Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The surface functionalization of magnetic nanoparticles (MNPs) has witnessed significant progress recently, revolutionizing their utility in multimodal imaging, drug delivery, and catalysis. This progression, spanning over the last decade, has unfolded in discernible phases, each marked by distinct advancements and paradigm shifts. In the early stage, emphasis was placed on foundational techniques, such as ligand exchange and organic coatings, establishing the groundwork for innovations to come.
- magnetic nanoparticles
- surface functionalization/engineering
- theranostics
- drug delivery
1. Introduction
Magnetic nanoparticles (MNPs) have emerged as versatile entities with profound implications across various scientific frontiers. Their unique physicochemical properties, including superparamagnetism and high surface area-to-volume ratios, have positioned them as compelling candidates in fields ranging from biomedicine [1][2] to catalysis [3][4]. Surface engineering approaches by means of surface modifying agents (such as amine, diimide, carboxyl, aldehyde, hydroxyl, etc.) have unlocked a new dimension of functionalities, allowing further modification by molecule attachment and thus driving recent advancements and expanding their potential [5].
Regarding multimodal imaging, the surface functionalization of MNPs has become a cornerstone in enhancing diagnostic accuracy [6][7][8]. By judiciously modifying the surface chemistry, researchers have endeavored to optimize their interaction with biological entities, improving biocompatibility and enhancing their imaging contrast properties. This has led to the development of MNPs with tailored surface coatings, thereby enabling the precise targeting of specific biomarkers and cell populations. The introduction of functional moieties onto the MNP surface has not only amplified their potential as magnetic resonance imaging (MRI) contrast agents but has also opened avenues for multimodal imaging techniques, merging the power of MRI with other imaging modalities such as fluorescence.
In drug delivery, the surface functionalization of MNPs has been pivotal in overcoming the challenges associated with efficient and targeted drug transport [9]. These modified surfaces allow for the conjugation of therapeutic agents, enabling controlled release profiles and enhancing the pharmacokinetics of drugs. Furthermore, surface functionalization offers the means to encapsulate drugs within protective shells, shielding them from premature degradation or clearance, while facilitating site-specific release at the intended destination. This approach has not only improved drug efficacy but has also mitigated off-target effects, bringing reasearchers one step closer to personalized medicine with a keen attention to the fate of the MNPs inside the human body [10]. The catalytic landscape has equally been reshaped by the ingenuity of MNP surface functionalization. Tailoring the surfaces of MNPs with catalytic moieties has yielded heterogeneous catalysts with unparalleled activity and selectivity. This has proven particularly advantageous in complex catalytic transformations, enabling efficient conversions with reduced side reactions. The advent of well-defined surface architectures has further enabled precise control over catalytic sites and reactions, fostering a synergy between catalysis and nanotechnology that promises to revolutionize chemical synthesis (Figure 1).
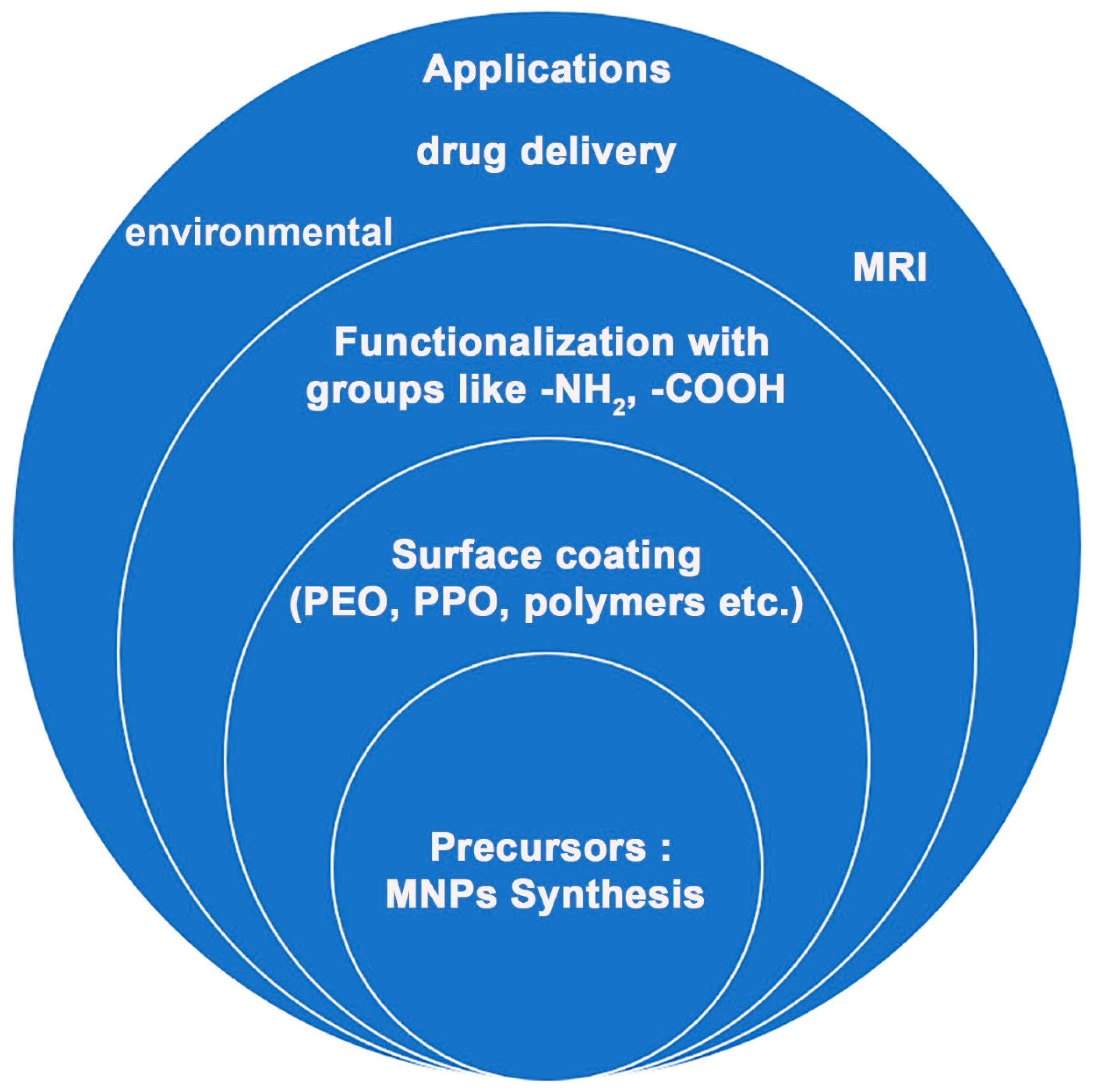
Figure 1. General schematic diagram of MNPs’ synthesis and functionalization.
2. Synthesis of Magnetic Nanoparticles
The synthesis of magnetic nanoparticles (MNPs) represents a key starting point in tailoring their properties for diverse applications. The field has witnessed a surge in innovative approaches to engineer MNPs with precise control over size, shape, crystallinity, and magnetic properties. While the MNP shape is not a main cause of oxidative stress that leads to apoptosis, it plays a critical role in cellular uptake. Controlling size and morphology (shape, aspect ratio) can be achieved for most iron oxides (including FeOOH, Fe2O3, and Fe3O4), given their prime magnetic feature that makes them suitable, among others, as magnetorheological fluids [11]. One strategy in this sense is to use size-controlling agents, which effectively also act as surface coating, like polyethyleneimine (PEI) [12]. A concise overview of the prominent synthesis methods and their influence on surface functionalization will consider the chemical and physical methods described below.
The chemical synthesis route remains a cornerstone in producing MNPs with tunable characteristics. Co-precipitation, a widely adopted method, involves the controlled precipitation of metal salts in the presence of reducing agents or surfactants; for instance, a mixture of cation precursors containing Fe2+ and Fe3+ in a stoichiometric ratio can be precipitated with hydroxide (NH4OH, NaOH) under a protective atmosphere to yield magnetite Fe3O4. This approach yields monodisperse MNPs with controllable sizes, making it a popular choice for subsequent functionalization. Similarly, thermal decomposition (polyol method) involves the decomposition of metal precursors at elevated temperatures, facilitating the formation of MNPs with narrow size distributions and high crystallinity, oftentimes allowing additional tuning of shape (cubic, hexagonal, etc.) and size (very small NPs of a few nm and very narrow PDI polydispersity index can be achieved through this route). These chemically synthesized MNPs offer versatile platforms for surface functionalization due to their well-defined surfaces and high crystallinity.
Physical methods, such as laser ablation and sputtering, have emerged as viable alternatives for producing MNPs tuned for specific applications. Laser ablation involves irradiating a target material with high-energy laser pulses, thereby inducing ablation and condensation of nanoparticles. This technique allows for precise control over size and composition, enabling tailored surface modifications. For instance, Franzel et al. reported the synthesis of superparamagnetic MNPs consisting of Fe3O4 and Fe3C upon laser ablation of an Fe foil in ethanol [13]. Superparamagnetism refers to the property exhibited by certain nanoparticles, particularly magnetic nanoparticles, that do not have a permanent magnetic moment but that can respond strongly to an external magnetic field, and it represents an important characteristic for applications like targeted drug delivery and magnetic resonance imaging (MRI). Further modification of the synthesis by altering reaction media (water, organic solvents) affords variation in the composition of MNPs, including core–shell structures of type iron–iron oxide, carbon coating, etc. Sputtering, on the other hand, relies on the ejection of target material atoms by energetic ion bombardment. This yields MNPs with minimal contamination suitable for subsequent surface engineering, like, for instance, FeCo NPs of very high saturation magnetization (226 emu/g) or multifunctional MNPs coated with PEG polyethylene glycol for improved solubility and enhanced biocompatibility [14]. Magnetization, as a core principle, refers to the property of a material to become magnetized in the presence of an external magnetic field. In the case of MNPs, their small size leads to unique magnetic behaviors. When subjected to an external magnetic field, the magnetic moments of individual nanoparticles align with the field, resulting in an overall magnetic polarization. This phenomenon is known as superparamagnetism. The level of magnetization is influenced by factors such as the size of the nanoparticles (sheer size, aspect ratio, shape, volume), their composition, and the strength of the applied magnetic field. Understanding and manipulating magnetization properties is essential in tailoring the behavior of magnetic nanoparticles for specific applications. The ability to control magnetization allows for precise targeting of nanoparticles to specific anatomical sites in magnetic drug delivery. Biocompatibility refers to the ability of a material or substance to function safely within a biological system without causing harm or adverse reactions; in this context, MNPs should not elicit harmful responses from the body’s tissues or immune system.
Biogenic synthesis has garnered attention for its eco-friendly approach and facile surface functionalization potential. Utilizing microorganisms, plants, or their extracts, this method harnesses the biological entities’ inherent ability to reduce metal ions and form MNPs. The resulting MNPs often exhibit unique surface functionalities due to the biomolecules involved in their synthesis, thus opening avenues for surface modifications [15]. Carvallo et al. reported the use of magnetotactic bacteria to synthesize magnetosomes coated with 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) or citric acid for use in magnetic hyperthermia applications [9][15]; the MNPs coated with citric acid showed a higher SAR-specific absorption rate and were thus better suited for biomedical applications [15]. When IONs were synthesized by magnetotactic bacteria and were further utilized to synthesize magnetosomes coated with citric acid or 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), the magnetosomes showed reduced magnetostatic interactions compared to those in neat magnetosomes [15].
The influence of synthesis parameters on MNP surfaces cannot be understated. The influence of the shape and size of MNPs on their MFH effect has been investigated recently, concluding that specific synthesis parameters should be met in order to produce the highest possible SAR and hence the desired effect in MFH; for instance, ellipsoidal NPs with the highest SAR were found to be those of 10 nm (equatorial size) and an aspect ratio of 2 [16]. The choice of precursor materials, solvents, and reaction conditions significantly affects the surface chemistry. Organic ligands or capping agents introduced during synthesis can impart initial surface functionalities, setting the stage for subsequent modifications [17]. In a typical synthesis, Fe(II) and Fe(III) precursors in a 1:2 molar ratio are added to a mixture of oleic acid (OA) and oleylamine (OAm) in a round-bottom flask and then heated up to 300 °C (depending on the solvent of choice, benzyl ether, dioctyl ether etc.), after which a dark brown solution is produced. After cooling and washing (typically with ethanol, C2H5OH), stirring and/or sonication with additional OA can successfully lead to the organic coating of MNPs, which can be resuspended in organic solvents (hexane or higher alkanes). It is essential to carry out the co-precipitation reaction under a protective inert gas atmosphere in order to avoid further oxidation of magnetite to hematite. Various modifications of the synthetic procedure are found throughout the literature, but the core principles remain the same. Notably, detailed procedures provide a reproducible method for the synthesis of magnetic nanoparticles, ensuring consistent results for subsequent applications in various fields.
The surface properties of magnetic nanoparticles (MNPs) are intricately linked to the synthesis parameters employed during their fabrication. These parameters include reaction temperature, precursor concentrations, surfactant types, and reaction times. The careful manipulation of these factors can yield MNPs with tailored surface characteristics, influencing their behavior in diverse applications. For instance, studies by Lu et al. [18] and Majidi et al. [19] systematically investigated the impact of reaction temperature on MNP surface functionalization while also serving as comprehensive reviews on synthetic methods for generating MNPs. The results demonstrated a notable increase in the density of surface functional groups as the temperature was elevated from 100 °C to above 200 °C. Moreover, reaction times have been shown to impact the size distribution and surface characteristics of MNPs [18].
Surfactant choice and concentration also exert significant control over MNP surface properties; for instance, the use of oleic acid (OA) as a surfactant resulted in a higher degree of surface coverage compared to other surfactants. The nature of the shell (organic/inorganic, magnetic or non-magnetic) has an influence on its magnetic properties, as the ligands can modify the anisotropy and the overall magnetic moment of the particles [18].
Furthermore, concentration gradients of precursor materials dictate the MNP surface composition, and oftentimes non-stoichiometric ratio is necessary in order to obtain pure Fe3O4 (magnetite). These examples highlight the critical role of quantitative data in elucidating the influence of synthesis parameters on MNP surfaces. By employing advanced characterization techniques and systematically varying synthesis conditions, researchers can precisely tailor MNP surface properties, opening avenues for enhanced performance in applications ranging from drug delivery to catalysis.
Regarding biogenic synthesis of magnetic nanoparticles, there are several potential concerns associated with their further use in a clinical setting. These concerns primarily revolve around safety, scalability, and regulatory approval. Biogenic synthesis relies on living organisms to produce nanoparticles, which introduces variability in terms of particle size, shape, and surface properties, and hence may not always meet the stringent standards required for clinical applications. Additionally, this type of synthesis can introduce impurities or contaminants that could pose risks in a clinical context, particularly if they include toxic substances or allergens. The use of biogenic synthesis methods may require extensive regulatory approval processes to ensure safety and efficacy; therefore, standardizing the synthesis process to meet regulatory standards can be challenging due to the biological variability inherent in living organisms. Scalability and reproducibility remain current challenges in order to meet clinical demand. The availability of specific organisms and their growth conditions may limit the production capacity, and replicating the exact conditions across different batches can be complex and oftentimes unpredictable. In some cases, the organisms used in biogenic synthesis may be genetically modified or engineered, which raises concerns about potential risks to the host organisms as well as the potential release of genetically modified organisms into the environment. Even though biogenic synthesis methods aim to produce NPs using biological entities, questions about the biocompatibility and potential toxicity of the resulting nanoparticles may arise. It is crucial to thoroughly evaluate the safety profile of biogenically synthesized nanoparticles for clinical use. Ethical consideration about the use of living organisms for nanoparticle synthesis also raises some issues related to the potential impact on ecosystems, especially if they are genetically modified or rare species. Lastly, biogenic synthesis may lead to nanoparticles with different stability profiles compared to their chemically synthesized counterparts. Understanding the long-term stability and storage conditions of biogenically synthesized nanoparticles is crucial for their clinical viability. In conclusion, while the biogenic synthesis of magnetic nanoparticles holds promise for various applications, including clinical ones, there are notable concerns regarding safety, scalability, regulatory approval, and ethical considerations. Thorough evaluation, standardization, and rigorous testing are essential steps in addressing these concerns and advancing biogenic synthesis methods towards clinical applications [18][19].
These synthesis methods collectively offer a toolkit for tailoring MNPs with distinct characteristics, laying the groundwork for subsequent surface functionalization. Characterization techniques such as TEM, XRD, FTIR, and spectroscopic methods provide critical insights into their structural and surface properties, guiding the choice of surface modification strategies. The synthesis route serves as a pivotal bridge between MNPs’ core composition and their surface chemistry, enabling a precise design for multimodal imaging, drug delivery, and catalytic applications.
The novel functionalization strategies represent significant advancements over existing methods in terms of both efficiency and effectiveness, tackling issues such as specificity, safety, biocompatibility, and clinical translation. In terms of efficiency, these novel strategies enable highly precise targeting of specific cells or tissues. This surpasses conventional methods, which often rely on passive accumulation through the enhanced permeability and retention (EPR) effect. The new functionalization techniques offer unprecedented control over drug release kinetics, surpassing traditional encapsulation methods that may have limited control over release rates. Surface modifications in the novel strategies also enhance catalytic activity through tailored active sites, surpassing generic functionalization which may not optimize the catalytic potential.
In terms of effectiveness, the novel strategies greatly enhance therapeutic precision, enabling drugs to be delivered precisely where they are needed. This is a significant improvement over traditional methods where off-target effects can be a concern. The new functionalization techniques often lead to enhanced stability and longevity of the functionalized nanoparticles, surpassing traditional coatings that may be less robust while also affording the incorporation of multiple functionalities onto a single nanoparticle, allowing for synergistic effects [8][20][21]. Theranostics refers to combined therapy and diagnostics and represents a class of technologies that combine therapeutic and diagnostic capabilities in a single system. Notably, MNPs used in theranostics can both deliver a drug to a specific site and also be imaged to monitor the drug’s distribution and therapeutic effects. This strategy outperforms existing methods that may be limited to a single function, and it is key to offering enhanced multifunctionality. In this context, MNPs are ideal candidates in theranostic platforms. For instance, MRI-guided NPs have been utilized in combined photodynamic therapy (PDT) and photothermal therapy (PTT), thus achieving chemical exchange on the tumor and, respectively, localized thermal damage at the tumor level [8]. Additional strides have been made in order to overcome biologic barriers such as the blood–brain barrier (BBB), and these consist of various functionalization of penetrating NPs with CPP (cell-penetrating peptides) such as hydrophilic (cationic; TAT, penetratin, R8), amphipathic (SynB, RGD, etc.) or hydrophobic (nonpolar, C105Y, PFV, Pep-7) [20]. An ongoing trend is to utilize MNPs in cell membrane-based biomimetic nanosystems for personalized disease theranostics including oncology, bacterial infections, brain diseases, and inflammatory diseases [21]. Theranostics exemplifies how the integration of multiple functionalities in MNPs can lead to highly effective multifunctional platforms. By combining targeted drug delivery with real-time imaging, researchers can develop personalized and optimized treatment strategies for cancer patients, minimizing side effects and maximizing therapeutic outcomes. This approach holds significant promise for the future of precision medicine in oncology [20][21].
Important advancements have been made in terms of specificity, with stimuli-responsive coatings in the novel strategies responding to specific cues in the microenvironment, thereby offering highly specific imaging capabilities. “Stimuli-responsive” describes materials, coatings, or systems that can change their properties or behavior in response to specific external stimuli; in relation to MNPs, this could refer to coatings that change their structure or release properties in response to factors like pH, temperature, or light, among other factors. Traditional contrast agents may lack this level of specificity. Meanwhile, recent strides often incorporate coatings or ligands that enhance biocompatibility, reducing potential toxicity concerns, which in turn surpasses older methods that may not have addressed biocompatibility to the same extent.
3. Surface Functionalization for Drug Delivery
The strategic surface functionalization of magnetic nanoparticles (MNPs) has revolutionized drug delivery, enabling precise and efficient therapeutic interventions. This section delves into the multifaceted enhancements brought about by surface engineering, including controlled drug release, targeted delivery, and overcoming biological and histohematic barriers [20]. Responsive and targeted drug delivery based on MNPs (magnetite Fe3O4 mainly) coated with polymers and biopolymers [22], biomolecules or macromolecules (5-fluorouracil (5-FU), oxaliplatin, and irinotecan), can be successfully used for the treatment of various types of cancer, such as in colorectal cancer therapy [23]. A brief timeline overview starting from the SiO2 coating of magnetite in 1985 is depicted in Figure 2 [23]. Inorganic shells such as SiO2 (silica) still remain one of the preferred surface functionalization routes, especially for MNPs like γ-Fe3O4 [24]. Recent advances in nanoscience greatly benefit from the introduction of NPs and magnetic iron oxide nanoparticles in particular, owing to their unique set of properties and many advantageous biomedical applications [25], extending their use to stem cell biotechnology [26].
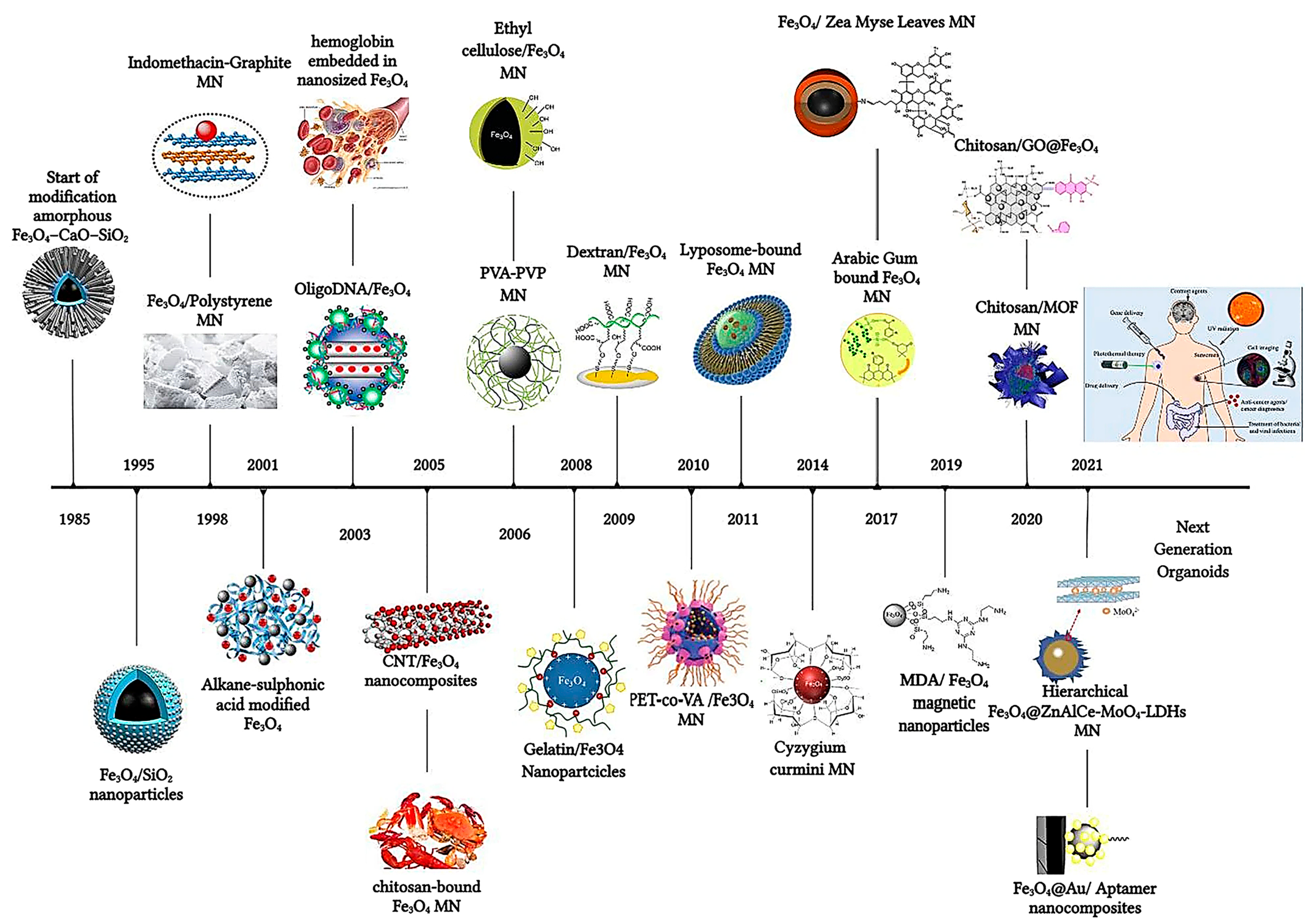
Figure 2. The timeline of magnetic nanoparticles in therapeutic and imaging application.
Various polymers have been employed for coating inorganic NPs (such as a Pt shell [27]), and MNPs in particular, such as hydrophilic polymers (like functional polyaspartamide [28]) that help them achieve water-solubility. Moreover, the stability of magnetite NPs obtained by Massart synthesis (co-precipitation of Fe(II) and Fe(III) precursors) was studied by Klekotka et al. [29], including their surface-functionalized counterparts, specifically SiO2-covered Fe3O4 (SiO2@Fe3O4) or magnetite grown on pre-formed Fe3O4 seeds (Fe3O4@Fe3O4) in polyol synthesis using oleyl amine/oleic acid as stabilizers and surface protection agents [29]. An overview of the main research direction currently being taken by the research community is given in Figure 3.
Figure 3. Representative research direction in functionalized magnetic nanoparticles.
Surface modifications offer unprecedented control over drug release kinetics. Drug-loaded MNPs encapsulated within stimuli-responsive polymers (pH, temperature) exhibit on-demand drug release triggered by specific microenvironment cues [30]. The rate and extent of drug release are characterized using in vitro release assays. Surface-functionalized MNPs offer unparalleled targeting precision. The conjugation of targeting ligands (antibodies, peptides) guides MNPs to specific cell receptors or tissues, minimizing off-target effects. In vitro cell binding assays and in vivo biodistribution studies validate targeted delivery efficiency. Biodistribution refers to how a substance, like a nanoparticle, is distributed throughout the body, and this encompasses where the substance moves, how much of it accumulates in different tissues or organs, and how long it stays there.
Tissue engineering and regeneration has been shown to be possible by Chitosan (CS)-based NP formulations, including CS/ferromagnetic scaffolds, owing to the efficient interaction of -NH2 groups (CS) with the MNPs’ polymeric coating [31]. Such effective CS-coating of the magnetite magnetic core also prevents further oxidation of magnetite Fe3O4 to hematite Fe2O3. Biomedical applications of MPNs and SPIONs in particular have been recently reviewed by Jiang et al. related to their synthesis, in vivo protein detection, magnetic heating/MFH [32], and in vivo imaging and drug delivery [1]. Treatment of degenerative diseases can potentially be modulated by Wnt signaling and has inspired Hu et al. to immobilize Wnt fragment peptides on MNPs with promising results on human embryonic kidney (HEK293) Luc-TCF/LEF reporter cell line [33].
Surface engineering influences cellular uptake mechanisms, thereby enhancing drug internalization. Ligand-decorated MNPs undergo receptor-mediated endocytosis, improving drug delivery to intracellular compartments. Confocal microscopy and flow cytometry quantify enhanced cellular uptake. Surface-engineered MNPs enable intracellular drug delivery. Cell-penetrating peptides facilitate direct entry into cells, bypassing endocytotic pathways. Intracellular imaging and drug efficacy assays validate efficient intracellular drug delivery. These surface modifications enable MNPs to traverse biological barriers [20]. Stealth coatings (PEG) reduce opsonization, prolonging blood circulation, and enable passive accumulation in tumor tissues via the enhanced permeability and retention (EPR) effect. Pharmacokinetic studies and tumor accumulation assays validate EPR-driven delivery.
Surface functionalization permits the simultaneous loading of multiple drugs onto MNPs. Conjugation of distinct drug molecules or payloads enables combination therapy, targeting multiple pathways simultaneously. In vitro cytotoxicity assays elucidate synergistic therapeutic effects. Surface-functionalized MNPs facilitate personalized medicine approaches. Patient-specific targeting ligands enable tailored drug delivery, minimizing adverse effects and enhancing therapeutic outcomes. Genomic and proteomic profiling guide the design of personalized MNPs, while biomimetic approaches [12] enhance the outcome in various diseases such as brain cancer and inflammatory and bacterial infections [21]. Biomimetic NPs can also be used as cost- and time-effective magnetic biosensors, and they can concentrate Gram-positive and Gram-negative microorganisms for easier bacterial detection to concentrations as low as 10 CFU/mL using qPCR [12].
MNPs equipped with responsive coatings release drugs in response to external stimuli (magnetic field, light) [30][34]. Magnetic hyperthermia-triggered drug release exploits localized heating induced by alternating magnetic fields. Temperature measurements validate controlled release [5][9][15][35][36]. Dual-functional MNPs merge drug delivery with therapeutic modalities. The conjugation of chemotherapeutic drugs with photosensitizers or gene therapy agents [37] offers synergistic treatment approaches. Cell viability assays and in vivo tumor regression studies demonstrate combination therapy efficacy.
Surface-functionalized MNPs have entered clinical trials, demonstrating promise in cancer therapy and beyond. The translation of these advanced platforms from preclinical studies to clinical practice requires rigorous safety assessments, optimization of targeting strategies, and scalable production techniques. While base nanoparticles pose several potential shortcomings and toxicity-related issues, functionalized NPs alleviate many of the main downsides preventing the use of MNP-based nanoplatforms: reactive oxygen species (ROS) generation, targeted delivery, and biodegradation. For instance, conjugated polymer nanoparticles (CPNs) modified with dopants (like iron oxide) behave as theranostic agents with prospects for multifunctionality in imaging and treatment [38]. Arias-Ramos et al. synthesized by nanoprecipitation conjugated polymer nanoparticles CPNs based on 2 nm-thick oleic acid-caped MNPs (NiFe2O4, Fe3O4 of ~5 nm) and fluorescent conjugated polymer (CP) poly(9,9-dioctylfluorene-alt-benzothiadiazole, F8BT) or polystyrene grafted with ethylene oxide functionalized with carboxyl groups (PS-EG-COOH), with good results towards glioblastoma (GBM, a common tumor of the central nervous system) using light delivery to the brain tissue by means of fiber optics [38].
Simulations using quantum chemistry (DFT) have also been performed, for instance on the system of tirapazamine (TPZ, anticancer drug) and the magnetic nanoparticle (MNP) Fe6(OH)18(H2O)6, where the interaction between the MNP and TPZ was shown to be facilitated by intraring N-atom, -NH2, and -NO groups present in the TPZ molecule, concluding that interaction energetics via the first two are more accessible than via the -NO moiety [39]. There were two envisioned pathways for the covalent binding of TPX onto MNPs via their surface -OH hydroxyl groups (Figure 4).
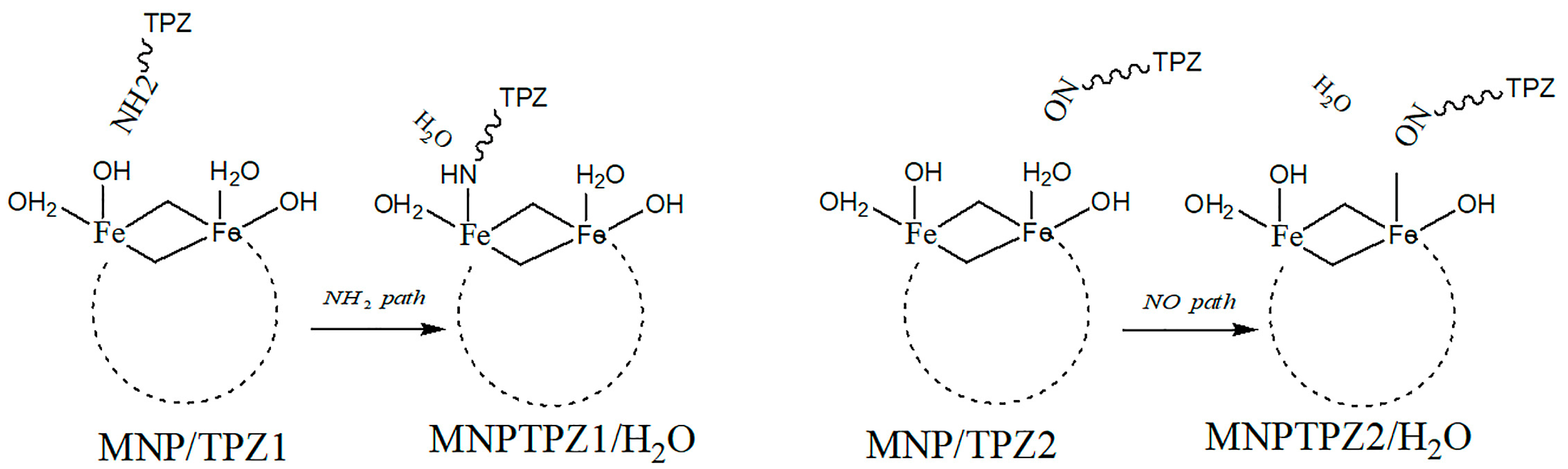
Figure 4. TPZ–MNPs binding via -NH2 or -NO mechanisms.
Other functionalization strategies focused on the polymer coating of magnetite NPs by atom transfer radical polymerization (ATRP) in order to yield magnetic functionalized supports of the general formula Fe3O4@MSN-PDMAEMA-FA of ~180 nm to be used for Dox (doxorubicin) drug loading Dox@Fe3O4@MSN-PDMAEMA(-FA). These drug delivery systems were tested against breast cancer cells (MCF-7) and resistant cancer cells (MCF-7 ADR) [40]. Beagan et al. used magnetic mesoporous silica nanoparticles (MMSNs) coated with pH-responsive polymer 2-Diethyl amino ethyl methacrylate (DEAEMA) grafted by surface-initiated ARGET atom transfer radical polymerization (ATRP); these surface-functionalized MMSNs were further modified by anionic groups’ functionalities that would then covalently bind FA (folic acid) as a targeting agent [40]. The functionalization strategy used is shown in Figure 5, and afforded long-circulation time for the smart drug delivery system.
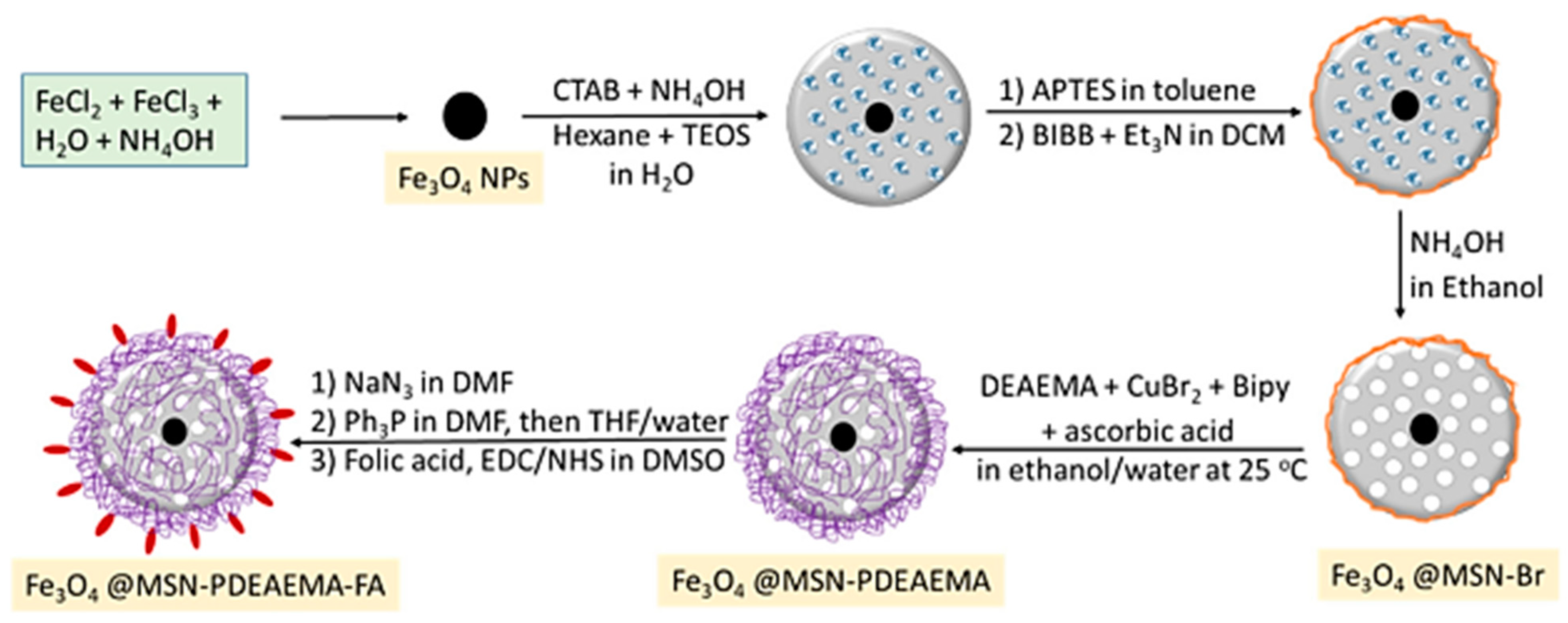
Figure 5. Synthesis procedure for poly(2-(diethylamino) ethylmethacrylate) brushes capped with folic acid grafted on magnetic mesoporous silica nanoparticle (MMSNs) surfaces via SI-ARGET ATRP.
The functionalization strategy outlined in Figure 5, involving the synthesis of poly(2-(diethylamino) ethylmethacrylate) (PDEAEMA) brushes capped with folic acid grafted on magnetic mesoporous silica nanoparticles (MMSNs) surfaces via SI-ARGET ATRP, offers several distinct advantages related to tailored surface functionality, grafting density, enhanced biocompatibility, specific targeting via folic acid, combined magnetic and mesoporous features, and potential for imaging applications. The use of surface-initiated atom transfer radical polymerization (SI-ARGET ATRP) enables precise control over the grafting process, allowing for customization of the surface with PDEAEMA brushes and folic acid, thereby providing tailored surface functionality. SI-ARGET ATRP is known for its ability to achieve high grafting densities, ensuring a densely packed layer of PDEAEMA brushes on the MMSNs surface. The incorporation of PDEAEMA brushes enhances the biocompatibility of the MMSNs. PDEAEMA is a pH-responsive polymer that becomes protonated under acidic conditions, mimicking the slightly acidic environment of cancer cells, and this property can aid in the selective targeting of cancer cells. More specifically, in an acidic environment, such as within the endosomes or lysosomes of cancer cells, the PDEAEMA brushes undergo protonation, leading to swelling and the subsequent release of encapsulated drugs. Through further conjugation via folic acid, this strategy targets cancer cells due to its high affinity for folate receptors, which are overexpressed on the surface of many cancer cells. The presence of folic acid on the MMSNs’ surface ensures specific binding and uptake by cancer cells, maximizing therapeutic efficacy [40]. The MMSNs possess both magnetic properties and a mesoporous structure, thereby allowing for magnetic guidance to target sites, and provide a high surface area for drug loading, thus enabling multifunctional drug delivery platforms. Furthermore, the magnetic properties of MMSNs offer the potential for imaging applications, such as magnetic resonance imaging (MRI). This dual functionality allows for theranostic applications, where therapy and imaging are integrated into a single platform, making this approach highly promising for targeted drug delivery in cancer therapy.
Magnetic nanoplatforms are currently enhanced by loading with enzymes, affording easier recovery and reutilization as well as improved stability and catalytic activity [41]. Belleti et al. synthesized hybrid Au/Fe3O4 NPs of ~15 nm mean diameter using L-cysteine (Cys) as the polymer capping agent or dithiol-terminated polyethylene glycol (PEG(SH)2), yielding NPs of type PEG(SH)2Au/F3O4NPs or CysAu/F3O4NPs [42]. These nanoparticle systems were further conjugated with luciferase enzymes able to catalyze bioluminescent reactions (Pyrearinus termitilluminans green-emitting click beetle luciferase, PyLuc and Phrixotrix hirtus red-emitting railroad worm luciferase, RELuc).
Various MNP systems have been designed to overcome embolism caused by these nanoparticles, which requires coating with biocompatible and non-cytotoxic polymers, such as poly (globalideco-ε-caprolactone) (PGlCL), modified with the amino acid cysteine (Cys) via a thiol-ene reaction (PGlCLCys) [43]. The reaction scheme utilized to produce a coating of SPIONS (SPION@PGlCLCys) and further conjugation with folic acid (FA) or the anti-cancer drug methotrexate (MTX) is described in Figure 6. Cysteine (Cys) was chosen specifically due to its good compatibility with SPIONs, which it binds to through carboxylic and thiol groups, while the selection of biomolecules (FA, MTX) was aimed at anticancer treatment. Experiments showed a 45% release of MTX within 72 h under enzymatic-triggered release and a reasonable reduction in tumor cell (breast carcinoma MDA-MB 231) viability of 20%. The tumoral cell viability remained high, although the MTX loading was quite low at 3.20 µg MTX/mg IONP [43].
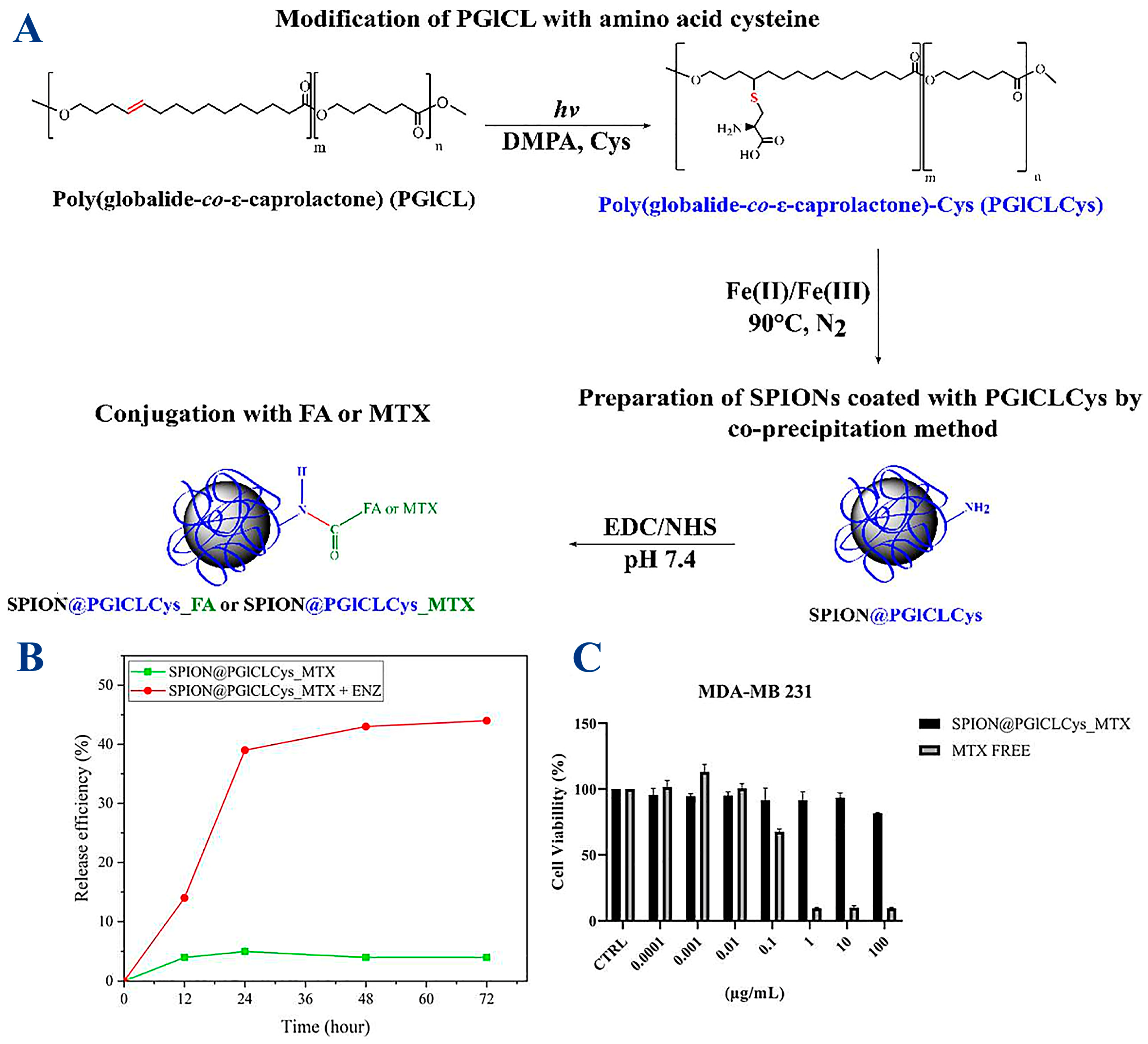
Figure 6. (A) Synthesis of PGICLCys and SPIONs and conjugation with folic acid (FA) or methotrexate (MTX). SPION@PGlCLCys_MTX: (B) drug delivery assay at lysosomal pH (pH 5.3) with or without protease (ENZ); (C) breast carcinoma-derived MDA-MB 231 cells viability after 72 h at different MTX concentrations.
The use of surface-functionalized magnetic nanoparticles (MNPs) in medical therapies shows great promise, but it is important to be aware of potential side effects and complications related to biocompatibility and toxicity, accumulation and biodistribution, retention and clearance, inflammatory/allergic response, potential interference, agglomeration, under/overdosing, unintended effect on nearby tissues/organs, rare element toxicity (present in some surface coatings), incomplete drug release, clinical translation, and regulatory approval.
Magnetization of the magnetic core is highly important, as it further dictates the final behavior of the magnetic nanoplatform when subjected to a magnetic field. It is noteworthy that other compounds such as nitrides γ′-Fe4N, synthesized through a consecutive reduction and nitridation strategy, have gained research momentum due to their superior magnetic properties when compared to typical IONPs (Figure 7) [44]. Such a new magnetic core (MS = 182.7 emu/g for γ′-Fe4N) could yield novel high-performance magnetic nanoplatforms.
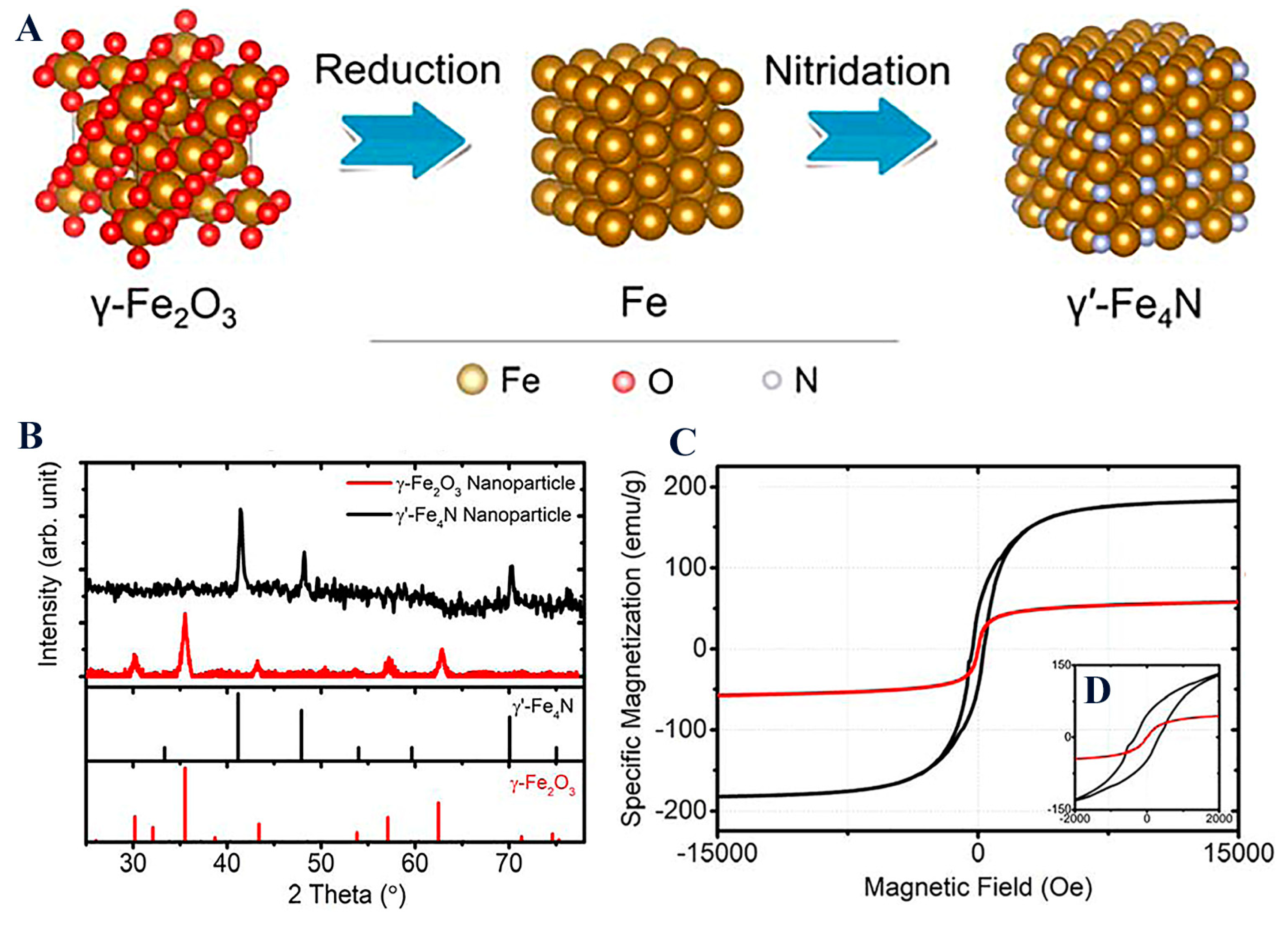
Figure 7. (A) Space-filling models of γ-Fe2O3, Fe, and γ′-Fe4N crystal structure transitions from the reduction and nitridation steps; (B) XRD patterns of γ-Fe2O3 and the synthesized γ′-Fe4N nanoparticles; (C) static magnetic hysteresis loops of γ-Fe2O3 and the synthesized γ′-Fe4N nanoparticles measured by PPMS with the external magnetic field swept from −15 to +15 kOe. The inset figure (D) shows hysteresis loops within a field range of ±2 kOe.
Depending on the composition and surface functionalization, MNPs may have different levels of biocompatibility, and some coatings or functional groups may induce toxicity or provoke an immune response. Therefore, thorough biocompatibility testing is crucial. Moreover, MNPs can accumulate in various tissues and organs, especially in the liver, spleen, and lymph nodes. Understanding and controlling their biodistribution is important in order to prevent potential long-term effects. The long-term retention of MNPs in the body, especially in critical organs, could lead to complications, so ensuring efficient clearance mechanisms is essential to mitigate potential risks. Some surface coatings may trigger inflammatory responses, which can lead to local or systemic reactions, potentially causing discomfort or more serious complications. Also, allergic reactions may occur within some individuals, ranging from mild skin irritation to severe anaphylactic responses. While some degree of interference can occur with other medical devices or implants, a more serious issue is related to the fate of the MNPs in the biological system. In targeted drug delivery, there is a risk of unintentional effects on neighboring healthy tissues if the targeting mechanisms are not sufficiently specific. These effects can be exacerbated by agglomeration of NPs, a phenomenon which occurs based on specific microenvironments and could lead to embolisms or blockages in blood vessels. A careful evaluation of the release profiles to ensure the correct dosage and distribution of MNPs is crucial: overdosing leads to toxicity, while underdosing results in ineffective therapy. In drug delivery applications, there may be challenges in achieving precise control over drug release rates, and this could lead to suboptimal therapeutic outcomes. In all cases, surface-functionalized MNPs have to meet regulatory standards for clinical translation, and this involves rigorous testing and approval processes.
It is important to note that rigorous pre-clinical testing and comprehensive risk assessments are essential steps in the development and application of surface-functionalized MNPs in medical therapies. Additionally, close monitoring of patients receiving MNP-based therapies is crucial in order to promptly identify and address any potential side effects or complications. Surface functionalization [45] has propelled MNPs into the forefront of drug delivery, redefining therapeutic precision. Rigorous characterization techniques, coupled with in vitro and in vivo validation studies, underscore the transformative potential of surface-engineered MNPs in revolutionizing drug delivery paradigms, offering novel avenues for personalized and targeted therapies [46].
This entry is adapted from the peer-reviewed paper 10.3390/coatings13101772
References
- Jiang, K.Y.; Zhang, L.L.; Bao, G. Magnetic iron oxide nanoparticles for biomedical applications. Curr. Opin. Biomed. Eng. 2021, 20, 100330.
- Shankar, M.; Kesavan, S.S.; Biswas, K. Exploring the Potentials of Magnetic Nanoscale Material for Different Biomedical Applications: A Review. Bionanoscience 2023.
- Matveeva, V.G.; Bronstein, L.M. Magnetic Nanoparticle-Containing Supports as Carriers of Immobilized Enzymes: Key Factors Influencing the Biocatalyst Performance. Nanomaterials 2021, 11, 2257.
- Palade, P.; Comanescu, C.; Radu, C. Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride. Materials 2023, 16, 427.
- Mehak; Thummer, R.P.; Pandey, L.M. Surface modified iron-oxide based engineered nanomaterials for hyperthermia therapy of cancer cells. Biotechnol. Genet. 2023.
- Pryazhnikov, D.V.; Kubrakova, I.V. Surface-Modified Magnetic Nanoscale Materials: Preparation and Study of Their Structure, Composition, and Properties. J. Anal. Chem. 2021, 76, 685–706.
- Ansari, M.J.; Kadhim, M.M.; Hussein, B.A.; Lafta, H.A.; Kianfar, E. Synthesis and Stability of Magnetic Nanoparticles. Bionanoscience 2022, 12, 627–638.
- Siddique, S.; Chow, J.C.L. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials 2022, 12, 2826.
- Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872–930.
- Senthilkumar, N.; Sharma, P.K.; Sood, N.; Bhalla, N. Designing magnetic nanoparticles for in vivo applications and understanding their fate inside human body. Coordin. Chem. Rev. 2021, 445, 214082.
- Gwon, H.; Park, S.; Lu, Q.; Choi, H.J.; Lee, S. Size effect of iron oxide nanorods with controlled aspect ratio on magneto-responsive behavior. J. Ind. Eng. Chem. 2023, 124, 279–286.
- Jimenez-Carretero, M.; Rodriguez-Lopez, J.; Ropero-Moreno, C.; Granada, J.; Delgado-Martin, J.; Martinez-Bueno, M.; Fernandez-Vivas, A.; Jimenez-Lopez, C. Biomimetic magnetic nanoparticles for bacterial magnetic concentration in liquids and qPCR-detection. Food Control 2023, 147, 109623.
- Franzel, L.; Bertino, M.F.; Huba, Z.J.; Carpenter, E.E. Synthesis of magnetic nanoparticles by pulsed laser ablation. Appl. Surf. Sci. 2012, 261, 332–336.
- Liu, J.; Su, D.; Wu, K.; Wang, J.P. High-moment magnetic nanoparticles. J. Nanopart. Res. 2020, 22, 66.
- Carvallo, C.; Fondet, A.; Le Fèvre, R.; Taverna, D.; Guyodo, Y.; Chebbi, I.; Dupuis, V.; Lagroix, F.; Khelfallah, M.; Guigner, J.-M.; et al. Magnetic and structural properties of biogenic magnetic nanoparticles along their production process for use in magnetic hyperthermia. J. Magn. Magn. Mater. 2023, 575, 170726.
- Iacob, N.; Kuncser, A.; Comanescu, C.; Palade, P.; Kuncser, V. Optimization of magnetic fluid hyperthermia with respect to nanoparticle shape-related parameters: Case of magnetite ellipsoidal nanoparticles. J. Nanopart. Res. 2020, 22, 138.
- Pedroso-Santana, S.; Fleitas-Salazar, N. The Use of Capping Agents in the Stabilization and Functionalization of Metallic Nanoparticles for Biomedical Applications. Part. Part. Syst. Charact. 2023, 40, 2200146.
- Lu, A.H.; Salabas, E.E.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244.
- Majidi, S.; Zeinali, F.; Samad, S.; Farkhani, M.; Soleymani, M.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734.
- Gareev, K.; Tagaeva, R.; Bobkov, D.; Yudintceva, N.; Goncharova, D.; Combs, S.E.; Ten, A.; Samochernych, K.; Shevtsov, M. Passing of Nanocarriers across the Histohematic Barriers: Current Approaches for Tumor Theranostics. Nanomaterials 2023, 13, 1140.
- Hao, H.S.; Chen, Y.; Wu, M.Y. Biomimetic nanomedicine toward personalized disease theranostics. Nano Res. 2021, 14, 2491–2511.
- Strassburg, S.; Mayer, K.; Scheibel, T. Functionalization of biopolymer fibers with magnetic nanoparticles. Phys. Sci. Rev. 2022, 7, 1091–1117.
- Darroudi, M.; Gholami, M.; Rezayi, M.; Khazaei, M. An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery. J. Nanobiotechnol. 2021, 19, 399.
- Mahajan, R.; Suriyanarayanan, S.; Nicholls, I.A. Improved Solvothermal Synthesis of gamma-Fe2O3 Magnetic Nanoparticles for SiO2 Coating. Nanomaterials 2021, 11, 1889.
- Elahi, N.; Rizwan, M. Progress and prospects of magnetic iron oxide nanoparticles in biomedical applications: A review. Artif. Organs 2021, 45, 1272–1299.
- Ferreira-Faria, I.; Yousefiasl, S.; Macario-Soares, A.; Pereira-Silva, M.; Peixoto, D.; Zafar, H.; Raza, F.; Faneca, H.; Veiga, F.; Hamblin, M.R.; et al. Stem cell membrane-coated abiotic nanomaterials for biomedical applications. J. Control Release 2022, 351, 174–197.
- Hur, J.U.; Shin, J.R.; Han, J.S.; Kim, Y.H.; An, G.S. Self-assembled core-shell Fe3O4-Pt nanoparticles via silylation/polymerization-based amino-functionalization. Colloid Interfac. Sci. 2022, 50, 100655.
- Das, P.; Jana, N.R. Biomedical Applications of Functional Polyaspartamide-Based Materials. ACS Appl. Polym. Mater. 2021, 3, 4791–4811.
- Klekotka, U.; Zambrzycka-Szelewa, E.; Satula, D.; Kalska-Szostko, B. Stability Studies of Magnetite Nanoparticles in Environmental Solutions. Materials 2021, 14, 5069.
- Low, L.E.; Lim, H.P.; Ong, Y.S.; Siva, S.P.; Sia, C.S.; Goh, B.H.; Chan, E.S.; Tey, B.T. Stimuli-controllable iron oxide nanoparticle assemblies: Design, manipulation and bio-applications. J. Control Release 2022, 345, 231–274.
- Zakhireh, S.; Barar, J.; Adibkia, K.; Beygi-Khosrowshahi, Y.; Fathi, M.; Omidain, H.; Omidi, Y. Bioactive Chitosan-Based Organometallic Scaffolds for Tissue Engineering and Regeneration. Topics Curr. Chem. 2022, 380, 13.
- Kwizera, E.A.; Stewart, S.; Mahmud, M.M.; He, X.M. Magnetic Nanoparticle-Mediated Heating for Biomedical Applications. J. Heat Trans.-T Asme 2022, 144, 030801.
- Hu, B.; Rotherham, M.; Farrow, N.; Roach, P.; Dobson, J.; El Haj, A.J. Immobilization of Wnt Fragment Peptides on Magnetic Nanoparticles or Synthetic Surfaces Regulate Wnt Signaling Kinetics. Int. J. Mol. Sci. 2022, 23, 10164.
- Mohapatra, A.; Uthaman, S.; Park, I.K. External and Internal Stimuli-Responsive Metallic Nanotherapeutics for Enhanced Anticancer Therapy. Front. Mol. Biosci. 2021, 7, 597634.
- Kothandaraman, H.; Kaliyamoorthy, A.; Rajaram, A.; Kalaiselvan, C.R.; Sahu, N.K.; Govindasamy, P.; Rajaram, M. Functionalization and Haemolytic analysis of pure superparamagnetic magnetite nanoparticle for hyperthermia application. J. Biol. Phys. 2022, 48, 383–397.
- Duong, H.D.T.; Yoon, S.H.; Nguyen, D.T.; Kim, K.S. Magnetic heating of water dispersible and size-controlled superparamagnetic cobalt iron oxide nanoparticles. Powder Technol. 2023, 427, 118720.
- Ghosal, K.; Chatterjee, S.; Thomas, S.; Roy, P. A Detailed Review on Synthesis, Functionalization, Application, Challenges, and Current Status of Magnetic Nanoparticles in the Field of Drug Delivery and Gene Delivery System. AAPS PharmSciTech 2022, 24, 25.
- Arias-Ramos, N.; Ibarra, L.E.; Serrano-Torres, M.; Yague, B.; Caverzan, M.D.; Chesta, C.A.; Palacios, R.E.; Lopez-Larrubia, P. Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors. Pharmaceutics 2021, 13, 1258.
- Avarand, S.; Morsali, A.; Heravi, M.M.; Beyramabadi, S.A. A quantum chemical study on the magnetic nanocarrier-tirapazamine drug delivery system. Nanosyst.-Phys. Chem. M 2021, 12, 167–174.
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic Acid-Terminated Poly(2-Diethyl Amino Ethyl Methacrylate) Brush-Gated Magnetic Mesoporous Nanoparticles as a Smart Drug Delivery System. Polymers 2021, 13, 59.
- Valls-Chivas, A.; Gomez, J.; Garcia-Peiro, J.I.; Hornos, F.; Hueso, J.L. Enzyme-Iron Oxide Nanoassemblies: A Review of Immobilization and Biocatalytic Applications. Catalysts 2023, 13, 980.
- Belleti, E.; Bevilaqua, V.R.; Brito, A.M.M.; Modesto, D.A.; Lanfredi, A.J.C.; Viviani, V.R.; Nantes-Cardoso, I.L. Synthesis of bioluminescent gold nanoparticle-luciferase hybrid systems for technological applications. Photoch. Photobio Sci. 2021, 20, 1439–1453.
- Beltrame, J.M.; Ribeiro, B.B.P.; Guindani, C.; Candiotto, G.; Felipe, K.B.; Lucas, R.; Zottis, A.D.; Isoppo, E.; Sayer, C.; de Araujo, P.H.H. Coating of SPIONs with a Cysteine-Decorated Copolyester: A Possible Novel Nanoplatform for Enzymatic Release. Pharmaceutics 2023, 15, 1000.
- Wu, K.; Liu, J.M.; Saha, R.; Ma, B.; Su, D.Q.; Chugh, V.K.; Wang, J.P. Stable and Monodisperse Iron Nitride Nanoparticle Suspension for Magnetic Diagnosis and Treatment: Development of Synthesis and Surface Functionalization Strategies. ACS Appl. Nano Mater. 2021, 4, 4409–4418.
- Xu, X.; Xiang, H.J.; Wang, Z.J.; Wu, C.J.; Lu, C.C. Doping engineering and functionalization of iron oxide nanoclusters for biomedical applications. J. Alloys Compd. 2022, 923, 166459.
- Upadhyay, K.; Tamrakar, R.K.; Thomas, S.; Kumar, M. Surface functionalized nanoparticle A boon to biomedical science. Chem.-Biol. Interact. 2023, 380, 110537.
This entry is offline, you can click here to edit this entry!
