Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The synthesis of primary amines via the reductive amination of alcohols involves a hydrogen-borrowing or hydrogen-transfer mechanism, which consists of three main steps: alcohol hydroxyl dehydrogenation, carbonyl imidization, and imine hydrogenation. Heterogeneous catalysts are widely used for this reaction because of their high performance and amenability to separation and reuse.
- competitive adsorption
- heterogeneous catalysts
- hydrogen-borrowing
1. Introduction
Recently, the field of the transformation of biomass derivatives has become a research hotspot, in which the amination of alcohols and carbonyls is becoming increasingly popular [1][2][3][4][5][6][7][8][9]. Amines are versatile products that have applications in pharmaceuticals, pesticides, rubbers, surfactants, crosslinkers, and curing agents. Some organic amines are also of biological and medical significance [10][11][12]. Among the various amines, primary amines are considered to be the most important class of organic amine compounds because of their derivatization characteristics. Primary amines are especially important as they can undergo various derivatization reactions. For instance, aniline is the foundation of the dye industry. Binary primary amines, such as ethylenediamine and 1,2-propylenediamine, have two amino groups that can polymerize and have widespread uses in the production of pesticides, coatings, chelating agents, insect repellents, soil amendments, and lubricants and as emulsifiers, antifreeze agents, and organic solvents [13][14]. Therefore, the study of primary amines is conducive to the development of a series of valuable products that are very important in theoretical research and industrial applications.
There are many methods for the synthesis of amines, such as the amination of halogenated hydrocarbons, the hydrogenation of nitrile compounds, the amination of olefins, the reduction of nitroaromatic compounds [15][16], and the reductive amination of aldehydes/ketones/alcohols [17][18][19][20][21][22][23]. With the continuous improvement of the petroleum industry and the production of biomass-based alcohol compounds, the reductive amination of alcohols is becoming the most promising and effective method because of its abundant source materials, low cost, and simple process [24][25][26]. Based on the reaction mechanism, the amination of alcohols can be divided into condensation amination and reductive amination.
Condensation amination uses dehydration catalysts like zeolite molecular sieves [27], metal oxides and their mixtures [28][29]. In 1909, Sabatier et al. first used ruthenium oxide as a catalyst to prepare a mixture of primary, secondary and tertiary amines from alcohol [30]. Since then, dehydration catalysts have been widely used in alcohol amination research and industrial production, e.g., for producing methylamines (methyleneamine, dimethylamine, trimethylamine, etc.) [31][32][33]. However, this method often requires a high reaction temperature that causes instability of alcohols and uncontrollable selectivity issues. Therefore, condensation amination is limited in low alcohol amination.
2. The Role of Heterogeneous Catalysts in the Reductive Amination of Alcohols
Heterogeneous metal catalysts have the advantages of relatively mild reaction conditions, high selectivity for primary amines, easy post-reaction treatment, and reusability. Therefore, they are increasingly being applied in the reductive amination of alcohols with NH3 to prepare primary amines. At present, the metal active components are noble metals, such as Ru, Rh, Pt, and Pd, and non-noble metals, such as Co, Ni, Cu, and Fe, which are commonly used in reaction systems for the reductive amination of alcohols with NH3 to produce primary amines.
2.1. Monometallic Catalysts
In catalytic hydrogenation/dehydrogenation reactions, the H-H bond or O-H bond is generally dissociated and adsorbed on the surface of the metal active site, allowing subsequent reactions to proceed. The activity of monometallic catalysts for the reductive amination of alcohols to primary amines is related to the relevant supports, which can be divided into three categories: amphoteric supports (such as γ-Al2O3, Y2O3, CeO2, ZrO2, and ZnO), acidic supports (such as TiO2 and Nb2O5), and alkaline supports (such as MgO and CaO). During the reductive amination reaction of alcohol, basic sites contribute to the extraction of alcohol protons, thereby increasing the rate of dehydrogenation, which is the rate-determining step of the reaction, while acidic sites are associated with hydrogen transfer.
2.1.1. Ni-Based Catalysts
Ni-based catalysts are most commonly used because of their good activity and high selectivity for primary amines in the reductive amination of alcohols.
As early as 1990, Baiker et al. [34] reported the reductive amination of 1-methoxy-2-propanol with NH3 over Ni/SiO2 catalyst. They studied the effects of reaction parameters such as the reaction temperature, the molar ratio of substrate to H2 and NH3, and the mass space velocity on the performance of the catalyst. The conversion of 1-methoxy-2-propanol reached 70%, and the selectivity for the primary amine product 2-amino-1-methoxypropane reached 82.9% under the conditions of a reaction temperature of 190 °C, a molar ratio of 1-methoxy-2-propanol/NH3/H2 of 1/8/2, and a space velocity of 40.5 g·g−1·h−1.Additionally, the catalyst was gradually deactivated when there was no H2 in the reaction system and recovered when H2 was re-introduced.
In order to study the effect of Ni loading on catalyst performance, Shin et al. [35] prepared five different loadings (4–27 wt%) of Ni/γ-Al2O3 using an equal volume impregnation method for the reductive amination of isopropanol with NH3 to isopropylamine. The results showed that when the Ni loading was 17 wt%, the metallic Ni had a better dispersion on the γ-Al2O3 support, and the metal had the highest reduction degree. Similarly, excess H2 could effectively prevent the catalyst from forming a phase transition of metal nitrides inhibiting the catalyst’s deactivation.
Shimizu et al. [36] applied heterogeneous metal catalysts in the hydrogen transfer amination of 2-octanol with NH3 to prepare primary amines. By comparing the different metals on the γ-Al2O3 support, it was found that Ni had better activity for catalyzing hydrogen transfer amination than Cu, Co, and other noble metals (Pt, Re, Au, etc.). In addition, they further studied the catalytic hydrogen transfer amination performance of different supported Ni catalysts and that of Ni powder and Raney Ni. They found that the activity of the Ni catalysts was related to the supports and attributed this to the different acid–base sites on the supports, in which the basic sites of alumina could facilitate the extraction of protons from alcohols to promote the dehydrogenation step, and the acidic sites could be related to the hydrogen transfer step. Afterwards, the research group also prepared a Ni/CaSiO3 catalyst using the ion exchange method, which has been successfully used in the hydrogen transfer amination of a variety of fatty alcohols and aromatic alcohols to generate primary amines [37].
Dumon et al. [38] compared the catalytic performance of Ni/Al2O3 and Pd/Al2O3 with regard to the reductive amination of n-butanol. The catalytic activity of Ni was predicted to be lower than that of Pd according to DFT (Density functional theory) calculations, with the corresponding energetic spans for the amination catalytic cycle being 1.22 eV and 1.13 eV, and these results contradicted the experimental findings indicating that Ni had a much stronger ability to catalyze alcohol dehydrogenation than Pd. Further DFT calculations revealed that the spectator NH3 adsorbates could promote the catalytic activity of the Ni catalyst: on the one hand, NH3 can promote the rupture of the O-H bond and the formation of amine intermediates on the Ni surface; on the other hand, as shown in Figure 1, NH3 can stabilize the imine on the surface of Pd through hydrogen bonding, which hinders the hydrogenation step of the imine.
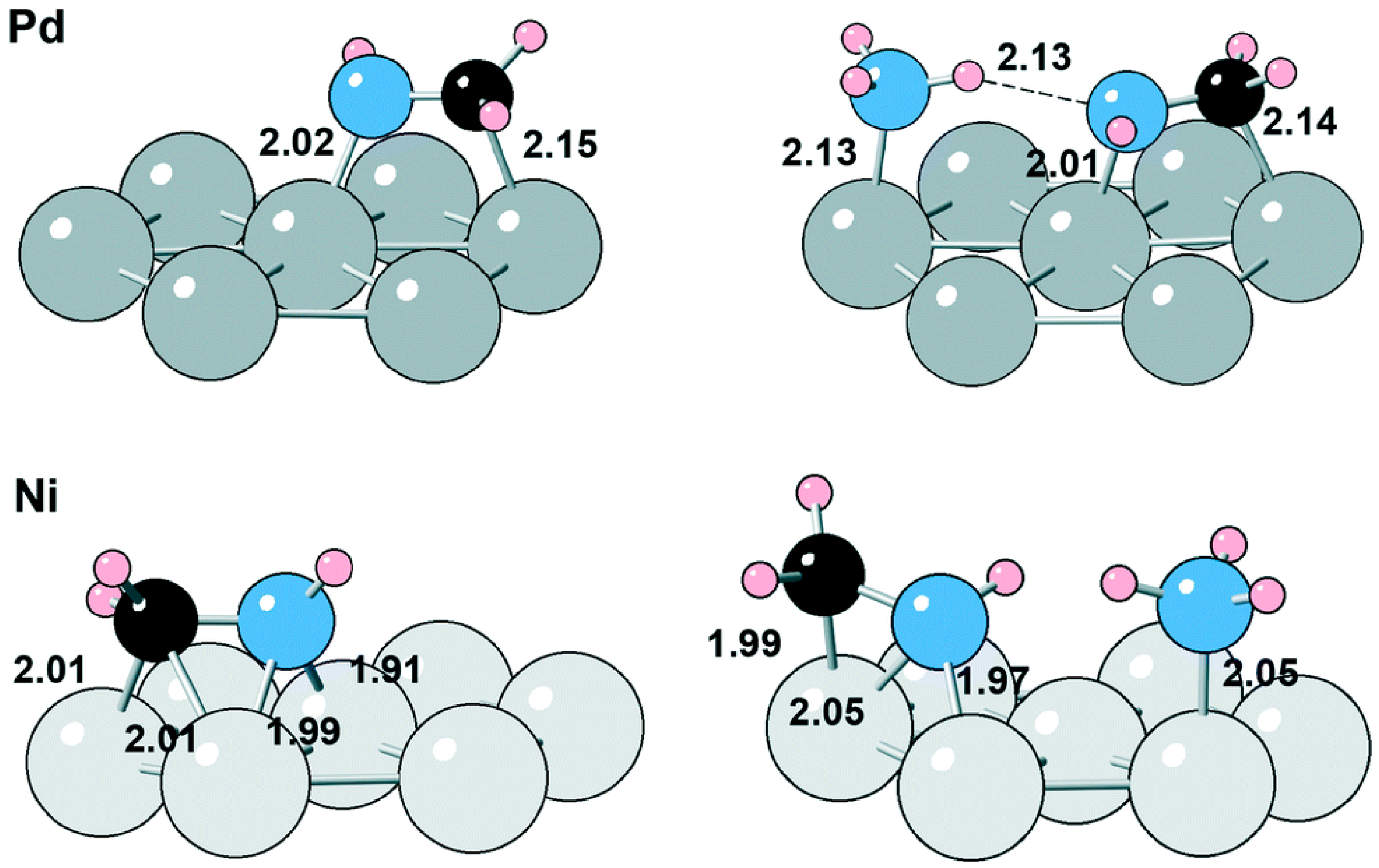
Figure 1. Imine adsorption in the absence and presence of NH3 on Pd and Ni. The distances are given in Å.
The research group worked on the production of furfuryl alcohol and its derivative products [39][40]. In the reductive amination of furfuryl alcohol, researchers compared catalysts with different metal components (Ru, Rh, Pt, Pd, Co, and Ni) and found that Ni-based catalysts displayed the best performance, which was attributed to the difference in competitive adsorption of NH3 and H2 on different metal surfaces [41]. Next, in the reductive amination of 5-hydroxymethylfurfural, researchers conducted a DFT calculation of the difference in the adsorption energy of metal for NH3 and H2 and found that the difference in the adsorption energy of Ni for these two gases was lower than that of noble metals and Co, indicating that NH3 will occupy less-active metal sites. This could be the reason why Ni-based catalysts show high performance in reductive amination [42].
Liu et al. [43] prepared a bifunctional Ni-based catalyst (Ni/SiO2-AE) containing nickel nanoparticles and Lewis acid sites derived from nickel phyllosilicates via a modified ammonia evaporation method and used it in the reductive amination reaction of phenol. As shown in Figure 2, the Ni/SiO2-AE catalyst enabled 89.4% phenol conversion and 86.0% selectivity for cyclohexylamine at 160 °C for 2 h, outperforming the catalysts formed via conventional deposition–precipitation (Ni/SiO2-DP) and wetness-impregnation (Ni/SiO2-WI). Based on the actual exposed metal surface area, the overturning frequencies (TOF) of Ni/SiO2-AE, Ni/SiO2-DP, and Ni/SiO2-WI were calculated as 71.7, 49.0, and 8.7 (h−1), respectively. Catalyst characterization results combining FTIR, XRD, TEM, and NH3-TPD indicated that the nickel phyllosilicate structure was beneficial for the construction of small nickel nanoparticles (3 nm) and abundant Lewis acid sites necessary for phenol amination. The Ni nanoparticles exsoluted from nickel phyllosilicates participated in the activation of dihydrogen molecules, and the Lewis acid sites generated by the coordinated unsaturated Ni2+ sites on the catalyst played a role in adsorbing and activating phenol. The synergistic effect of the dual functions enhanced the activity of the phenol amination reaction.
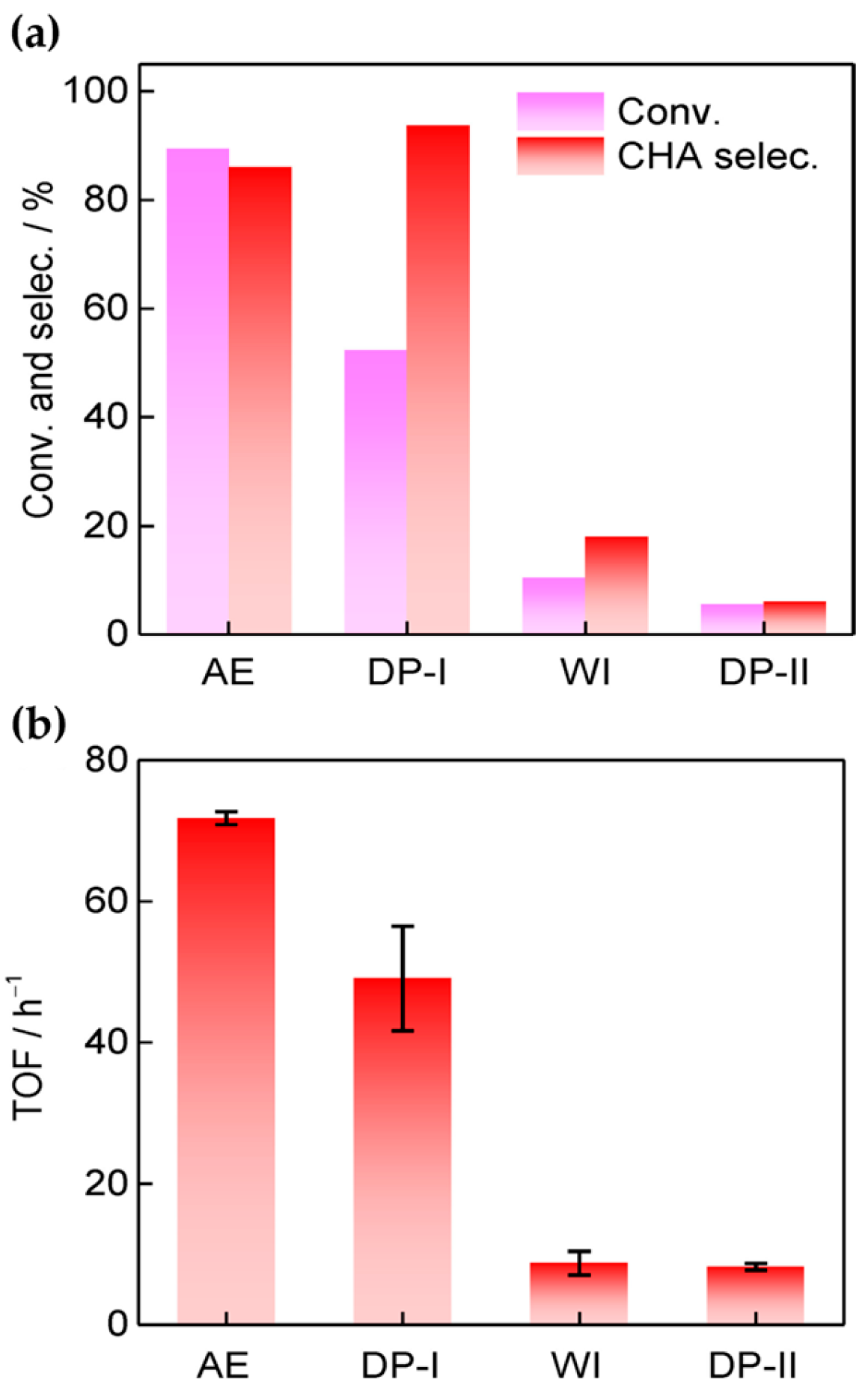
Figure 2. (a) The catalytic performance of catalysts for the amination of phenol to cyclohexamine (CHA). (b) TOF values for catalysts developed using different preparation methods with respect to phenol amination.
From the above studies, it can be seen that temperature, H2 pressure, NH3 pressure, the structure of the substrate alcohol, catalysts, and other factors all have important effects on the catalytic reaction in the reductive amination of alcohols with NH3 to produce primary amines. In the reductive amination of alcohols, a higher temperature is favorable for the dehydrogenation step, but an excessive temperature will cause side reactions of the substrate alcohol (such as cracking, dehydration, etc.), leading to a selectivity decrease for primary amines. Adding excess NH3 to the reaction system can inhibit the formation of secondary and tertiary amines and thus increase the selectivity of primary amines. In addition, although the reductive amination of alcohol does not consume H2, its presence can prevent the formation of metal nitrides and thus prevent the catalyst from deactivating. At the same time, the specific surface area, pore volume, and pH of the catalyst support will affect the activity and selectivity of the catalyst by affecting its dispersion and anti-sintering ability.
2.1.2. Co-Based Catalysts
Shin et al. [44] found that Co/Al2O3 had good activity and selectivity for the reaction of isopropanol with NH3 that leads to the production of isopropylamine. With the increase in the Co loading, the conversion of isopropanol and the yield of isopropylamine increased significantly until the Co loading reached 23%. Additionally, with the increase in NH3 partial pressure, the conversion of isopropanol was basically constant, the selectivity for isopropylamine gradually increased, and the selectivity for the dehydration by-product acetone decreased (Figure 3). This is because excess NH3 promotes the reductive amination of acetone, which increases selectivity for isopropylamine.
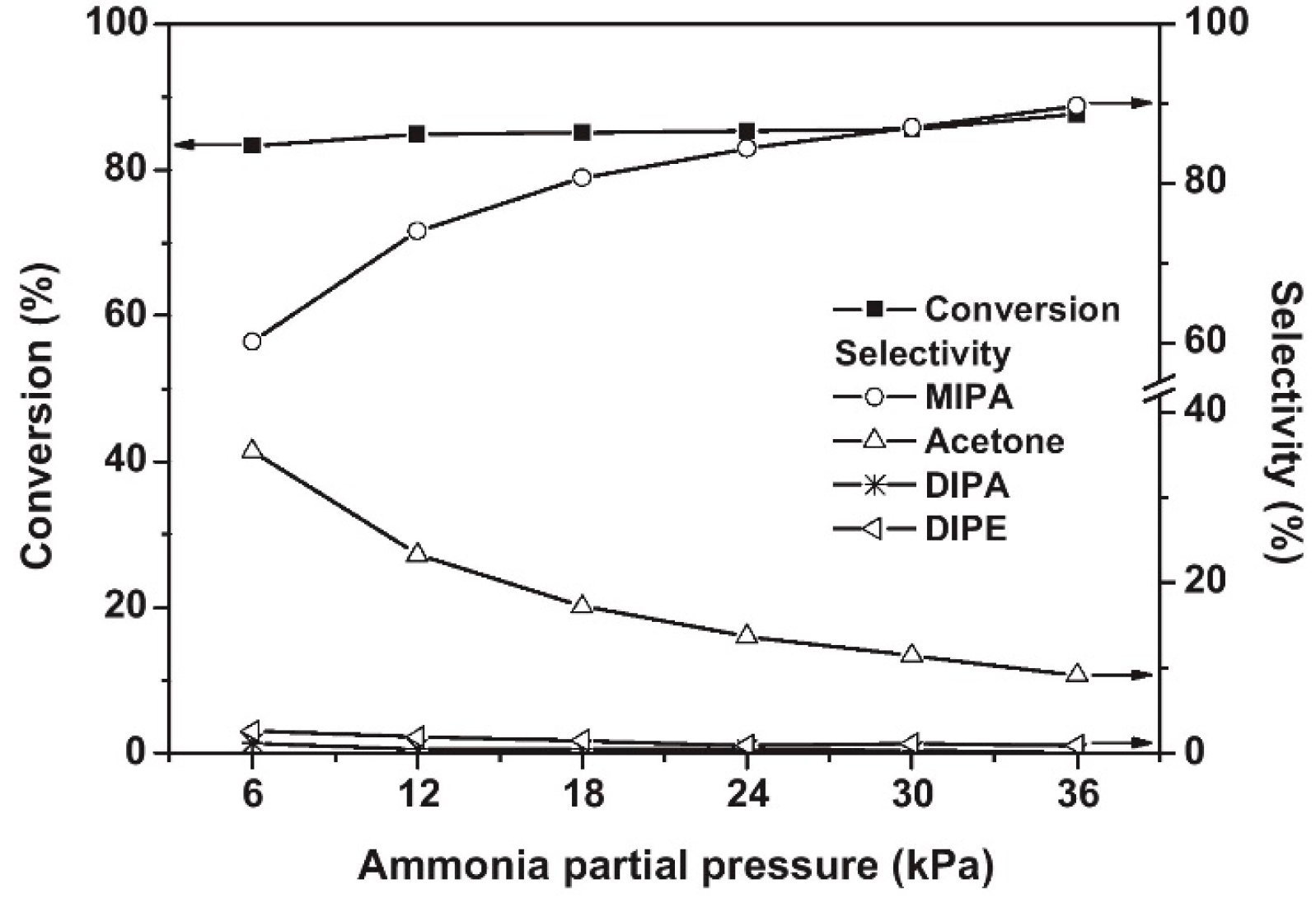
Figure 3. Influence of ammonia partial pressure on the reductive amination of 2-propanol over Co(23)Al2O3. Reaction conditions: T = 210 °C, WHSV (h−1) = 4.29; feed composition of 2-propanol/H2 (mol %) = 1/8.
Lei et al. [45] prepared a Co catalyst supported on γ-Al2O3 for the liquid-phase ammonolysis of ethylene glycol to produce high-value-added ethanolamine and explored the effect of different metal Co particle sizes on the selectivity of ethanolamine in the reductive amination of ethylene glycol. Among the sizes analyzed, Co nanoparticles above 4 nm showed the highest ethanolamine selectivity (73.6%) and the highest ammonolysis rate based on the total Co content (Figure 4a). Smaller Co nanoparticles (above 2 nm) were not only intrinsically less active in the ammonolysis of ethylene glycol but also exhibited a higher selectivity for glycolaldehyde due to an insufficient ability to catalyze the condensation reaction between glycolaldehyde and NH3.
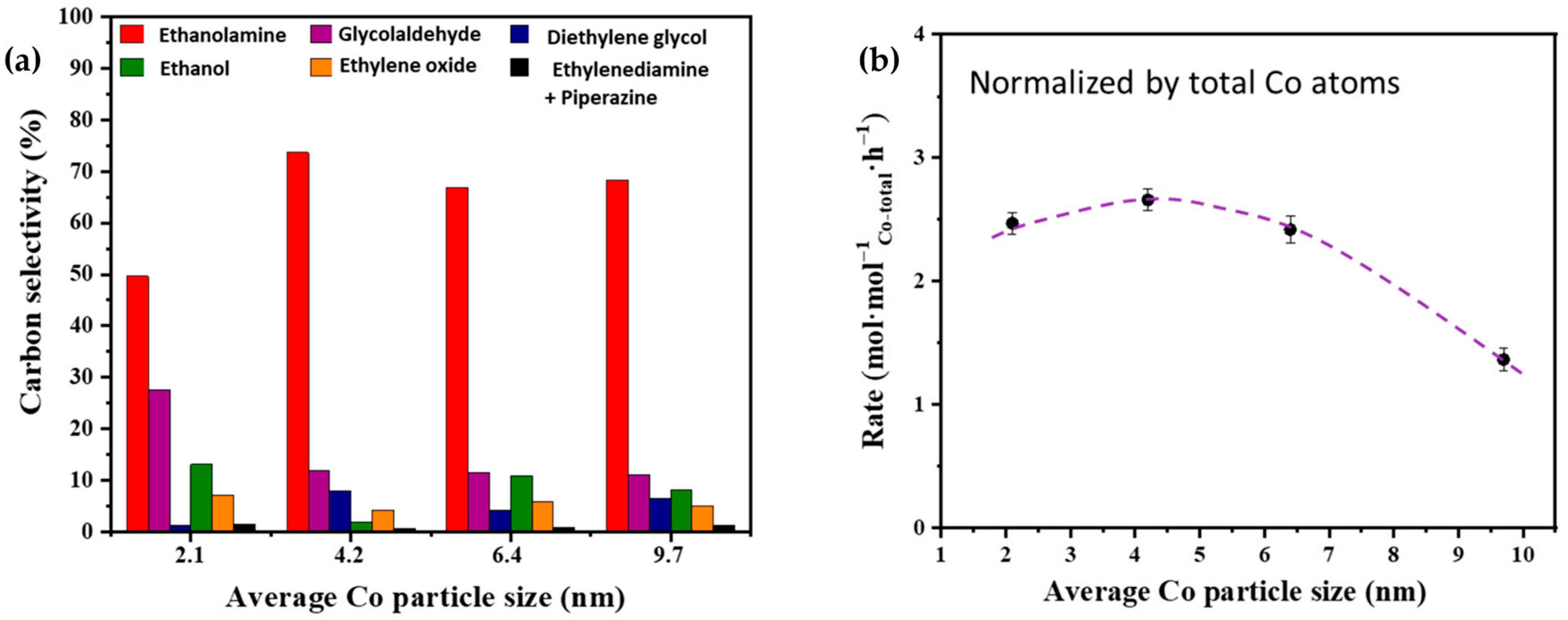
Figure 4. (a) Effects of Co particle size on the carbon selectivity of ammonolysis of ethylene glycol on Co/γ-Al2O3 catalysts. Red represents ethanolamine; purple represents glycolaldehyde; blue represents diethylene glycol; green represents ethanol; orange represents ethylene oxide; black represents ethylenediamine + piperazine. (b) Effects of Co particle size on the rate of ethylene glycol conversion over Co/γ-Al2O3 catalysts normalized by the total Co atoms. The dot represents the rate of ethylene glycol conversion.
2.1.3. Cu/Fe-Based Catalysts
Chary et al. [46] employed a constant volume impregnation method to prepare a series of Cu/ZrO2 catalysts with different loadings (1–15 wt%) for catalyzing the reductive amination of cyclohexanol. Firstly, 2.5 wt% loading Cu was supported on ZrO2, Nb2O5, and TiO2 to screen for a suitable support. The highest yield of 57% cyclohexylamine was obtained over the catalyst supported on ZrO2, which contained acid, alkali, reduction, and oxidation sites at the same time. The catalyst with a Cu loading of 3.5 wt% had the highest dispersion and the best catalytic performance. Moreover, the acidity and basicity of the catalysts were studied by catalyzing the dehydrogenation of cyclohexanol and contrasted with the results of NH3-TPD. It was discovered that acidic sites of moderate strength were favorable for the dehydration of cyclohexanol, but acidic sites of weak strength were beneficial for the dehydrogenation of cyclohexanol. Additionally, in their previous study [47], it was found that with the increase in the Cu loading, the alkalinity of the catalyst increased and the acidity of the catalyst decreased, and both of them tended to be stable with a higher Cu loading (more than 5.0 wt%).
Zhang et al. [48] studied catalysts supported on γ-Al2O3 to catalyze the reaction between ethanol and NH3 and found that the selectivity for ethylamine in the product was not high when incorporating the metal component Cu. Furthermore, when the NH3/ethanol molar ratio was low, aldehyde and acetonitrile accounted for the majority of the byproducts.
In early research, Kliger et al. [49] studied the mechanism of the reductive amination of alcohols using metal Fe catalysts and proposed an analogous hydrogen transfer mechanism.
For the reductive amination of alcohols, Cu and Fe are not usually used as active components alone but instead are generally used together with Co, Ni, and other metals as bimetallic/multimetallic catalysts.
2.1.4. Noble Metal-Based Catalysts
Noble metals are widely used in the reaction of aldehydes/ketones/alcohols with organic amines to produce secondary or tertiary amines due to their excellent hydro-/dehydrogenation activity. However, they are rarely used in the preparation of primary amines via the reductive amination of aldehydes/ketones/alcohols with NH3. In a series of studies on the reductive amination of isophorone nitrile, furfuryl alcohol, and 5-hydroxymethylfurfural, the research group found that noble metal catalysts had no advantages over Ni and Co in terms of activity and selectivity [4][7][50][51]. For example, in the reductive amination of furfuryl alcohol and 5-hydroxymethylalcohol, researchers found that the yield of primary amine was very low when using Ru, Rh, Pt, and Pd as metal active components, which could be attributed to the competitive adsorption of hydrogen and NH3 on the metal surface [41][42].
Among the catalysts for the reductive amination of alcohols with NH3, Ru is the most used noble metal. Rose et al. [52] employed Ru/C, Pd/C, Pt/C, and other commercial catalysts to catalyze the reductive amination of biomass diols (isomannitol, isosorbide, etc.) with ammonium hydroxide to produce monoamine or diamine. It was found that under the reaction conditions of 140–180 °C with water as a solvent, Pd/C was basically inactive, and Pt/C had low activity. Ru/C proved to be the most active catalyst: using this catalyst, the total yield of monoamine and diamine reached 45% under the reaction conditions of 1 MPa of H2, 1 g of 25 wt% ammonium hydroxide, 170 °C, and 6 h.
Ruiz et al. [53] studied the catalytic performance of different noble metal catalysts (Ru/C, Pd/C, Ir/C, Pt/C, and Os/C) on the reductive amination of dodecanol with NH3 to produce dodecylamine. Under the reaction conditions of 150 °C, 0.4 MPa of NH3, 0.2 MPa of H2, and 24 h, the yield of dodecylamine was 83.8% over the Ru/C catalyst; the results are shown in Figure 5c. The authors studied the effects of H2 pressure and NH3 pressure on the reaction. In the case of excess NH3 (applied to suppress the formation of secondary amines), changing the pressure of NH3 had no significant effect on the conversion of dodecanol and selectivity for dodecylamine. When H2 was not introduced, the conversion of dodecanol and the selectivity for dodecylamine were relatively low, and the main by-product was dodeconitrile. After the introduction of H2 into the reaction system, the conversion of dodecanol and the selectivity for dodecylamine both increased. However, when the H2 pressure was too high, dodecanol was cracked, producing a large amount of undecane. Moreover, it was found that the deactivation of the Ru/C catalyst occurred due to surface coking. Ruiz et al. [54] also studied the direct amination of dodecanol with NH3 and H2 over the catalysts of Rh, Pt, Ir, Ru, Ni, Cu, and Co supported on SiO2. The results showed that Ru and Ir catalysts had greater activity, while Ni and Co catalysts exhibited better selectivity in alcohol reductive amination.
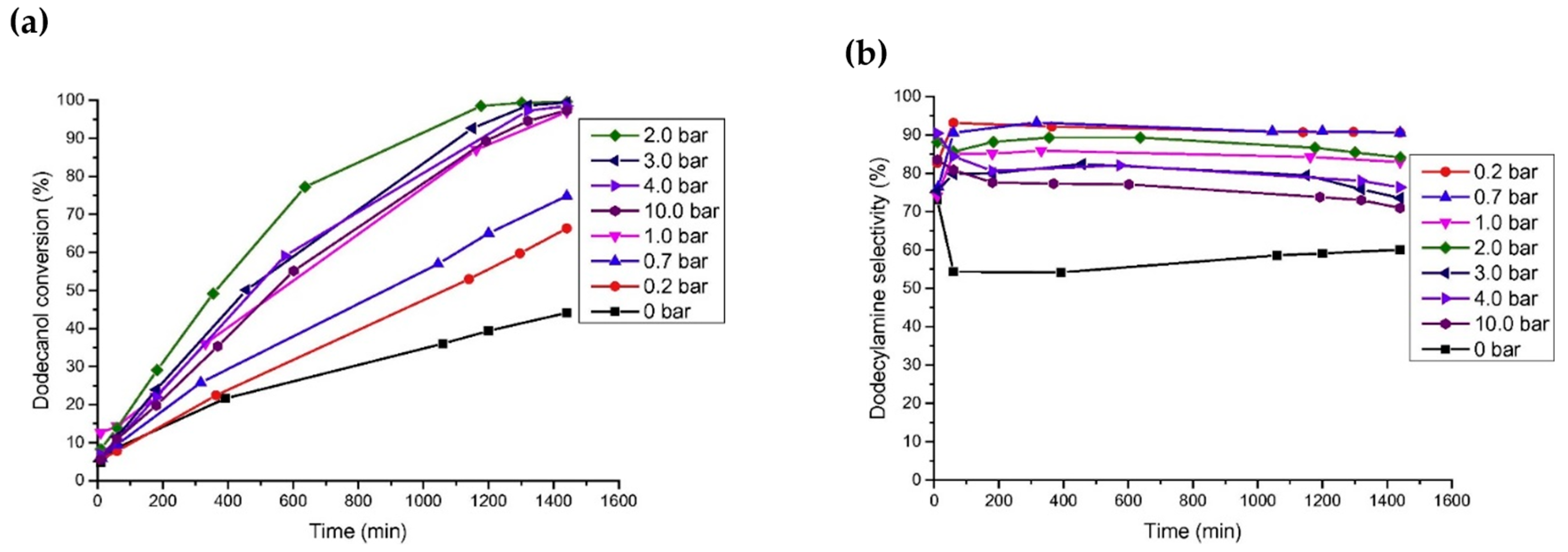
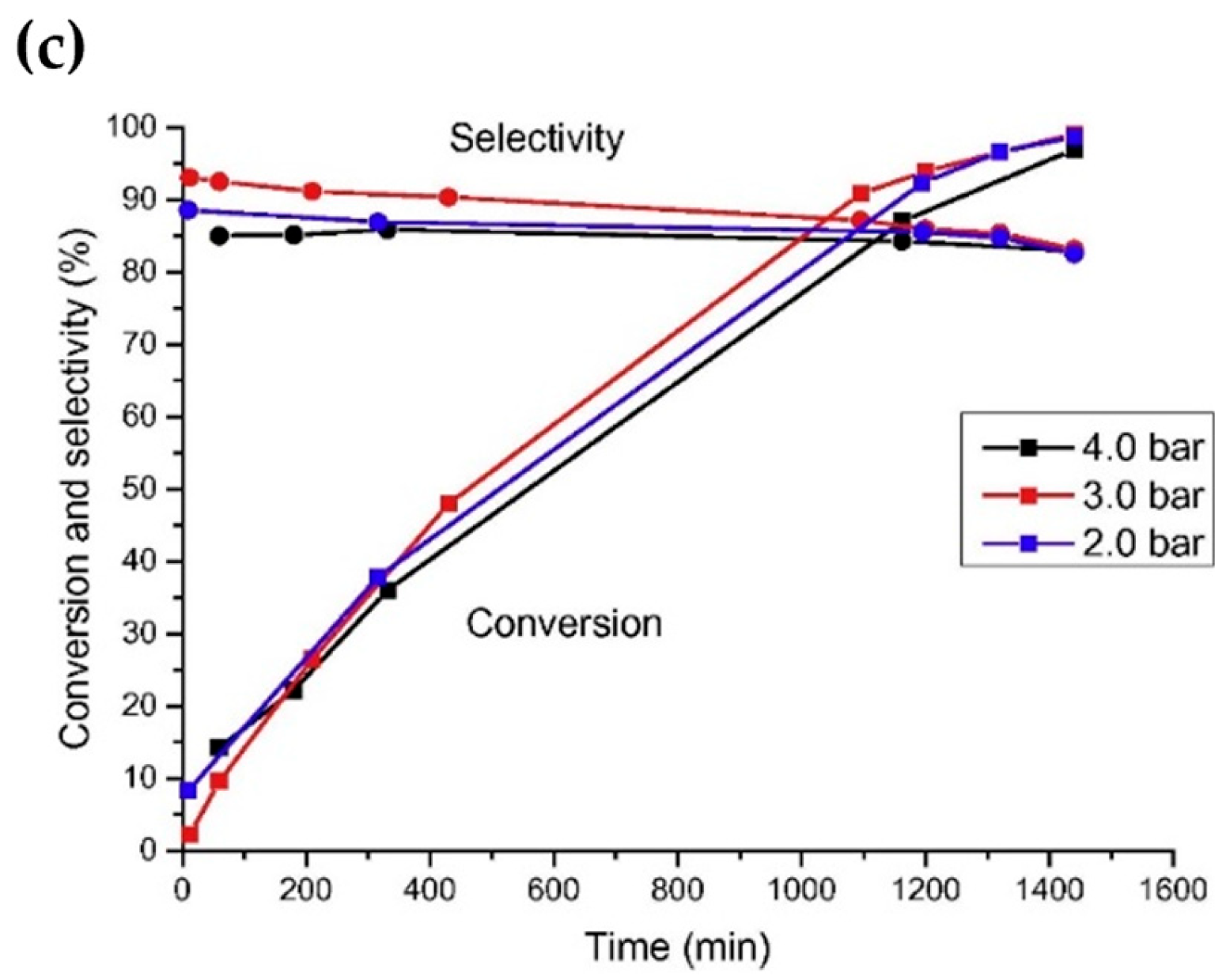
Figure 5. (a) Influence of hydrogen pressure on dodecanol conversion over Ru/C at 150 °C. (b) Influence of hydrogen pressure on selectivity toward dodecylamine over Ru/C at 150 °C. (c) Influence of ammonia pressure on dodecanol conversion and selectivity toward dodecylamine.
Fang et al. [55] investigated the reaction of 1-octanol with ammonia for the synthesis of 1-octylamine and examined the catalytic activity of loaded Ru catalysts on different acidic carriers including zeolites with various topologies and Si/Al ratios, in which the Ru/HBEA (Si/Al = 25) catalysts with 5 wt% Ru achieved a greater than 90% conversion of 1-octanol and 90% selectivity for 1-octylamine (Figure 6a). The authors attributed this result to the presence of moderately strong Brønsted/Lewis acid centers near the Ru nanoparticles located on the outer surface of HBEA.
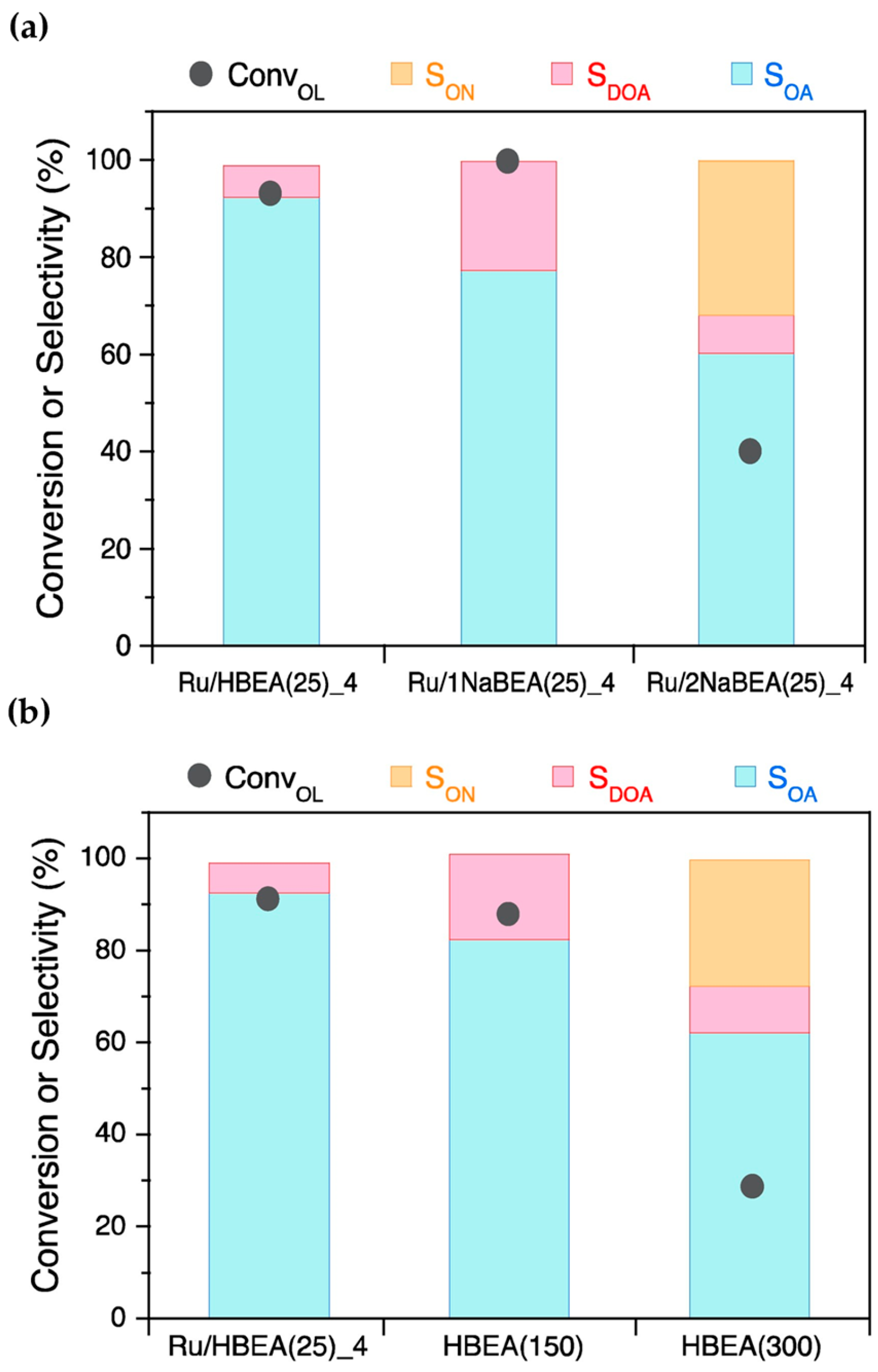
Figure 6. (a) Catalytic performance of Ru/BEA(25)_4 at different H+-exchange degrees in the direct amination reaction of 1-octanol (OL) with NH3. (b) Catalytic performance of Ru/BEA (x) as a function of Si/Al ratio (x) with x = 25, 150 and 300.
2.2. Bimetallic/Multimetallic Catalysts
Compared with monometallic metal catalysts, bimetallic/multimetallic metal catalysts have the following main advantages in terms of their catalytic mechanisms:
-
Due to the addition of a second metal, the metals will interact with each other and have different electronic and chemical properties from the corresponding single metals, thus showing better catalytic activity, selectivity, and stability.
-
The addition of a second metal component can promote the reduction of the first metal precursor and improve the dispersion of metal particles on the support, strengthening the dehydrogenation site, thereby facilitating better catalytic activity and higher product selectivity.
-
The addition of a second metal can enable the formation of a highly dispersed bimetallic alloy on the nanostructure scale. The synergistic effect of a bimetallic alloy is as follows: the introduction of the second metal affects the electron cloud density of the first metal, which is beneficial to the in situ reduction of the first metal, thereby improving catalyst activity.
-
The introduction of a second metal component can inhibit the competitive adsorption of NH3 at the main metal catalytic sites, and more main metal catalytic sites can be used to catalyze the dehydrogenation reaction of alcohol, thus improving the dehydrogenation activity of the catalyst.
These properties are particularly important for catalytic processes that require high activity and high product selectivity [23][56][57].
Ni-Based Catalysts
Cui et al. [58] prepared a Ni-based multimetallic catalyst, NiCuFeOx, using a co-precipitation method, which was stable in air and was successfully used to catalyze the reductive amination of alcohols with NH3 for producing primary and secondary amines. When studying the reaction between alcohol and NH3, a superior yield of primary amines was obtained when ammonium carbonate was used as the NH3 source. It is worth noting that the reaction was carried out under hydrogen-free conditions, which meant a hydrogen transfer mechanism was successfully achieved. The catalyst could be reused multiple times in the same reaction. This study provides a new idea for the use of the hydrogen transfer amination of alcohol with NH3 to prepare primary amines over heterogeneous metal catalysts.
Shin et al. [59] studied the effect of metal loading and support morphology on the reductive amination of isopropanol with NH3 and then further investigated the effect of the Ni/Fe molar ratio in Ni-Fe/γ-Al2O3 on the activity of the catalyst. They prepared 17 wt% Ni-Fe/γ-Al2O3 catalysts with different Fe/Ni molar ratios (0–0.7) through the same volume impregnation method. XRD characterization revealed that a Ni-Fe alloy had formed in the reduced catalyst. H2-TPR characterization showed that the reduction degree of Ni in the bimetallic catalyst significantly increased, and the reduction temperature was decreased compared to that of Ni/γ-Al2O3. Furthermore, XPS characterization showed that the content of Ni0 in the catalyst changed with the change in the Ni/Fe molar ratio. When the Ni/Fe molar ratio was 0.3, the content of Ni0 on the catalyst’s surface reached a peak, and the activity and selectivity of the catalyst were the greatest, with no obvious deactivation after 100 h of reaction in the presence of hydrogen.
The research group prepared a series of Ni-M/γ-Al2O3 (M = Mn, Re, Ce, Mo, Cr, Zr, Cu, Zn, La, and V) catalysts using the co-impregnation method for a 5-hydroxymethylfurfural “one-pot” reductive amination in order to produce 2,5-bis(aminomethyl)furan [60]. The results showed that the Mn-added catalyst displayed the best performance in the reaction. Researchers considered that the addition of Mn endowed the Ni with higher surface dispersion and chemical stability through the dilution and electron transfer of Ni on the support surface.
Ma et al. [61] prepared Al2O3-supported Ni and Ni-Re catalysts using an impregnation method for the reductive amination of monoethanolamine. Compared with the Ni/Al2O3 catalyst, the Ni-Re/Al2O3 catalyst showed high activity and excellent stability and catalyzed the reaction to reach a superior ethanediamine selectivity. According to NH3-TPD, CO2-TPD, and PY-IR characterizations, the Ni-Re/Al2O3 catalyst exhibited a smaller Ni particle size (4–6 nm), more Ni0 sites, and higher acid strength due to the introduction of Re. Moreover, the authors believed that the O atom in the -OH group was preferably adsorbed on the ReOx site during the amination of monoethanolamine due to its stronger oxygen affinity with Re compared to Ni. Compared with the bare Ni0 surface, the dissociation energy of proton transfer and β-H elimination of monoethanolamine was significantly reduced on the Ni0 surface modified by low-valence-state (<3) ReOx. As a result, the adsorption structure on the ReOx-Ni0 surface facilitated the dehydrogenation process [62].
Chang et al. [63][64] added CeO2 as a component to prepare Ni-CeO2/Al2O3 to catalyze the synthesis of polypropylene diamine from polypropylene glycol. By increasing the content of Ce from 0 wt% to 2.5 wt%, 7.5% wt%, and 15 wt%, the total degrees of amine conversion increased from 64.5% to 66.8%, 71.1%, and 74.4% respectively, indicating that the catalytic performance was enhanced by the addition of CeO2. This result may be attributed to the formation of a Ni-Ce-O interface on the nanostructure scale, providing a synergistic approach to promoting polypropylene diamine desorption (Figure 7).
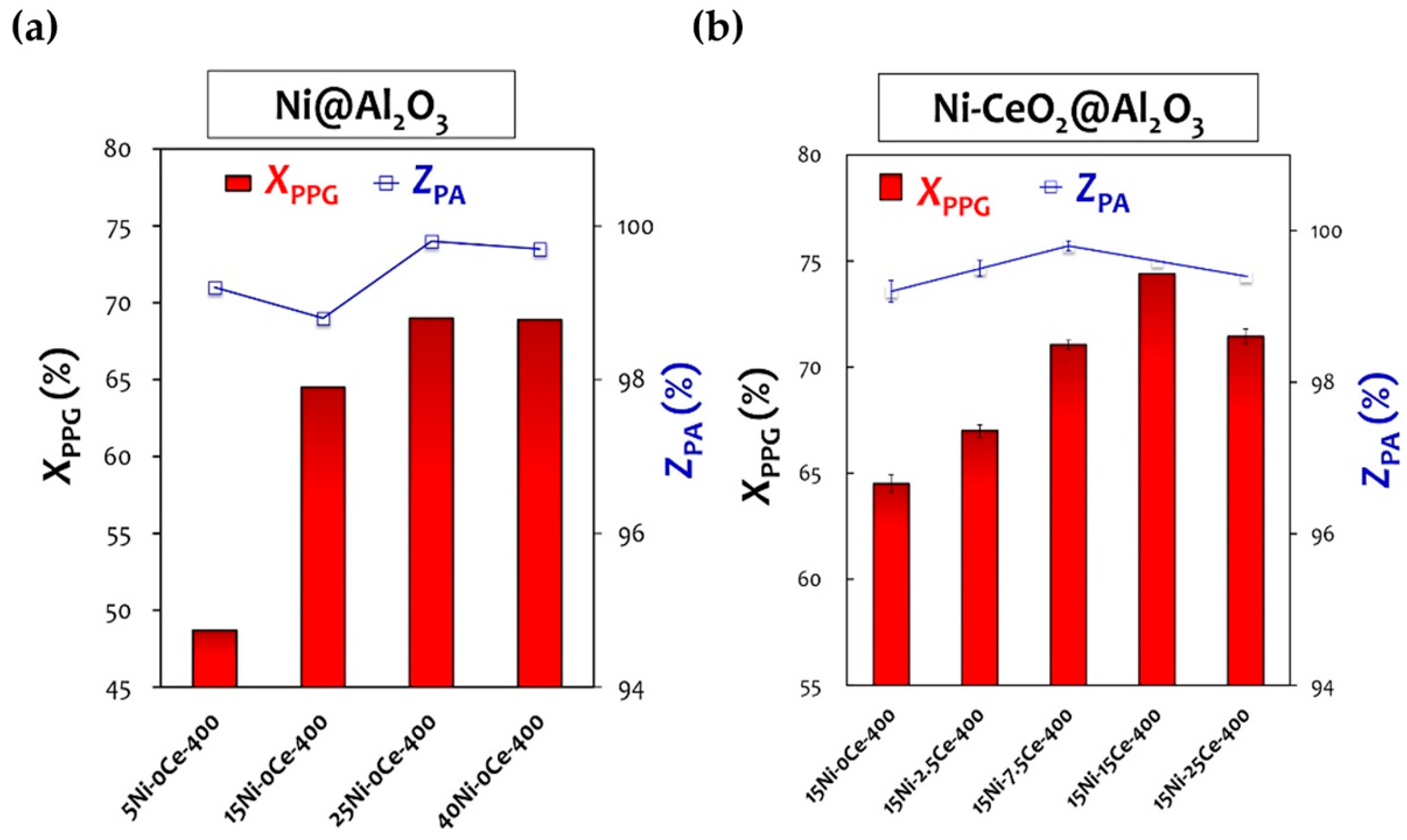
Figure 7. The conversion to total amine compound ratio (XPPG) and the selectivity toward primary amine (ZPA) using Ni@Al2O3 (a) and Ni-CeO2@Al2O3 samples (b).
Then, Chang et al. [64] used Ni-CeO2/Al2O3 to achieve a 99% selectivity and a 77% yield of polypropylene diamine. As shown in the SEM characterization presented in Figure 8, Ni and Ce had a uniform distribution in the mixed nanostructures, which meant that the Ni-Ce-O interface had been successfully generated. They believed that such oxygen vacancies at the Ni-Ce-O interface could promote the adsorption of the substrate and thus resist the competitive adsorption of NH3 on the Ni surface. The Ni-Ce-O interface also provided additional active sites for the dehydrogenation of polypropylene glycol, shortening the reaction time. Hardly any Ni3N crystals were found via characterization, suggesting that the addition of CeO2 also inhibited the formation of nitrides on the surface of the catalyst. Furthermore, the addition of CeO2 increased the acidity of the Ni-based catalysts, and the acidity increment could improve the stability of the Ni catalyst materials and weaken the NH3 adsorption at the Ni-Ce-O interface, thus preventing the irreversible adsorption of NH3/amines throughout the catalytic reaction process.
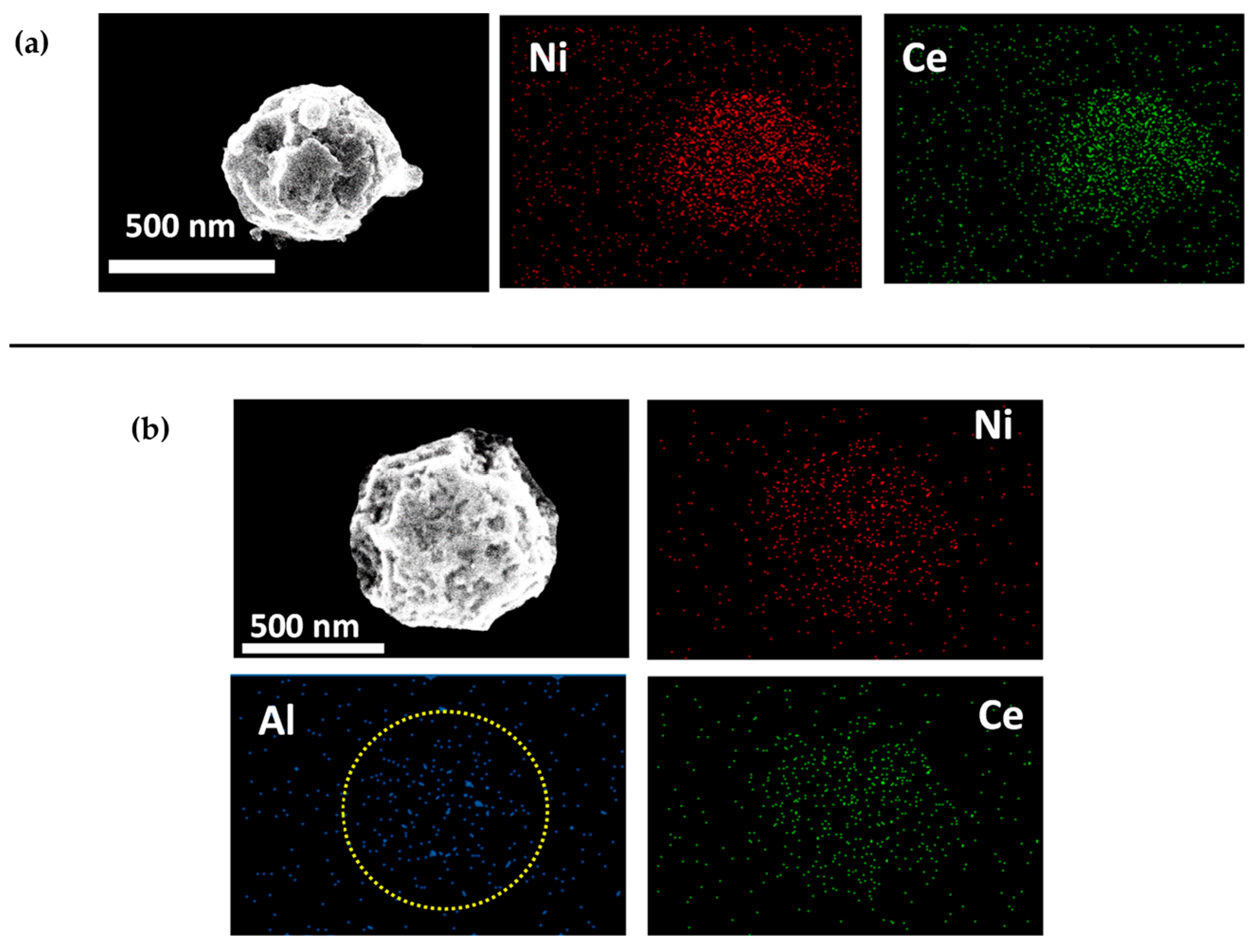
Figure 8. Representative SEM images with EDS elemental mapping. (a) 1Ni-0.5Ce-0Al. (b) 1Ni-0.5Ce-5Al. The yellow circle indicated that the mesoporous structure of 1Ni-0.5Ce-5Al was observed, indicating a successful formation of Al2O3 nanoparticle cluster (NPC) as support material of Ni-CeO2 hybrid nanoparticle (NP) via gas-phase evaporation-induced self-assembly.
Wei et al. [65] employed a non-precious-metal catalyst, namely, NiCu/MgAlO, for the reductive amination reaction of ethanol to prepare ethylamine. As shown in Figure 9d, the bimetallic 9%Ni-3%Cu/MgAlO catalyst achieved 98.7% ethanol conversion and high selectivity (98.9%) for ethylamines under optimal reaction conditions. The characterization results combining XRD, XPS, H2-TPR, SEM, TEM, BET, and other catalyst characterization tools indicated that highly dispersed active Ni and Cu nanoparticles and a uniform particle size played significant roles in achieving the excellent catalytic performance and stability of the NiCu/MgAlO catalysts.
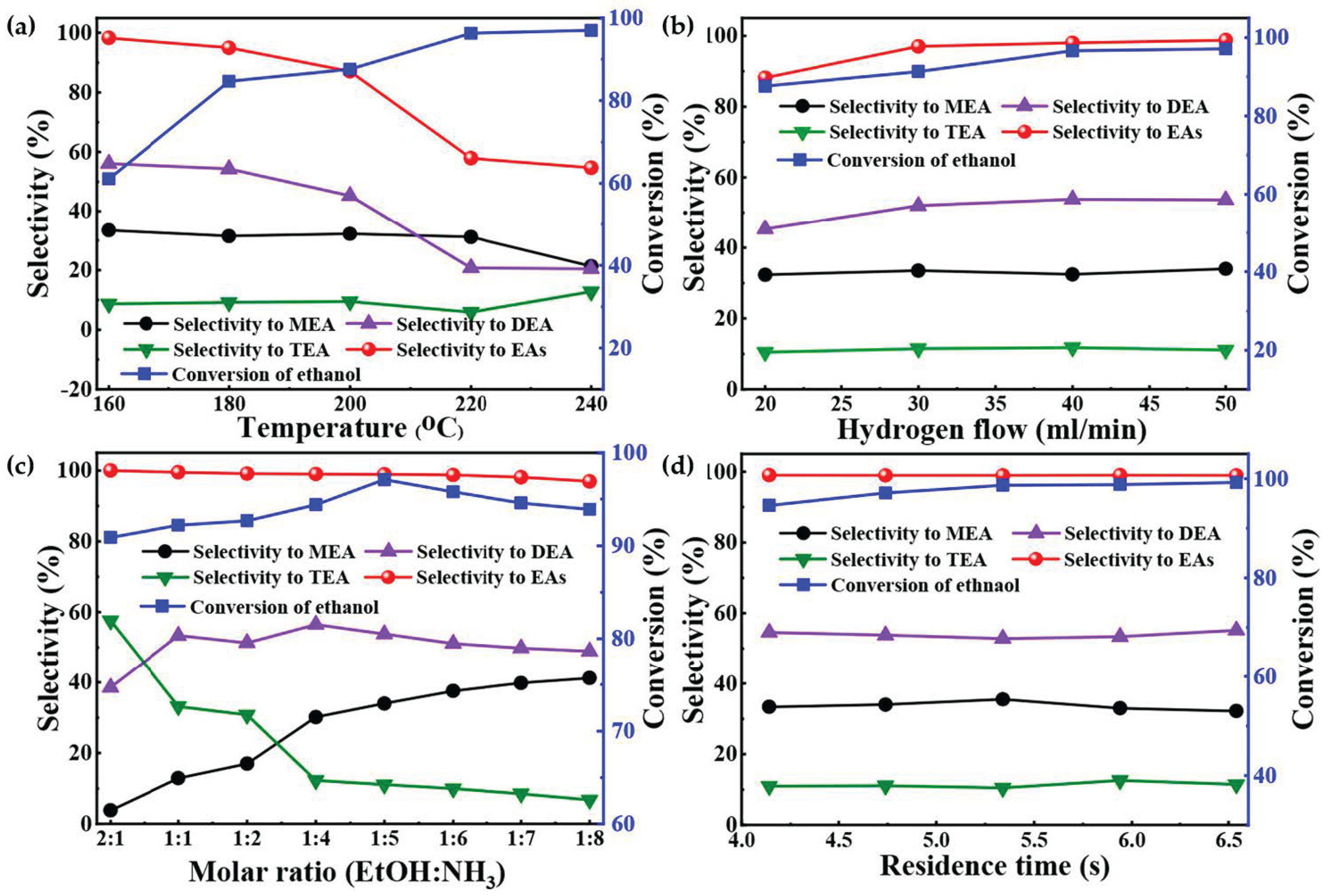
Figure 9. Amination results of ethanol with NH3 over 9% Ni-3% Cu/MgAlO under different reaction conditions. (a) Molar ratio of ethanol to NH3 is 1:5, flow rate of H2 is 20 mL/min, flow rate of NH3 is 38 mL/min, residence time is 5.34 s, and time on stream is 6 h. (b) Temperature is 200 °C, molar ratio of ethanol to NH3 is 1:5, flow rate of NH3 is 38 mL/min, residence time is 5.34 s, and time on stream is 6 h. (c) Temperature is 200 °C, flow rate of H2 is 50 mL/min, flow rate of NH3 is 38 mL/min, residence time is 5.34 s, and time on stream is 6 h. (d) Temperature is 200 °C, molar ratio of ethanol to NH3 is 1:5, flow rate of H2 is 50 mL/min, flow rate of NH3 is 38 mL/min, and time on stream is 6 h.
This entry is adapted from the peer-reviewed paper 10.3390/catal13101350
References
- Dong, C.; Wang, H.; Du, H.; Peng, J.; Cai, Y.; Guo, S.; Zhang, J.; Samart, C.; Ding, M. Ru/HZSM-5 as an efficient and recyclable catalyst for reductive amination of furfural to furfurylamine. Mol. Catal. 2020, 482, 110755.
- Irrgang, T.; Kempe, R. 3d-Metal catalyzed N- and C-alkylation reactions via borrowing hydrogen or hydrogen autotransfer. Chem. Rev. 2019, 119, 2524–2549.
- Kar, A.K.; Chauhan, A.; Srivastava, R. A CoPd nanoalloy embedded N-doped porous carbon catalyst for the selective reduction and reductive amination of levulinic acid using formic acid in water. Sustain. Energ. Fuels 2023, 7, 1855–1869.
- Liu, Y.X.; Zhou, K.; Lu, M.; Wang, L.C.; Wei, Z.J.; Li, X.N. Acidic/basic oxides-supported cobalt catalysts for one-pot synthesis of isophorone diamine from hydroamination of isophorone nitrile. Ind. Eng. Chem. Res. 2015, 54, 9124–9132.
- Saini, M.K.; Kumar, S.; Li, H.; Babu, S.A.; Saravanamurugan, S. Advances in the catalytic reductive amination of furfural to furfural amine: The momentous role of active metal sites. ChemSusChem 2022, 15, e202200107.
- Wan, Y.; Lee, J.M. Recent advances in reductive upgrading of 5-hydroxymethylfurfural via heterogeneous thermocatalysis. ChemSusChem 2022, 15, e202102041.
- Wei, Z.J.; Liu, H.Y.; Zhou, K.; Shu, H.M.; Liu, Y.X. Supported Co/activated carbon catalysts for the one-pot synthesis of isophorone diamine from hydroamination of isophorone nitrile. React. Kinet. Mech. Catal. 2019, 127, 931–943.
- Xiao, Y.; Lim, C.W.; Chang, J.; Yuan, Q.; Wang, L.; Yan, N. Electrocatalytic amino acid synthesis from biomass-derivable keto acids over ball milled carbon nanotubes. Green Chem. 2023, 25, 3117–3126.
- Zou, H.; Chen, J. Efficient and selective approach to biomass-based amine by reductive amination of furfural using Ru catalyst. Appl. Catal. B-Environ. 2022, 309, 121262.
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.P.; Boutevin, B. Biobased amines: From synthesis to polymers; present and future. Chem. Rev. 2016, 116, 14181–14224.
- Pelckmans, M.; Renders, T.; Van de Vyver, S.; Sels, B.F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 2017, 19, 5303–5331.
- Zhang, J.; Yang, J.; Li, X.; Liu, H.; Wang, A.; Xia, C.; Chen, J.; Huang, Z. Efficient synthesis of pharmaceutical intermediates from biomass-derived aldehydes and ketones over robust NixAl nanocatalysts. ACS Sustain. Chem. Eng. 2022, 10, 5526–5537.
- Laval, S.; Dayoub, W.; Favre-Reguillon, A.; Berthod, M.; Demonchaux, P.; Mignani, G.; Lemaire, M. A mild and efficient method for the reduction of nitriles. Tetrahedron Lett. 2009, 50, 7005–7007.
- Willis, M.C. Palladium-catalyzed coupling of ammonia and hydroxide with aryl halides: The direct synthesis of primary anilines and phenols. Angew. Chem. Int. Ed. 2007, 46, 3402–3404.
- Liang, L.Y.; Kung, Y.H.; Hsiao, V.K.; Chu, C.C. Reduction of nitroaromatics by gold nanoparticles on porous silicon fabricated using metal-assisted chemical etching. Nanomaterials 2023, 13, 1805.
- Poulose, A.C.; Zoppellaro, G.; Konidakis, I.; Serpetzoglou, E.; Stratakis, E.; Tomanec, O.; Beller, M.; Bakandritsos, A.; Zboril, R. Fast and selective reduction of nitroarenes under visible light with an earth-abundant plasmonic photocatalyst. Nat. Nanotechnol. 2022, 17, 485–492.
- Fu, X.P.; Han, P.; Wang, Y.Z.; Wang, S.; Yan, N. Insight into the roles of ammonia during direct alcohol amination over supported Ru catalysts. J. Catal. 2021, 399, 121–131.
- Patil, S.; Bedre, A.; Gade, V.; Jopale, M.; Bhagat, R.; Pise, A. One-pot protocol for the reductive amination of aldehydes using thiamine hydrochloride as a green catalyst under solvent-free condition. Synth. Commun. 2023, 53, 1545–1558.
- Patil, S.M.; Bedre, A.V.; Gade, V.B.; Jopale, M.K. Metal-free, an effective and one-pot protocol for the reductive amination of aldehydes using glycerol as a green solvent. J. Chem. Sci. 2023, 135, 50.
- Sun, R.; Ma, S.S.; Zhang, Z.H.; Zhang, Y.Q.; Xu, B.H. Ruthenium-catalyzed reductive amination of ketones with nitroarenes and nitriles. Org. Biomol. Chem. 2023, 21, 1450–1456.
- Zhang, J.; Yin, J.; Duan, X.; Zhang, C.; Zhang, J. Continuous reductive amination to synthesize primary amines with high selectivity in flow. J. Catal. 2023, 420, 89–98.
- Zheng, B.X.; Xu, J.; Song, J.L.; Wu, H.H.; Mei, X.L.; Zhang, K.L.; Han, W.Y.; Wu, W.; He, M.Y.; Han, B.X. Nanoparticles and single atoms of cobalt synergistically enabled low-temperature reductive amination of carbonyl compounds. Chem. Sci. 2022, 13, 9047–9055.
- Zhou, K.; Xie, R.; Xiao, M.; Guo, D.; Cai, Z.; Kang, S.; Xu, Y.; Wei, J. Direct amination of biomass-based furfuryl alcohol and 5-(aminomethyl)-2-furanmethanol with NH3 over hydrotalcite-derived nickel catalysts via the hydrogen-borrowing strategy. ChemCatChem 2021, 13, 2074–2085.
- Kobayashi, H.; Ohta, H.; Fukuoka, A. Conversion of lignocellulose into renewable chemicals by heterogeneous catalysis. Catal. Sci. Technol. 2012, 2, 869–883.
- Kumar, A.; Daw, P.; Milstein, D. Homogeneous catalysis for sustainable energy: Hydrogen and methanol economies, fuels from biomass, and related topics. Chem. Rev. 2022, 122, 385–441.
- Likhar, P.R.; Arundhathi, R.; Kantam, M.L.; Prathima, P.S. Amination of alcohols catalyzed by copper-aluminium hydrotalcite: A green synthesis of amines. Eur. J. Org. Chem. 2009, 2009, 5383–5389.
- Veefkind, V.A.; Lercher, J.A. Zeolite catalysts for the selective synthesis of mono- and diethylamines. J. Catal. 1998, 180, 258–269.
- Klyuev, M.V.; Khidekel, M.L. Catalytic amination of alcohols, aldehydes, and ketones. Russ. Chem. Rev. 1980, 49, 28–53.
- Niu, F.; Wang, Q.; Yan, Z.; Kusema, B.T.; Khodakov, A.Y.; Ordomsky, V.V. Highly efficient and selective N-alkylation of amines with alcohols catalyzed by in situ rehydrated titanium hydroxide. ACS Catal. 2020, 10, 3404–3414.
- Sabatier, P.; Reid, E.E. Catalysis in Organic Chemistry; D. Van Nostrand Company: New York, NY, USA, 1922; Volume 72, pp. 33–38.
- Cai, X.; Ke, Y.; Wang, B.; Zeng, Y.; Chen, L.; Li, Y.; Bai, G.; Yan, X. Efficient catalytic amination of diols to diamines over Cu/ZnO/gamma-Al2O3. Mol. Catal. 2021, 508, 111608.
- Jeon, H.Y.; Shin, C.H.; Jung, H.J.; Hong, S.B. Catalytic evaluation of small-pore molecular sieves with different framework topologies for the synthesis of methylamines. Appl. Catal. A-Gen. 2006, 305, 70–78.
- Maeda, N.; Meemken, F.; Hungerbuhler, K.; Baiker, A. Selectivity-controlling factors in catalytic methanol amination studied by isotopically modulated excitation IR spectroscopy. ACS Catal. 2013, 3, 219–223.
- Bassili, V.A.; Baiker, A. Catalytic amination of 1-methoxy-2-propanol over silica supported nickel: Study of the influence of the reaction parameters. Appl. Catal. 1990, 65, 293–308.
- Cho, J.H.; Park, J.H.; Chang, T.S.; Kim, J.E.; Shin, C.H. Reductive amination of 2-propanol to monoisopropylamine over Ni/γ-Al2O3 catalysts. Catal. Lett. 2013, 143, 1319–1327.
- Shimizu, K.; Kon, K.; Onodera, W.; Yamazaki, H.; Kondo, J.N. Heterogeneous Ni catalyst for direct synthesis of primary amines from alcohols and ammonia. ACS Catal. 2013, 3, 112–117.
- Shimizu, K.I.; Kanno, S.; Kon, K.; Siddiki, S.H.; Tanaka, H.; Sakata, Y. N-alkylation of ammonia and amines with alcohols catalyzed by Ni-loaded CaSiO3. Catal. Today 2014, 232, 134–138.
- Dumon, A.S.; Wang, T.; Ibanez, J.; Tomer, A.; Yan, Z.; Wischert, R.; Sautet, P.; Pera-Titus, M.; Michel, C. Direct n-octanol amination by ammonia on supported Ni and Pd catalysts: Activity is enhanced by “spectator” ammonia adsorbates. Catal. Sci. Technol. 2018, 8, 611–621.
- Wei, Z.J.; Liu, Y.X.; Thushara, D.; Ren, Q.L. Entrainer-intensified vacuum reactive distillation process for the separation of 5-hydroxylmethylfurfural from the dehydration of carbohydrates catalyzed by a metal salt-ionic liquid. Green Chem. 2012, 14, 1220–1226.
- Wei, Z.J.; Xia, S.W.; Chen, M.T.; Lu, M.; Liu, Y.X. Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran over a Cu-acetonitrile complex. New J. Chem. 2019, 43, 7600–7605.
- Liu, Y.X.; Zhou, K.; Shu, H.M.; Liu, H.Y.; Lou, J.T.; Guo, D.C.; Wei, Z.J.; Li, X.N. Switchable synthesis of furfurylamine and tetrahydrofurfurylamine from furfuryl alcohol over RANEY® nickel. Catal. Sci. Technol. 2017, 7, 4129–4135.
- Zhou, K.; Liu, H.Y.; Shu, H.M.; Xiao, S.W.; Guo, D.C.; Liu, Y.X.; Wei, Z.J.; Li, X.N. A comprehensive study on the reductive amination of 5-hydroxymethylfurfural into 2,5-bisaminomethylfuran over raney Ni through DFT calculations. ChemCatChem 2019, 11, 2649–2656.
- Liu, X.Y.; Wang, X.; Qiu, J.R.; Chen, W.K.; Fu, X.; Ye, L.M.; Yuan, Y.Z. Selective amination of phenol to cyclohexylamine over metal-acid bifunctional catalysts derived from nickel phyllosilicates. ChemCatChem 2023, 15, e202300306.
- Cho, J.H.; Park, J.H.; Chang, T.S.; Seo, G.; Shin, C.H. Reductive amination of 2-propanol to monoisopropylamine over Co/γ-Al2O3 catalysts. Appl. Catal. A-Gen. 2012, 417–418, 313–319.
- Lei, X.C.; Gu, G.D.; Hu, Y.F.; Wang, H.S.; Zhang, Z.X.; Wang, S. Structural requirements for chemoselective ammonolysis of ethylene glycol to ethanolamine over supported cobalt catalysts. Catalysts 2021, 11, 736.
- Chary, K.V.; Seela, K.K.; Naresh, D.; Ramakanth, P. Characterization and reductive amination of cyclohexanol and cyclohexanone over Cu/ZrO2 catalysts. Catal. Commun. 2008, 9, 75–81.
- Sagar, G.V.; Rao, P.V.; Srikanth, C.S.; Chary, K.V. Dispersion and reactivity of copper catalysts supported on Al2O3-ZrO2. J. Phys. Chem. B 2006, 110, 13881–13888.
- Zhang, Y.; Zhang, Y.; Feng, C.; Qiu, C.; Wen, Y.; Zhao, J. Amination of ethanol to acetonitrile over Ni-doped Co/γ-Al2O3 catalyst. Catal. Commun. 2009, 10, 1454–1458.
- Kliger, G.A.; Shuikin, A.N.; Glebov, L.S.; Mikaya, A.I.; Loktev, S.M.; Zaikin, V.G. Mechanism of the hydroamination of alcohols and carbonyl compounds on a fused iron catalyst. Bull. Acad. Sci. USSR Div. Chem. Sci. 1990, 39, 1750–1753.
- Liu, Y.X.; Zhou, K.; Lu, M.; Wang, L.C.; Wei, Z.J. Synthesis of isophorone diamine and its reaction condition optimization. J. Chem. Eng. Chin. Univ. 2015, 29, 616–620.
- Zhou, K.; Liu, Y.X.; Lu, M.; Wei, Z.J. Activated carbon supported cobalt for one-pot synthesis of isophorone diamine from hydroamination of isophorone nitrile. In Proceedings of the 16th ICC, Beijing, China, 3–8 July 2016.
- Niemeier, J.; Engel, R.V.; Rose, M. Is water a suitable solvent for the catalytic amination of alcohols? Green Chem. 2017, 19, 2839–2845.
- Ruiz, D.; Aho, A.; Saloranta, T.; Eranen, K.; Warna, J.; Leino, R.; Murzin, D.Y. Direct amination of dodecanol with NH3 over heterogeneous catalysts Catalyst screening and kinetic modelling. Chem. Eng. J. 2017, 307, 739–749.
- Ruiz, D.; Aho, A.; Maki-Arvela, P.; Kumar, N.; Oliva, H.; Murzin, D.Y. Direct amination of dodecanol over noble and transition metal supported silica catalysts. Ind. Eng. Chem. Res. 2017, 56, 12879–12888.
- Fang, L.; Yan, Z.; Wu, J.; Bugaev, A.; Lamberti, C.; Pera-Titus, M. Highly selective Ru/HBEA catalyst for the direct amination of fatty alcohols with ammonia. Appl. Catal. B-Environ. 2021, 286, 119942.
- Li, P.; Huang, H.; Wang, Z.; Hong, Z.; Xu, Y.; Zhao, Y. Reductive amination of n-hexanol to n-hexylamine over Ni-Ce/gamma-Al2O3 catalysts. Front. Chem. Sci. Eng. 2023, 17, 82–92.
- He, L.; Huang, Y.; Liu, X.Y.; Li, L.; Wang, A.; Wang, X.; Mou, C.Y.; Zhang, T. Structural and catalytic properties of supported Ni–Ir alloy catalysts for H2 generation via hydrous hydrazine decomposition. Appl. Catal. B-Environ. 2014, 147, 779–788.
- Cui, X.; Dai, X.; Deng, Y.; Shi, F. Development of a general non-noble metal catalyst for the benign amination of alcohols with amines and ammonia. Chem. Eur. J. 2013, 19, 3665–3675.
- Hong, E.; Bang, S.; Cho, J.H.; Jung, K.D.; Shin, C.H. Reductive amination of isopropanol to monoisopropylamine over Ni-Fe/γ-Al2O3 catalysts: Synergetic effect of Ni-Fe alloy formation. Appl. Catal. A-Gen. 2017, 542, 146–153.
- Zhou, K. Catalyst design and catalytic reaction in the synthesis of diamines by reductive amination of carbonyl and hydroxyl. Ph.D. Dissertation, Zhejiang University of Technology, Hangzhou, China, 2017.
- Ma, L.; Yan, L.; Lu, A.H.; Ding, Y.J. Effect of Re promoter on the structure and catalytic performance of Ni-Re/Al2O3 catalysts for the reductive amination of monoethanolamine. RSC Adv. 2018, 8, 8152–8163.
- Ma, L.; Sun, K.J.; Luo, M.; Yan, L.; Jiang, Z.; Lu, A.H.; Ding, Y.J. Role of ReOx species in Ni-Re/Al2O3 catalyst for amination of monoethanolamine. J. Phys. Chem. C 2018, 122, 23011–23025.
- Chang, H.Y.; Lai, G.H.; Lin, C.Y.; Lee, C.Y.; Chia, C.C.; Hwang, C.L.; Chang, H.M.; Tsai, D.H. Reductive amination of polypropylene glycol using Ni-CeO2@Al2O3 with high activity, selectivity and stability. Catal. Commun. 2019, 127, 15–19.
- Chang, H.Y.; Lai, G.H.; Tsai, D.H. Aerosol route synthesis of Ni-CeO2-Al2O3 hybrid nanoparticle cluster for catalysis of reductive amination of polypropylene glycol. Adv. Powder Technol. 2019, 30, 2293–2298.
- Wei, Y.; You, K.; Xu, W.; Ou, X.; Zhao, F.; Chen, Z.; Yan, D.; Zhang, X.; Luo, H. Highly efficient reductive amination of ethanol to ethylamines over non-noble metallic NiCu/MgAlO catalyst. Ind. Eng. Chem. Res. 2023, 62, 4947–4954.
This entry is offline, you can click here to edit this entry!
