoside | 50–600 µg/mL | In Vitro Both the compounds alone or in combinations were added and incubated with HCT-116 and HT-29 human colorectal cancer cells for 24 h. |
Both showed potential for binding with and inhibiting immune checkpoints, PD-1 and PD-L1, which can activate the immune response in the tumor microenvironment and induce cancer cell death. | [70] |
| Anthocyanin-rich mix | 40 mg/kg BW | In Vivo High-fat-diet-induced obesity, dyslipidemia, insulin resistance, and steatosis in C57BL/6J mice. |
Increased glucagon-like peptide-2 levels, the intestinal hormone that upregulates tight junction protein expression. | [71] |
| Anthocyanin-rich mix (AC mix) | 5 μg/mL | In Vitro Caco-2 cell monolayers were pre-incubated for 24 h with IFNγ (10 ng/mL) to upregulate the TNFα receptor. |
PCA, C3G, and the AC mix, fully prevented p65, ERK1/2, and MLC phosphorylation. D3G inhibited TNFα-triggered p65 phosphorylation; Peo3G had no effect. | |
| Protocatechuic acid (PCA) | 0.5 μM | |||
| Delphinidin-3-O-glucoside (D3G) | 0.5 μM | |||
| Cyanidin-3-O-glucoside (C3G) | 1 μM | |||
| Peonidin-3-O-glucoside (Peo3G) | 0.1 μM | |||
| Cyanidin-3-glucoside-rich anthocyanin extract of Lonicera caerulea fruit | 0–0.8 mg/mL | In Vitro Human hepatoma cell line SMMC-7721. |
Inhibits proliferation of the tumor cells. | [72] |
| 50, 100 and 200 mg/kg BW | In Vivo Tumor-bearing male Kunming strain mice model was established using the murine H22 hepatoma cells line. Treated with sample for 15 days. |
Extract effectively combats tumours by dynamically adjusting the redox balance and enhancing immunoregulatory activity. | ||
| Cyanidin-3-O-glucoside | 20 and 40 μM | In Vitro TNF-α-stimulated intestinal cells tested on endothelial cell activation by using cocultures (Caco-2, HUVEC, and mononuclear cells) |
Inhibited NF-κB pathway in epithelial cells. | [73] |
| Cyanidin chloride and cyanidin 3-O-glucoside | 0–25 μM | In Vitro LPS- (100 ng/mL) or IFNγ- (10 ng/mL) induction in immortalized mouse microglial (bv-2) cells pretreated with samples. |
Both compounds reduced ROS production in a dose-dependent manner. Cyanidin chloride showed a maximum decrease in NO production. | [74] |
| Cyanidin-3-glucoside chloride | 1.25–5.0 μg/mL | In Vitro EL-4 T cells were pretreated with various concentrations of the sample for 1 h and stimulated with PMA/ionomycin for 16 h. |
Suppressed Th2 activation through the downregulation of Th2 cytokines and the GATA3 transcription factor. | [75] |
| Black raspberry Ethanol extract | 0–200 μg/mL | In Vitro Normal donor peripheral blood mononuclear cells were induced with IL-6 and GM-CSF (10 ng/mL) and were pretreated with samples for 7 days. |
Limited expansion of myeloid-derived suppressor cells (MDSC). Attenuated IL-6-mediated phosphorylation of STAT3 and IL-2-induced STAT5 phosphorylation. Pretreatment of immune cells inhibited MDSC expansion and IL-6-mediated STAT3 signalling. |
[76] |
| Cyanidin-3-rutinoside | 0–200 μM | |||
| Delphinidin-3-glucoside | 0–100 μM | In Vitro BM185 cells were incubated with test samples for 48 h. |
Inhibited cell proliferation dose-dependently. | [77] |
| Cyanidin-3-rutinoside | 0–100 μM | |||
| Anthocyanin-rich berry extract | 10–100 μg/mL | |||
| Cyanidin-3-rutinoside | 50 mg/kg/d | In Vivo Eight-week-old female Balb/c mice challenged with BM185 cells treated with samples for 21 days. |
Moderate induction of caspases. | [77] |
| Anthocyanin-rich berry extract | 500 mg/kg/d | |||
| Cyanidin glycosides-rich juice (total polyphenol = 236 mg) | 330 mL/d | Clinical trial Randomized, crossover study divided into five periods each lasting two weeks. |
Enhanced antioxidant status, reduced oxidative DNA damage, and stimulated immune cell functions (IL-2 production). |
[78] |
| Proanthocyanidins | 30 µM | In Vitro LPS (10 ng/mL) or IL-4 (1 ng/mL) activated murine J774 macrophages. LPS (10 ng/mL) or IL-4 (1 ng/mL) activated human U-937 monocytes. |
Enhanced the expression of Arg-1 and MRC-1 in a dose-dependent manner. Enhanced the phosphorylation of STAT6. Increased CCL-17 expression. |
[79] |
| Delphinidin 3-O-β-d-glycoside | 50 µM | In Vitro LPS-induced mesenchymal stem cells. |
Modified the immunomodulatory properties and increased the IL-10 production. | [80] |
| Black lentil water extract | 600 mg/kg BW | In Vivo Azoxymethane/dextran sodium sulphate-induced C57BL/6 mice model. |
Modulated cytokines are involved in mediating communication between immune cells and anti-inflammatory action. Reduced colon mucosa of markers like IL-1β and IL-6 and gene expression, such as CXCR2 and CSF3. |
[81] |
| Delphinidin 3-O-(2-O-β-D-glucopyranosyl-α-L-arabinopyranoside rich lentil extract | 41 mg/kg BW | |||
| Delphinidin chloride | 20 and 40 µM | In Vitro CD4+ T cells were purified from the spleen of C57BL/6 mice and were activated with 1 µg/mL of plate-bound anti-CD3 and plate-bound anti-CD28 antibodies in the presence samples for 3 days. |
Promoted the differentiation of Tregs and enhanced immune response control. | [82] |
| 1.5 and 3 mg/kg | In Vivo allograft model Five-week-old male C57BL/6 mice were subcutaneously injected with a fixed number of P815 cells |
Reduced activated T cells by promoting Treg differentiation. | ||
| Delphinidin | 10−2 g/L | In Vitro T-lymphocytes from non-metabolic-syndrome and metabolic syndrome patients were stimulated for 24 h with the sample and 5 µg/mL of PHA. |
Inhibited Ca2+ signalling via reduced store-operated Ca2+ entry and release and the subsequent decrease of HDAC and NFAT activations. Also inhibited ERK1/2 activation and suppressed differentiation of T cells toward Th1, Th17, and Treg without affecting Th2 subsets. Prevented the PHA-induced proliferation in cells isolated from WT. Inhibited HDAC activity by a mechanism downstream of its effect on Ca2+ signaling. |
[83] |
| Peripheral blood mononuclear cells isolated either from 57BL/6 females ERα Wild-Type or Knock-Out mice stimulated for 48 h with 5 µg/mL of PHA. | ||||
| T-lymphocytes stimulated with thapsigargin (1 µM). | ||||
| Delphinidin | 0–20 µM | In Vitro Normal Human epidermal keratinocytes treated with rhIL-22 and TPA. |
Suppressed key kinases involved in psoriasis pathogenesis and alleviated IMQ-induced murine psoriasis-like disease, suggesting a novel PI3K/AKT/mTOR pathway modulator for psoriasis. | [84] |
| Delphinidin | 0–50 µM | In Vitro Caspase activation in human prostate LNCaP and Du145 cells treated with sample for 12 h. |
Sensitized prostate cancer cells to TRAIL-induced apoptosis by inducing DR5, causing caspase-mediated HDAC3 cleavage. | [85] |
| Delphinidin | 0–50 µM | In Vitro MH7A and Jurkat cells were treated with the sample and cultured for 2 h with TNFα or LPS. |
Inhibited NF-ĸB expression, thereby decreasing cytokine expression. | [86] |
| Resveratrol and peonidin 3-O-glucoside rich red grape vine leaf extract | 100 μg/mL | In Vitro THP-1 cells were stimulated with 2 μg/mL Cy3-labeled DNA for 4 h. |
Restricted the AIM2 inflammasome activation by preventing DNA entry. | [87] |
| 140 mg/kg | In Vivo Treated in IMQ-induced BALB/c mice (6–9 weeks old) model for 5 days. |
Inhibited proinflammatory caspase-1 activation, IL-1β maturation, and IL-17 production. | ||
| Malvidin-3-O-glucoside | 10 µM | In Vitro Murine microglia primary cultures primed and activated with LPS. |
Targets NLRP3, NLRC4, and AIM2 inflammasomes, subsequently reducing caspase-1 and IL-1β protein levels. Stopped the release of IL-1β in the hippocampus. |
[88] |
| 12.5 mg/kg/d | In Vivo Chronic unpredictable stress was subjected to the mice for 28 days, consisting of different stressors. A 2-week pretreatment was followed. |
|||
| Malvidin-3-O-glucoside | 0.5 µg/kg/d | In Vivo C57BL/6 male mice were treated with the sample for 2 weeks before and throughout repeated social defeat stress and then performed social avoidance/interaction testing. |
Modulated synaptic plasticity by increasing histone acetylation of the regulatory sequences of the Rac1 gene. | [89] |
4. Gut Microbiota in the Immunity and Metabolism of Anthocyanins
5. Nutraceutical Potential of Anthocyanins
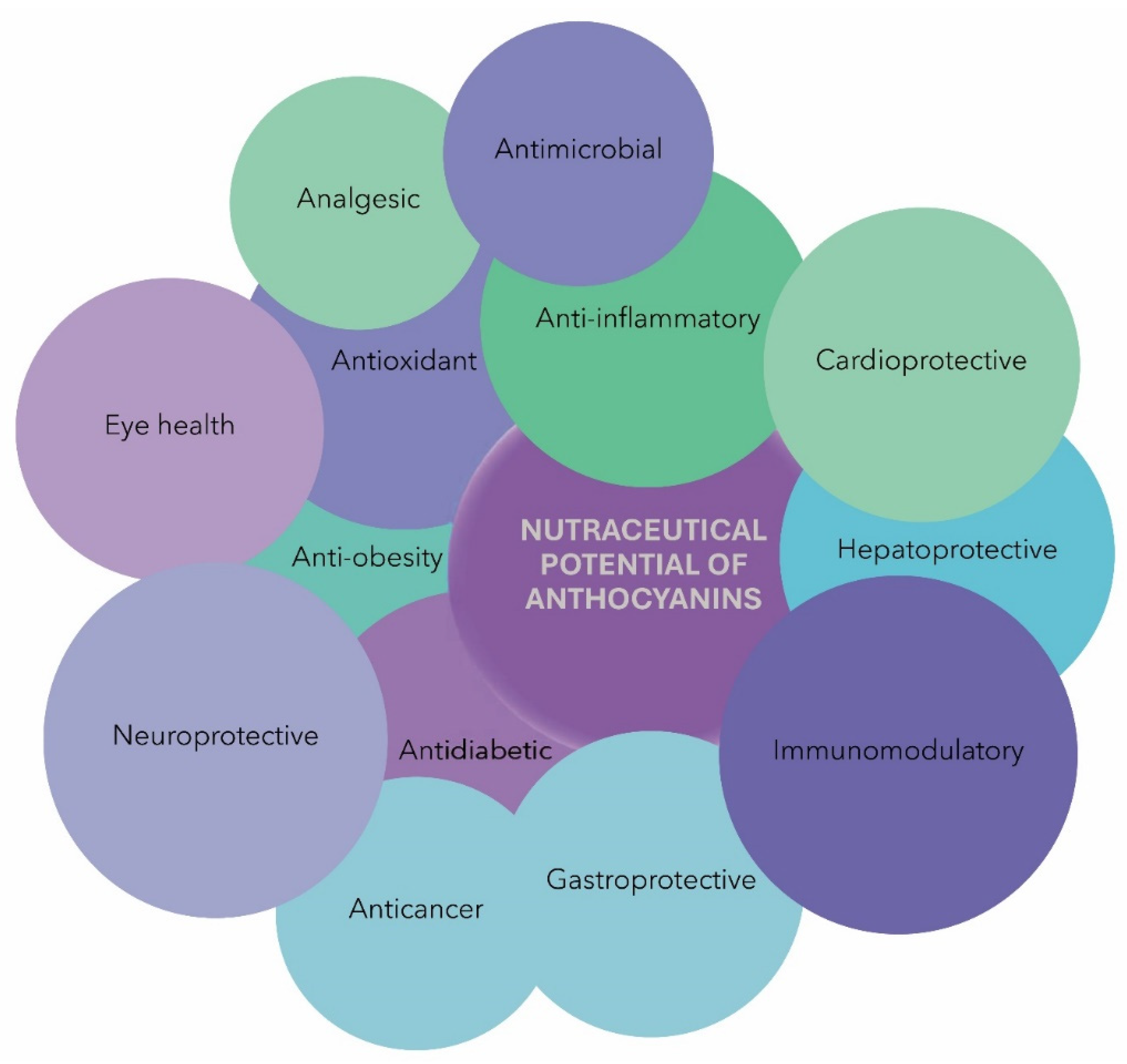
This entry is adapted from the peer-reviewed paper 10.3390/nu15194152
References
- Mei, H.; Dong, X.; Wang, Y.; Tang, L.; Hu, Y. Managing patients with cancer during the COVID-19 pandemic: Frontline experience from Wuhan. Lancet Oncol. 2020, 21, 634–636.
- Mastrandrea, L.D. An overview of organ-specific autoimmune diseases including immunotherapy. Immunol. Investig. 2015, 44, 803–816.
- Xu, H.; Jia, S.; Xu, H. Potential therapeutic applications of exosomes in different autoimmune diseases. Clin. Immunol. 2019, 205, 116–124.
- Rusek, P.; Wala, M.; Druszczyńska, M.; Fol, M. Infectious agents as stimuli of trained innate immunity. Int. J. Mol. Sci. 2018, 19, 456.
- Mrak, D.; Tobudic, S.; Koblischke, M.; Graninger, M.; Radner, H.; Sieghart, D.; Hofer, P.; Perkmann, T.; Haslacher, H.; Thalhammer, R.; et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann. Rheum. Dis. 2021, 80, 1345–1350.
- Wang, Y.; Jia, A.; Bi, Y.; Wang, Y.; Yang, Q.; Cao, Y.; Li, Y.; Liu, G. Targeting myeloid-derived suppressor cells in cancer immunotherapy. Cancers 2020, 12, 2626.
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558.
- Ouyang, W.; O’Garra, A. IL-10 family cytokines IL-10 and IL-22: From basic science to clinical translation. Immunity 2019, 50, 871–891.
- Galon, J.; Bruni, D. Tumor immunology and tumor evolution: Intertwined histories. Immunity 2020, 52, 55–81.
- Macedo, A.C.; de Faria, A.O.V.; Ghezzi, P. Boosting the immune system, from science to myth: Analysis the infosphere with google. Front. Med. 2019, 6, 165.
- Wagner, D.N.; Marcon, A.R.; Caulfield, T. “Immune boosting” in the time of COVID: Selling immunity on instagram. Allergy Asthma Clin. Immunol. 2020, 16, 76.
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl. Health Stat. Report. 2015, 79, 1–16.
- Grand View Research, Dietary Supplements Market Size, Share & Trend Analysis Report by Ingredient (Botanicals, Vitamins, Minerals, Amino Acids, Enzymes), by Product, by Application, by End-use, and Segment Forecasts, 2018–2024. 2018. Available online: https://web.archive.org/web/20190115112358/https://www.grandviewresearch.com/industry-analysis/dietary-supplements-market (accessed on 2 June 2023).
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Buenz, E.J.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.-J.R.; Izzo, A.A.; Maffia, P.; et al. Future directions for the discovery of natural product-derived immunomodulating drugs: An IUPHAR positional review. Pharmacol. Res. 2022, 177, 106076.
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Front. Immunol. 2021, 12, 637553.
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Publisher correction: Harnessing innate immunity in cancer therapy. Nature 2019, 576, E3.
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-derived nutraceuticals and immune system modulation: An evidence-based overview. Vaccines 2020, 8, 468.
- Maheshwari, S.; Kumar, V.; Bhadauria, G.; Mishra, A. Immunomodulatory potential of phytochemicals and other bioactive compounds of fruits: A Review. Food Front. 2022, 3, 221–238.
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Corrigendum: Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2018, 9, 1178.
- Gupta, B.; Singh, V.R.; Verma, S.; Meshram, N.; Dhruw, L.; Sharma, R.; Ghosh, K.K.; Gupta, R.C. Plant and Food Derived Immunomodulators as Nutraceuticals for Performance Enhancing Activities. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 593–601.
- Kim, J.H.; Kim, D.H.; Jo, S.; Cho, M.J.; Cho, Y.R.; Lee, Y.J.; Byun, S. Immunomodulatory functional foods and their molecular mechanisms. Exp. Mol. Med. 2022, 54, 1–11.
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins: Anthocyanins and human health. Phytother. Res. 2016, 30, 1265–1286.
- do Rosario, V.A.; Chang, C.; Spencer, J.; Alahakone, T.; Roodenrys, S.; Francois, M.; Weston-Green, K.; Hölzel, N.; Nichols, D.S.; Kent, K.; et al. Anthocyanins attenuate vascular and inflammatory responses to a high fat high energy meal challenge in overweight older adults: A cross-over, randomized, double-blind clinical trial. Clin. Nutr. 2021, 40, 879–889.
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. In Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–35.
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and proanthocyanidins: Chemical structures, food sources, bioactivities, and product development. Food Rev. Int. 2022, 39, 4581–4609.
- Kozłowska, A.; Dzierżanowski, T. Targeting inflammation by anthocyanins as the novel therapeutic potential for chronic diseases: An update. Molecules 2021, 26, 4380.
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622.
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. Biosynthesis and Stability of Anthocyanins. In Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Riaz, M., Zia-Ul-Haq, M., Saad, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 71–86.
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089.
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451.
- Calderaro, A.; Barreca, D.; Bellocco, E.; Smeriglio, A.; Trombetta, D.; Laganà, G. Colored Phytonutrients: Role and Applications in the Functional Foods of Anthocyanins. In Phytonutrients in Food: From Traditional to Rational Usage; Nabavi, S.M., Suntar, I., Barreca, D., Khan, H., Eds.; Elsevier: Amsterdam, The Netherlands; Woodhead Publishing: Sawston, UK, 2020; pp. 177–195.
- Onyilagha, J.C.; Grotewold, E. The biology and structural distribution of surface flavonoids. Recent Res. Dev. Plant Sci. 2004, 2, 53–71.
- Harbone, J.B. The Flavonoids: Recent Advances. In Plant Pigments; Goodwin, T.W., Ed.; Academic Press: San Diego, CA, USA, 1988; pp. 299–343.
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749.
- Sasaki, N.; Nishizaki, Y.; Ozeki, Y.; Miyahara, T. The role of acyl-glucose in anthocyanin modifications. Molecules 2014, 19, 18747–18766.
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615.
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128.
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500.
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144.
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30.
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Touillaud, M.; Kaaks, R.; Teucher, B.; Mattiello, A.; Grioni, S.; et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2011, 106, 1090–1099.
- Bellocco, E.; Barreca, D.; Laganà, G.; Calderaro, A.; El Lekhlifi, Z.; Chebaibi, S.; Smeriglio, A.; Trombetta, D. Cyanidin-3- O -Galactoside in ripe pistachio (Pistachia vera L. Variety Bronte) Hulls: Identification and evaluation of its antioxidant and cytoprotective activities. J. Funct. Foods 2016, 27, 376–385.
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225.
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: A review. Front. Chem. 2018, 6, 52.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; Anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Rev. Int. 2021, 37, 759–811.
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An insight into anti-inflammatory activities and inflammation related diseases of anthocyanins: A review of both in vivo and in vitro investigations. Int. J. Mol. Sci. 2021, 22, 11076.
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food Chem. Toxicol. 2020, 135, 110922.
- Samarpita, S.; Ganesan, R.; Rasool, M. Cyanidin prevents the hyperproliferative potential of fibroblast-like synoviocytes and disease progression via targeting IL-17A cytokine signalling in rheumatoid arthritis. Toxicol. Appl. Pharmacol. 2020, 391, 114917.
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, microbiome and health benefits in aging. Molecules 2021, 26, 537.
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of anthocyanin on intestinal health: A systematic review. Nutrients 2021, 13, 1331.
- Chen, K.; Kortesniemi, M.K.; Linderborg, K.M.; Yang, B. Anthocyanins as promising molecules affecting energy homeostasis, inflammation, and gut microbiota in type 2 diabetes with special reference to impact of acylation. J. Agric. Food Chem. 2023, 71, 1002–1017.
- Kapoor, P.; Tiwari, A.; Sharma, S.; Tiwari, V.; Sheoran, B.; Ali, U.; Garg, M. Effect of anthocyanins on gut health markers, firmicutes-bacteroidetes ratio and short-chain fatty acids: A systematic review via meta-analysis. Sci. Rep. 2023, 13, 1729.
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2023; ahead of print.
- Kumazawa, Y.; Takimoto, H.; Matsumoto, T.; Kawaguchi, K. Potential use of dietary natural products, especially polyphenols, for improving type-1 allergic symptoms. Curr. Pharm. Des. 2014, 20, 857–863.
- Percival, S.S. Grape consumption supports immunity in animals and humans. J. Nutr. 2009, 139, 1801S–1805S.
- Park, K.H.; Gu, D.R.; So, H.S.; Kim, K.J.; Lee, S.H. Dual role of cyanidin-3-glucoside on the differentiation of bone cells. J. Dent. Res. 2015, 94, 1676–1683.
- Park, S.-H.; Kim, J.-Y.; Cheon, Y.-H.; Baek, J.M.; Ahn, S.-J.; Yoon, K.-H.; Lee, M.S.; Oh, J. Protocatechuic acid attenuates osteoclastogenesis by downregulating JNK/c-Fos/NFATc1 signaling and prevents inflammatory bone loss in mice: PCA attenuates osteoclastogenesis. Phytother. Res. 2016, 30, 604–612.
- Jara, E.; Hidalgo, M.A.; Hancke, J.L.; Hidalgo, A.I.; Brauchi, S.; Nuñez, L.; Villalobos, C.; Burgos, R.A. Delphinidin activates NFAT and induces IL-2 production through SOCE in T cells. Cell Biochem. Biophys. 2014, 68, 497–509.
- Hou, D.-X.; Kai, K.; Li, J.-J.; Lin, S.; Terahara, N.; Wakamatsu, M.; Fujii, M.; Young, M.R.; Colburn, N. Anthocyanidins inhibit activator protein 1 activity and cell transformation: Structure-activity relationship and molecular mechanisms. Carcinogenesis 2003, 25, 29–36.
- Hou, D.-X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure–activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425.
- Kiselova-Kaneva, Y.; Nashar, M.; Roussev, B.; Salim, A.; Hristova, M.; Olczyk, P.; Komosinska-Vassev, K.; Dincheva, I.; Badjakov, I.; Galunska, B.; et al. Sambucus Ebulus (Elderberry) fruits modulate inflammation and complement system activity in humans. Int. J. Mol. Sci. 2023, 24, 8714.
- Semmarath, W.; Mapoung, S.; Umsumarng, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Cyanidin-3-O-glucoside and peonidin-3-o-glucoside-rich fraction of black rice germ and bran suppresses inflammatory responses from SARS-CoV-2 spike glycoprotein S1-induction in vitro in A549 lung cells and THP-1 macrophages via inhibition of the NLRP3 inflammasome pathway. Nutrients 2022, 14, 2738.
- Li, J.; Zou, C.; Liu, Y. Amelioration of ovalbumin-induced food allergy in mice by targeted rectal and colonic delivery of cyanidin-3-o-glucoside. Foods 2022, 11, 1542.
- Amer, S.A.; Al-Khalaifah, H.S.; Gouda, A.; Osman, A.; Goda, N.I.A.; Mohammed, H.A.; Darwish, M.I.M.; Hassan, A.M.; Mohamed, S.K.A. Potential effects of anthocyanin-rich roselle (Hibiscus sabdariffa L.) extract on the growth, intestinal histomorphology, blood biochemical parameters, and the immune status of broiler chickens. Antioxidants 2022, 11, 544.
- Trinei, M.; Carpi, A.; Menabo’, R.; Storto, M.; Fornari, M.; Marinelli, A.; Minardi, S.; Riboni, M.; Casciaro, F.; DiLisa, F.; et al. Dietary intake of cyanidin-3-glucoside induces a long-lasting cardioprotection from ischemia/reperfusion injury by altering the microbiota. J. Nutr. Biochem. 2022, 101, 108921.
- Shaw, O.M.; Hurst, R.D.; Cooney, J.; Sawyer, G.M.; Dinnan, H.; Martell, S. Boysenberry and apple juice concentrate reduced acute lung inflammation and increased M2 macrophage-associated cytokines in an acute mouse model of allergic airways disease. Food Sci. Nutr. 2021, 9, 1491–1503.
- Aloud, B.M.; Petkau, J.C.; Yu, L.; McCallum, J.; Kirby, C.; Netticadan, T.; Blewett, H. Effects of cyanidin 3-o-glucoside and hydrochlorothiazide on T-cell phenotypes and function in spontaneously hypertensive rats. Food Funct. 2020, 11, 8560–8572.
- Wang, H.; Li, S.; Zhang, G.; Wu, H.; Chang, X. Potential therapeutic effects of cyanidin-3-o-glucoside on rheumatoid arthritis by relieving inhibition of CD38+ NK cells on Treg cell differentiation. Arthritis Res. Ther. 2019, 21, 220.
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, delphinidin-3-o-glucoside and cyanidin-3-o-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560.
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269.
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-tumor properties of anthocyanins from Lonicera caerulea ‘Beilei’ fruit on human hepatocellular carcinoma: In vitro and in vivo study. Biomed. Pharmacother. 2018, 104, 520–529.
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Busà, R.; Saija, A.; Speciale, A. Cyanidin-3-o-glucoside modulates the in vitro inflammatory crosstalk between intestinal epithelial and endothelial cells. Mediators Inflamm. 2017, 2017, 3454023.
- Simonyi, A.; Chen, Z.; Jiang, J.; Zong, Y.; Chuang, D.Y.; Gu, Z.; Lu, C.-H.; Fritsche, K.L.; Greenlief, C.M.; Rottinghaus, G.E.; et al. Inhibition of microglial activation by elderberry extracts and its phenolic components. Life Sci. 2015, 128, 30–38.
- Pyo, M.Y.; Yoon, S.J.; Yu, Y.; Park, S.; Jin, M. Cyanidin-3-glucoside suppresses Th2 cytokines and GATA-3 transcription factor in EL-4 T cells. Biosci. Biotechnol. Biochem. 2014, 78, 1037–1043.
- Mace, T.A.; King, S.A.; Ameen, Z.; Elnaggar, O.; Young, G.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Knobloch, T.J.; Weghorst, C.M.; et al. Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signaling. Cancer Immunol. Immunother. 2014, 63, 889–900.
- Köchling, J.; Schmidt, M.; Rott, Y.; Sagner, M.; Ungefroren, H.; Wittig, B.; Henze, G. Can anthocyanins improve maintenance therapy of Ph(+) acute lymphoblastic leukaemia? Eur. J. Haematol. 2013, 90, 291–300.
- Bub, A.; Watzl, B.; Blockhaus, M.; Briviba, K.; Liegibel, U.; Müller, H.; Pool-Zobel, B.L.; Rechkemmer, G. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J. Nutr. Biochem. 2003, 14, 90–98.
- Ryyti, R.; Hämäläinen, M.; Leppänen, T.; Peltola, R.; Moilanen, E. Phenolic compounds known to be present in lingonberry (Vaccinium Vitis-Idaea L.) enhance macrophage polarization towards the anti-inflammatory M2 phenotype. Biomedicines 2022, 10, 3045.
- Neves, B.R.O.; de Freitas, S.; Borelli, P.; Rogero, M.M.; Fock, R.A. Delphinidin-3-O-glucoside in vitro suppresses NF-ΚB and changes the secretome of mesenchymal stem cells affecting macrophage activation. Nutrition 2023, 105, 111853.
- Mazewski, C.; Luna-Vital, D.; Berhow, M.; Gonzalez de Mejia, E. Reduction of colitis-associated colon carcinogenesis by a black lentil water extract through inhibition of inflammatory and immunomodulatory cytokines. Carcinogenesis 2020, 41, 790–803.
- Hyun, K.H.; Gil, K.C.; Kim, S.G.; Park, S.-Y.; Hwang, K.W. Delphinidin chloride and its hydrolytic metabolite gallic acid promote differentiation of regulatory T cells and have an anti-inflammatory effect on the allograft model. J. Food Sci. 2019, 84, 920–930.
- Dayoub, O.; Le Lay, S.; Soleti, R.; Clere, N.; Hilairet, G.; Dubois, S.; Gagnadoux, F.; Boursier, J.; Martínez, M.C.; Andriantsitohaina, R. Estrogen receptor α/HDAC/NFAT axis for delphinidin effects on proliferation and differentiation of T lymphocytes from patients with cardiovascular risks. Sci. Rep. 2017, 7, 9378.
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Satyshur, K.A.; Syed, D.N.; Dodwad, S.-J.M.; Chaves-Rodriquez, M.-I.; Longley, B.J.; et al. Dual inhibition of PI3K/Akt and MTOR by the dietary antioxidant, delphinidin, ameliorates psoriatic features in vitro and in an imiquimod-induced psoriasis-like disease in mice. Antioxid. Redox Signal. 2017, 26, 49–69.
- Ko, H.; Jeong, M.-H.; Jeon, H.; Sung, G.-J.; So, Y.; Kim, I.; Son, J.; Lee, S.-W.; Yoon, H.-G.; Choi, K.-C. Delphinidin sensitizes prostate cancer cells to TRAIL-induced apoptosis, by inducing DR5 and causing caspase-mediated HDAC3 cleavage. Oncotarget 2015, 6, 9970–9984.
- Seong, A.-R.; Yoo, J.-Y.; Choi, K.; Lee, M.-H.; Lee, Y.-H.; Lee, J.; Jun, W.; Kim, S.; Yoon, H.-G. Delphinidin, a specific inhibitor of histone acetyltransferase, suppresses inflammatory signaling via prevention of NF-ΚB acetylation in fibroblast-like synoviocyte MH7A cells. Biochem. Biophys. Res. Commun. 2011, 410, 581–586.
- Chung, I.-C.; Yuan, S.-N.; OuYang, C.-N.; Hu, S.-I.; Lin, H.-C.; Huang, K.-Y.; Lin, W.-N.; Chuang, Y.-T.; Chen, Y.-J.; Ojcius, D.M.; et al. EFLA 945 restricts AIM2 inflammasome activation by preventing DNA entry for psoriasis treatment. Cytokine 2020, 127, 154951.
- Iban-Arias, R.; Sebastian-Valverde, M.; Wu, H.; Lyu, W.; Wu, Q.; Simon, J.; Pasinetti, G.M. Role of polyphenol-derived phenolic acid in mitigation of inflammasome-mediated anxiety and depression. Biomedicines 2022, 10, 1264.
- Wang, J.; Hodes, G.E.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M.; et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 2018, 9, 477.
- Eisenstein, M. Microbiome: Bacterial broadband. Nature 2016, 533, S104–S106.
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533.
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104 (Suppl. S3), S48–S66.
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533.
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141.
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441.
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.-O.; Carbonero, F. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018, 59, 160–172.
- Bresciani, L.; Angelino, D.; Vivas, E.I.; Kerby, R.L.; García-Viguera, C.; Del Rio, D.; Rey, F.E.; Mena, P. Differential catabolism of an anthocyanin-rich elderberry extract by three gut microbiota bacterial species. J. Agric. Food Chem. 2020, 68, 1837–1843.
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24.
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2019, 9, 2.
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224.
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991.
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894.
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188.
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66.
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18.
- Zhao, R.; Shen, G.X. Impact of anthocyanin component and metabolite of saskatoon berry on gut microbiome and relationship with fecal short chain fatty acids in diet-induced insulin resistant mice. J. Nutr. Biochem. 2023, 111, 109201.
- Dong, A.; Lin, C.-W.; Echeveste, C.E.; Huang, Y.-W.; Oshima, K.; Yearsley, M.; Chen, X.; Yu, J.; Wang, L.-S. Protocatechuic acid, a gut bacterial metabolite of black raspberries, inhibits adenoma development and alters gut microbiome profiles in Apcmin/+ mice. J. Cancer Prev. 2022, 27, 50–57.
- Chen, W.; Zhu, R.; Ye, X.; Sun, Y.; Tang, Q.; Liu, Y.; Yan, F.; Yu, T.; Zheng, X.; Tu, P. Food-derived cyanidin-3-o-glucoside reverses microplastic toxicity via promoting discharge and modulating the gut microbiota in mice. Food Funct. 2022, 13, 1447–1458.
- Mu, J.; Xu, J.; Wang, L.; Chen, C.; Chen, P. Anti-inflammatory effects of purple sweet potato anthocyanin extract in DSS-induced colitis: Modulation of commensal bacteria and attenuated bacterial intestinal infection. Food Funct. 2021, 12, 11503–11514.
- Liu, X.; Wang, L.; Jing, N.; Jiang, G.; Liu, Z. Biostimulating gut microbiome with bilberry anthocyanin combo to enhance anti-PD-L1 efficiency against murine colon cancer. Microorganisms 2020, 8, 175.
- Nakano, H.; Wu, S.; Sakao, K.; Hara, T.; He, J.; Garcia, S.; Shetty, K.; Hou, D.-X. Bilberry anthocyanins ameliorate NAFLD by improving dyslipidemia and gut microbiome dysbiosis. Nutrients 2020, 12, 3252.
- Liu, F.; Wang, T.T.Y.; Tang, Q.; Xue, C.; Li, R.W.; Wu, V.C.H. Malvidin 3--glucoside modulated gut microbial dysbiosis and global metabolome disrupted in a murine colitis model induced by dextran sulfate sodium. Mol. Nutr. Food Res. 2019, 63, 1900455.
- Tan, J.; Li, Y.; Hou, D.-X.; Wu, S. The effects and mechanisms of cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants 2019, 8, 479.
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309.
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Colour and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181.
- Tamura, H.; Yamagami, A. Antioxidative activity of monoacylated anthocyanins isolated from Muscat Bailey a grape. J. Agric. Food Chem. 1994, 42, 1612–1615.
- Tsuda, T.; Shiga, K.; Ohshima, K.; Kawakishi, S.; Osawa, T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem. Pharmacol. 1996, 52, 1033–1039.
- Brown, J.E.; Kelly, M.F. Inhibition of lipid peroxidation by anthocyanins, anthocyanidins and their phenolic degradation products. Eur. J. Lipid Sci. Technol. 2007, 109, 66–71.
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011, 11, 770–789.
- Speciale, A.; Anwar, S.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol. Nutr. Food Res. 2013, 57, 1979–1987.
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-B and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358.
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich Açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093.
- Hassimotto, N.M.A.; Moreira, V.; do Nascimento, N.G.; Souto, P.C.M.d.C.; Teixeira, C.; Lajolo, F.M. Inhibition of carrageenan-induced acute inflammation in mice by oral administration of anthocyanin mixture from wild mulberry and cyanidin-3-glucoside. Biomed Res. Int. 2013, 2013, 146716.
- Cui, H.-X.; Chen, J.-H.; Li, J.-W.; Cheng, F.-R.; Yuan, K. Protection of anthocyanin from Myrica rubra against cerebral ischemia-reperfusion injury via modulation of the TLR4/NF-ΚB and NLRP3 pathways. Molecules 2018, 23, 1788.
- Hou, Y.; Wang, Y.; He, Q.; Li, L.; Xie, H.; Zhao, Y.; Zhao, J. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 2018, 336, 32–39.
- Putta, S.; Yarla, N.S.; Peluso, I.; Tiwari, D.K.; Reddy, G.V.; Giri, P.V.; Kumar, N.; Malla, R.; Rachel, V.; Bramhachari, P.V.; et al. Anthocyanins: Multi-target agents for prevention and therapy of chronic diseases. Curr. Pharm. Des. 2018, 23, 6321–6346.
- Zhou, P.; Pan, Y.; Yang, W.; Yang, B.; Ou, S.; Liu, P.; Zheng, J. Hepatoprotective effect of cyanidin-3-o-glucoside–lauric acid ester against H2O2-induced oxidative damage in LO2 cells. J. Funct. Foods 2023, 107, 105642.
- Romualdo, G.R.; de Souza, I.P.; de Souza, L.V.; Prata, G.B.; Fraga-Silva, T.F.d.C.; Sartori, A.; Borguini, R.G.; Santiago, M.C.P.d.A.; Fernandes, A.A.H.; Cogliati, B.; et al. Beneficial effects of anthocyanin-rich peels of Myrtaceae fruits on chemically-induced liver fibrosis and carcinogenesis in mice. Food Res. Int. 2021, 139, 109964.
- Zuo, A.; Wang, S.; Liu, L.; Yao, Y.; Guo, J. Understanding the effect of anthocyanin extracted from Lonicera Caerulea L. on alcoholic hepatosteatosis. Biomed. Pharmacother. 2019, 117, 109087.
- Popović, D.; Kocić, G.; Katić, V.; Zarubica, A.; Veličković, L.J.; Ničković, V.P.; Jović, A.; Veljković, A.; Petrović, V.; Rakić, V.; et al. Anthocyanins protect hepatocytes against CCl4-induced acute liver injury in rats by inhibiting pro-inflammatory mediators, polyamine catabolism, lipocalin-2, and excessive proliferation of Kupffer cells. Antioxidants 2019, 8, 451.
- Nussbaum, L.; Hogea, L.M.; Călina, D.; Andreescu, N.; Grădinaru, R.; Ştefănescu, R. Modern treatment approaches in psychoses. pharmacogenetic, neuroimagistic and clinical implications. Farmacia 2017, 65, 75–81.
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583.
- Russo, M.V.; McGavern, D.B. Immune surveillance of the CNS following infection and injury. Trends Immunol. 2015, 36, 637–650.
- Min, J.; Yu, S.-W.; Baek, S.-H.; Nair, K.M.; Bae, O.-N.; Bhatt, A.; Kassab, M.; Nair, M.G.; Majid, A. Neuroprotective effect of cyanidin-3-o-glucoside anthocyanin in mice with focal cerebral ischemia. Neurosci. Lett. 2011, 500, 157–161.
- Cásedas, G.; González-Burgos, E.; Smith, C.; López, V.; Gómez-Serranillos, M.P. Regulation of redox status in neuronal SH-SY5Y cells by blueberry (Vaccinium myrtillus L.) juice, cranberry (Vaccinium macrocarpon A.) juice and cyanidin. Food Chem. Toxicol. 2018, 118, 572–580.
- Pacheco, S.M.; Soares, M.S.P.; Gutierres, J.M.; Gerzson, M.F.B.; Carvalho, F.B.; Azambuja, J.H.; Schetinger, M.R.C.; Stefanello, F.M.; Spanevello, R.M. Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J. Nutr. Biochem. 2018, 56, 193–204.
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via P38/JNK pathway against Aβ1–42-induced oxidative stress. J. Nanobiotechnology 2017, 15, 12.
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-KB/JNK/GSK3β signaling pathway. Nanomedicine 2017, 13, 2533–2544.
- Mulabagal, V.; Lang, G.A.; DeWitt, D.L.; Dalavoy, S.S.; Nair, M.G. Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour cherries. J. Agric. Food Chem. 2009, 57, 1239–1246.
- Joseph, J.A.; Arendash, G.; Gordon, M.; Diamond, D.; Shukitt-Hale, B.; Morgan, D.; Denisova, N.A. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr. Neurosci. 2003, 6, 153–162.
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012, 78, 1138–1145.
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734.
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000.
- Ockermann, P.; Headley, L.; Lizio, R.; Hansmann, J. A Review of the properties of anthocyanins and their influence on factors affecting cardiometabolic and cognitive health. Nutrients 2021, 13, 2831.
- Yang, Y.; Andrews, M.C.; Hu, Y.; Wang, D.; Qin, Y.; Zhu, Y.; Ni, H.; Ling, W. Anthocyanin extract from black rice significantly ameliorates platelet hyperactivity and hypertriglyceridemia in dyslipidemic rats induced by high fat diets. J. Agric. Food Chem. 2011, 59, 6759–6764.
- García-Conesa, M.-T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.; et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018, 19, 694.
- Luís, Â.; Domingues, F.; Pereira, L. Association between berries intake and cardiovascular diseases risk factors: A systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018, 9, 740–757.
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High Anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196.
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594.
- Joo, H.; Choi, S.; Lee, Y.; Lee, E.; Park, M.; Park, K.; Kim, C.-S.; Lim, Y.; Park, J.-T.; Jeon, B. Anthocyanin-rich extract from Red Chinese Cabbage alleviates vascular inflammation in endothelial cells and APO E−/− Mice. Int. J. Mol. Sci. 2018, 19, 816.
- Wang, D.; Wei, X.; Yan, X.; Jin, T.; Ling, W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2010, 58, 12722–12728.
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S. Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J. Nutr. 2010, 140, 1628–1632.
- Petroni, K.; Trinei, M.; Fornari, M.; Calvenzani, V.; Marinelli, A.; Micheli, L.A.; Pilu, R.; Matros, A.; Mock, H.-P.; Tonelli, C.; et al. Dietary cyanidin 3-glucoside from purple corn ameliorates doxorubicin-induced cardiotoxicity in mice. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 462–469.
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries containing anthocyanins with enhanced methylation profiles are more effective at ameliorating high fat diet-induced metabolic damage. Food Chem. Toxicol. 2018, 111, 445–453.
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F. Antioxidant and anti-inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355.
- Hardie, D.G.; Ashford, M.L.J. AMPK: Regulating energy balance at the cellular and whole body levels. Physiology 2014, 29, 99–107.
- López, M. Hypothalamic AMPK and energy balance. Eur. J. Clin. Investig. 2018, 48, e12996.
- Park, S.; Kang, S.; Jeong, D.-Y.; Jeong, S.-Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 6.
- Wu, T.; Jiang, Z.; Yin, J.; Long, H.; Zheng, X. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet-treated mice. Int. J. Food Sci. Nutr. 2016, 67, 257–264.
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions: Anthocyanin-rich foods in obesity control. Biofactors 2017, 43, 507–516.
- Zhang, B.; Kang, M.; Xie, Q.; Xu, B.; Sun, C.; Chen, K.; Wu, Y. Anthocyanins from Chinese Bayberry extract protect β cells from oxidative stress-mediated injury via HO-1 upregulation. J. Agric. Food Chem. 2011, 59, 537–545.
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469.
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653.
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512.
- Tomay, F.; Marinelli, A.; Leoni, V.; Caccia, C.; Matros, A.; Mock, H.-P.; Tonelli, C.; Petroni, K. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med. 2019, 17, 237.
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508.
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243.
- Dreesen, O.; Brivanlou, A.H. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007, 3, 7–17.
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62.
- Pratheeshkumar, P.; Son, Y.-O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-ΚB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137.
- Choi, Y.J.; Choi, Y.J.; Kim, N.; Nam, R.H.; Lee, S.; Lee, H.S.; Lee, H.-N.; Surh, Y.-J.; Lee, D.H. Açaí berries inhibit colon tumorigenesis in azoxymethane/dextran sulfate sodium-treated mice. Gut Liver 2017, 11, 243–252.
- Fragoso, M.F.; Romualdo, G.R.; Vanderveer, L.A.; Franco-Barraza, J.; Cukierman, E.; Clapper, M.L.; Carvalho, R.F.; Barbisan, L.F. Lyophilized Açaí pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis while its main anthocyanin has the potential to affect the motility of colon cancer cells. Food Chem. Toxicol. 2018, 121, 237–245.
- Long, H.-L.; Zhang, F.-F.; Wang, H.-L.; Yang, W.-S.; Hou, H.-T.; Yu, J.-K.; Liu, B. Mulberry anthocyanins improves thyroid cancer progression mainly by inducing apoptosis and autophagy cell death. Kaohsiung J. Med. Sci. 2018, 34, 255–262.
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020, 11, 1300.
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Dunger, N.; Messmann, H.; Hartmann, A.; Endlicher, E. Anthocyanins prevent colorectal cancer development in a mouse model. Digestion 2017, 95, 275–280.
- Chen, J.; Zhu, Y.; Zhang, W.; Peng, X.; Zhou, J.; Li, F.; Han, B.; Liu, X.; Ou, Y.; Yu, X. Delphinidin induced protective autophagy via MTOR pathway suppression and AMPK pathway activation in HER-2 positive breast cancer cells. BMC Cancer 2018, 18, 342.
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic effects of anthocyanins for vision and eye health. Molecules 2019, 24, 3311.
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Lab. Investig. 2012, 92, 102–109.
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food 2012, 15, 818–823.
- Ohguro, H.; Ohguro, I.; Katai, M.; Tanaka, S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica 2012, 228, 26–35.
- Thiraphatthanavong, P.; Wattanathorn, J.; Muchimapura, S.; Wipawee, T.-M.; Wannanon, P.; Terdthai, T.-U.; Suriharn, B.; Lertrat, K. Preventive effect of Zea mays L. (Purple Waxy Corn) on experimental diabetic cataract. Biomed Res. Int. 2014, 2014, 507435.
- Paik, S.-S.; Jeong, E.; Jung, S.W.; Ha, T.J.; Kang, S.; Sim, S.; Jeon, J.H.; Chun, M.-H.; Kim, I.-B. Anthocyanins from the seed coat of black soybean reduce retinal degeneration induced by N-methyl-N-nitrosourea. Exp. Eye Res. 2012, 97, 55–62.
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui a chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242.
- Iturriaga, L.; Olabarrieta, I.; de Marañón, I.M. Antimicrobial assays of natural extracts and their inhibitory effect against Listeria innocua and fish spoilage bacteria, after incorporation into biopolymer edible films. Int. J. Food Microbiol. 2012, 158, 58–64.
- Côté, J.; Caillet, S.; Doyon, G.; Dussault, D.; Sylvain, J.-F.; Lacroix, M. Antimicrobial effect of cranberry juice and extracts. Food Control 2011, 22, 1413–1418.
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507.
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156.
