Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Several bacterial strains have acquired significant antibiotic resistance and can, therefore, become difficult to contain. To counteract such trends, relational databases can be a powerful tool for supporting the decision-making process. The case of Klebsiella pneumoniae diffusion in a central region of Italy was analyzed as a case study. A specific relational database is shown to provide very detailed and timely information about the spatial–temporal diffusion of the contagion, together with a clear assessment of the multidrug resistance of the strains.
- infectious diseases
- hotspot
- relational databases
- Klebsiella
1. The Spread of Infections
Humans infected by a pathogen, depending on the state of the infection, may or may not transmit the pathogen. An individual who can transmit the pathogen is classified as a carrier [1][2] A passive carrier means a host that is carrying the pathogen can transmit it to another host, but that generally they are not infected [3]. For example, a healthcare professional who does not wash their hands properly after the examination of an infected patient could transmit the pathogen to another patient without becoming infected themselves [4]. On the contrary, an active carrier is an infected host that can directly transmit the infection but can show or not show the symptoms of the disease. Indeed, it can transmit the pathogen during the incubation period or in the recovery period [5][6][7]. Asymptomatic carriers are defined as those active carriers that do not show symptoms of the disease [8].
Pathogenic microorganisms, during their evolution, have developed different mechanisms for transmission. Among them, direct and indirect contact transmission represent two of the most diffused mechanisms. Person–person transmission is a form of direct contact transmission [9][10]. In this case, the agent is transmitted by physical contact between two individuals through actions such as touching, sexual intercourse, or droplet spread [11][12][13] Among the places that attract the interest of epidemiologists are hospitals and nursing settings due to high levels of disease transmission. Consequently, special efforts must be exerted to limit the risks of infection spreading in these environments. Infections acquired in healthcare facilities are called nosocomial or healthcare-associated infections (HAIs) [14][15] To classify an infection as an HAI, the patient must have been admitted to the healthcare facility for a reason other than the infection. In the case of nosocomial diseases, higher rates of transmission may be caused by characteristics of the environment itself, characteristics of the population, or both [16]. HAIs are often connected with surgery or other invasive procedures that facilitate access of the pathogen to the host [17][18] Indeed, in these settings, patients suffering from a primary disease are often afflicted by compromised immunity and are more susceptible to secondary infections and opportunistic pathogens [5][6].
2. Klebsiella Pneumoniae
Klebsiella pneumoniae is a non-motile, Gram-negative, lactose fermenting, rod-shaped, and encapsulated bacterium. It is widely diffused in the world, mainly in tropical and sub-tropical areas [19]. K. pneumoniae is the most clinically relevant member of the Klebsiella genus, a member of Enterobacteriaceae family [20][21][22][23]. Klebsiella bacteria are ubiquitous and probably have two common habitats, one is the natural environment, where they are found in surface water, soil and plants. The other habitat is represented by the mucosal surfaces of mammals such as humans, horses, and swine [24]. In humans, K. pneumoniae is present as a saprophyte in the nasopharynx and in the intestinal tract.
Normally, individuals carry K. pneumoniae asymptomatically [25][26]. However, in immunocompromised individuals, especially in infants and elderly, K. pneumoniae can cause severe hospital-acquired infections [27][28][29] such as pneumonia and urinary tract infections. In addition, certain hypervirulent K. pneumoniae strains can cause community-acquired infections [8][30][31]. The acquisition of antibiotic resistance genes and intrinsic resistance to several classes of antibiotics limit treatment options for infections caused by this [32][33][34].
Currently, K. pneumoniae strains producing extended spectrum beta-lactamases (ESBLs) and carbapenemases have spread globally [35][36]. These types of enzymes inactivate β-lactams, a class of antibiotics that is normally the basis for effective treatment of patients suffering from infections with K. pneumoniae. Consequently, the antimicrobial peptide (AMP) and colistin (also known as polymyxin E) have been reintroduced to treat infections with multidrug-resistant K. pneumoniae [12]. The rise of these multidrug-resistant isolates will require the development of novel classes of antibiotics or alternative treatment strategies.
3. Antibiotic Resistance
The treatment of bacterial infections depends heavily on effective antimicrobial therapy. The combination of the wide use of inappropriate therapies and the delayed administration of effective antibiotics have been associated with a higher mortality rate in patients with severe infections (especially nosocomial patients) due to the diffusion of resistances [37]. For these reasons, nosocomial ESBL-producing K. pneumoniae infection has been considered an emerging threat. K. pneumoniae is a major cause of nosocomial infections and is responsible for roughly 15% of Gram-negative infections in hospital intensive care units (ICUs), primarily affecting immunocompromised patients [34][38]. In these cases, the presence of drug-resistant pathogens would adversely affect the treatment outcome [14].
Data on healthcare-associated infections reported by the Centers for Diseases Control and Prevention (CDC) from 2007 indicates that the overall prevalence of ESBL-producing K. pneumoniae isolates varies considerably depending on the geographic region [38][39]. According to the CDC, the geographical distribution of ESBL-producing K. pneumoniae is as follows: Eastern Europe (39.3%), Korea (26.2%), USA (16%), and Canada (3.6%) [16][39][40][41].
In Italy, as reported by the Istituto Superiore di Sanità (ISS), Gram-bacteria have shown increased levels of resistance during the period 2006–2008, especially to specific classes of antibiotics (such as fluoroquinolones, aminopenicillin, aminoglycoside, and carbapenems). From 2009 to 2011, a worrying trend was registered where the percentage of strains of K. pneumoniae resistant to carbapenems presented a 1.3% increase in 2009, a 16% increase in 2010, and a 26.7% increase in 2011. In order to stop this escalation, it is, therefore, necessary to pay particular attention to optimizing antibiotic therapies and to rationalizing antibiotic-sensitivity tests [42][43][44][45][46].
4. Relational Databases as Decision Support Systems
Relational databases became the dominant model in the 1980s. The term “relational database” was defined by E. F. Codd at IBM in 1970 [47]. The formal and most complete definition of what constitutes a relational database is based on Codd’s 12 rules. However, no commercial implementations of the relational model conform to all of Codd’s requirements [48]. The term has, therefore, gradually been used to identify a broader class of database systems which, at a minimum, have to present the following characteristics:
Presentation of the data to the user as relations: the presentation format is tabular, i.e., consists of a collection of tables with each table comprising a set of rows and columns.
IBM began developing System R, a research project aimed at developing a prototype relational database management system (RDBMS) in 1974. The first software package commercialized as an RDBMS was Multics Relational Data Store (June 1976). The first version of Oracle was released in 1979 by Relational Software, which is now the Oracle Corporation.
Relational databases are organized according to the relational model, which consists of an intuitive way of representing data in tables. In practically all implementations, each row in the table of a relational database is a record with a unique ID called the key. The columns of the table contain the attributes of the data where, in principle, each record usually holds a specific value for each attribute. Thanks to its logical structure and practical implementation, a relational database allows the easy finding specific information. The retrieved information can be easily sorted on the basis of any field from each record. Moreover, it is easy to compare information thanks to the arrangement of data in columns. Relational databases are built using a specific computer language, called structured query language (SQL), which is the established standard for database interoperability.
The power and flexibility of relational databases has become very valuable to support the decision-making process in the sanitary or healthcare context at different levels. First, they can help significantly in the phase of monitoring the general situation and, in the specific case of the spread of infectious diseases, in detecting hotspots and outbreaks in a timely manner. Relational databases can also provide extremely useful information to organize interventions and prepare for them. In the present application, for example, they can be a very effective basis for managing the storage and reserves of antibiotics. These software tools have also great potential in providing support to the organization of prevention strategies. Most of these aspects will be exemplified by the analysis of the database described in the following subsection.
The software used to analyze the data presented in this work was the package ACCESS (Microsoft® Access®) and Microsoft® Excel®.
5. The Database
Anonymized data were provided by A.S.L. Frosinone (the local Healthcare Service of Frosinone city) for the region of central Italy shown in Figure 1. It covers the period that spanned from January 2014 to March 2016. For each patient, the available information was progressive code, acceptance date, initials of the name, date of birth, sex- origin, material, bacterial charge, results of antibiogram, and antibiograms.
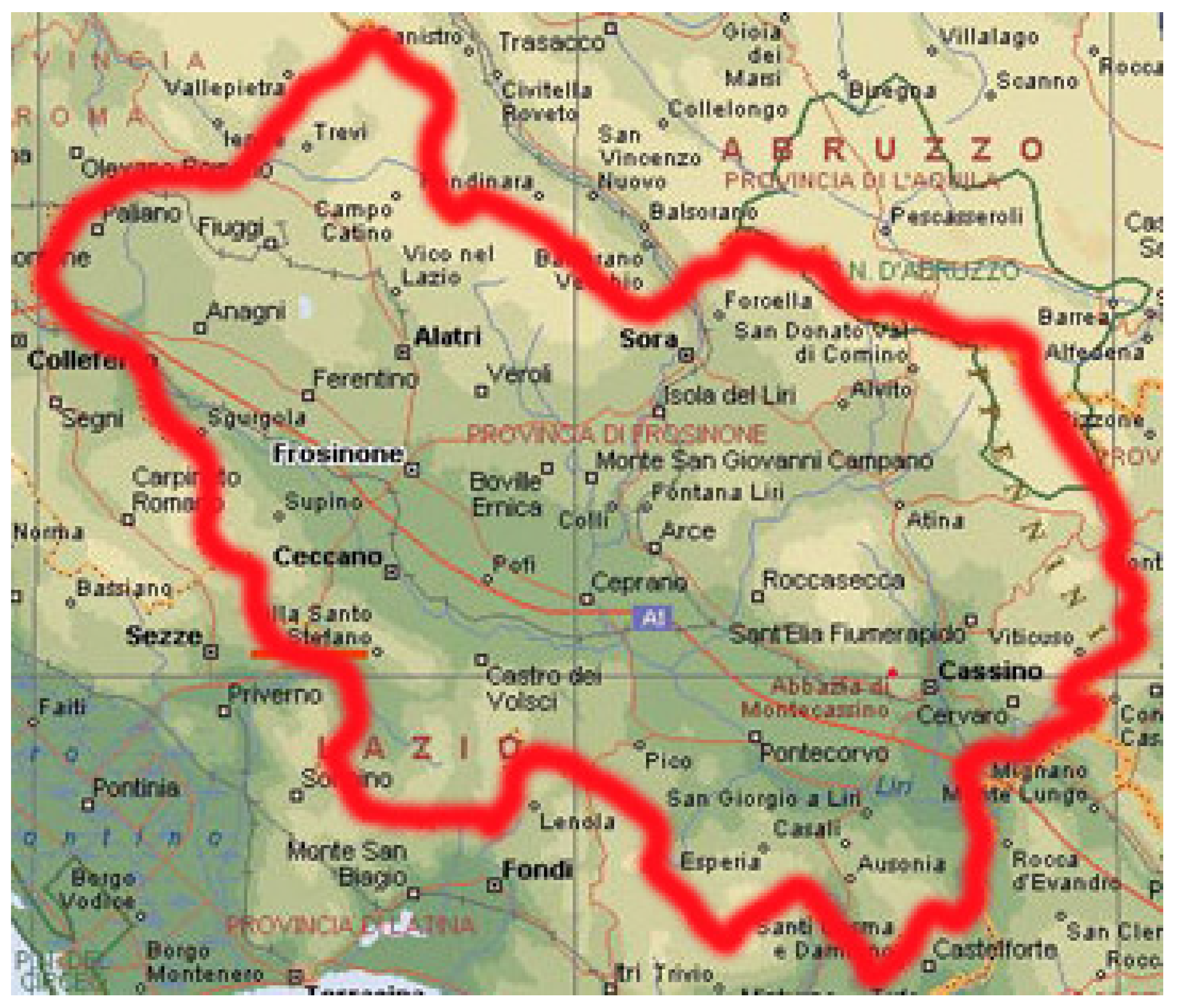
Figure 1. Provinces and municipalities of central Italy from which the patients affected by K. pneumoniae and included in the database came from.
The antibiogram test utilizes a variety of culture methods which work by exposing bacteria to antibiotics. Culture methods typically consist of measuring the diameter of areas without bacterial growth, the so-called zones of inhibition, around paper discs containing antibiotics on agar culture dishes. The culture dishes had been previously inoculated evenly with bacteria. The lowest concentration of an antibiotic which stops the growth of bacteria is the minimum inhibitory concentration and can be determined by observing the size of the zone of inhibition.
The blood samples of the patients were tested to assess the resistance to the following antibiotics ampicillin, amoxicillin, piperacillin, cefoxitin, ceftazidime, cefepime, ertapenem, imipenem, meropenem, tigecycline, fosfomycin, cyprofloxacin, amikacin, gentamicin, and colystin. To quantify the level of resistance, three classes were defined: (1) sensitive S, (2) intermediate I, and (3) resistant R. A bacterial strain is classified as susceptible to a certain antibiotic when it is inhibited in vitro by a concentration of the drug which corresponds to a high likelihood of therapeutic success. The sensitivity of a bacterial strain to a given antibiotic belongs to the intermediate category when it is inhibited in vitro by a drug concentration which presents an uncertain therapeutic effect. A bacterial strain is categorized as resistant to a given antibiotic when it is inhibited in vitro by a drug concentration, which tends to result in therapeutic failure.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12040784
References
- Schmidt, H.; Hensel, M. Pathogenicity Islands in Bacterial Pathogenesis. Clin. Microbiol. Rev. 2004, 17, 14–56.
- Alberts, B.; Johnson, A.; Lewis, J.; Walter, P.; Raff, M.; Roberts, K. Molecular Biology of the Cell 4th Edition: International Student Edition; Garland Science: Wolverhampton, UK, 2002.
- Mack, A.; Relman, D.A.; Choffnes, E.R. (Eds.) Antibiotic Resistance: Implications for Global Health and Novel Intervention Strategies: Workshop Summary; National Academies Press: Washington, DC, USA, 2011.
- Akova, M.; Daikos, G.; Tzouvelekis, L.; Carmeli, Y. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin. Microbiol. Infect. 2012, 18, 439–448.
- Friis, R.H.; Sellers, T. Epidemiology for Public Health Practice; Jones & Bartlett Publishers: Boston, MA, USA, 2013.
- Euzéby, J.P. List of Bacterial Names with Standing in Nomenclature: A Folder Available on the Internet. Int. J. Syst. Evol. Microbiol. 1997, 47, 590–592.
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, S14459.
- Riggs, M.M.; Sethi, A.K.; Zabarsky, T.F.; Eckstein, E.C.; Jump, R.L.; Donskey, C.J. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility res-idents. Clin. Infect. Dis. 2007, 45, 992–998.
- Abbott, L.S. Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and Other Enterobacteriacae. In Manuel of Clinical Microbiology; Murray, P.R., Baron, J.O.E., Jorgensen, J.H., Candry, M.L., Pfaller, M.A., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 705–711.
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208.
- Tulchinsky, T.H.; Varavikova, E.A. The New Public Health; Academic Press: Cambridge, MA, USA, 2014.
- Thomas, R.J. Particle size and pathogenicity in the respiratory tract. Virulence 2013, 4, 847–858.
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707.
- Eisenberg, J.N.; Desai, M.A.; Levy, K.; Bates, S.J.; Liang, S.; Naumoff, K.; Scott, J.C. Environmental Determinants of Infectious Disease: A Framework for Tracking Causal Links and Guiding Public Health Research. Environ. Health Perspect. 2007, 115, 1216–1223.
- Duffy, J.; Sievert, D.; Rebmann, C.; Kainer, M.; Lynfield, R.; Smith, P.; Fridkin, S. Effective State-Based Surveillance for Multidrug-Resistant Organisms Related to Health Ca re-Associated Infections. Public Health Rep. 2011, 126, 176–185.
- Guggenbichler, J.P.; Assadian, O.; Boeswald, M.; Kramer, A. Incidence and clinical implication of nosocomial infec-tions associated with implantable biomaterials–catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenh. Interdiszip. 2011, 6.
- Barton, A. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. AORN J. 2009, 90, 601–602.
- Battersby, S. (Ed.) Clay’s Handbook of Environmental Health; Routledge: New York, NY, USA, 2016.
- Lawlor, M.S.; Hsu, J.; Rick, P.D.; Miller, V.L. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol. Microbiol. 2005, 58, 1054–1073.
- Paterson, D.L. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am. J. Med. 2006, 119, S20–S28.
- Paterson, D.L.; Bonomo, R.A. Extended-Spectrum β-Lactamases: A Clinical Update. Clin. Microbiol. Rev. 2005, 18, 657–686.
- Peterson, J.W. Bacterial pathogenesis. In Med. Microbiol.; 1996; 592. Available online: https://pubmed.ncbi.nlm.nih.gov/21413346/ (accessed on 12 April 2023).
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283.
- Podshum, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603.
- Sydnor, E.R.M.; Perl, T.M. Hospital Epidemiology and Infection Control in Acute-Care Settings. Clin. Microbiol. Rev. 2011, 24, 141–173.
- Sun, Y.; Patel, A.; SantaLucia, J.; Roberts, E.; Zhao, L.; Kaye, K.; Rao, K.; Bachman, M.A. Measurement of Klebsiella Intestinal Colonization Density To Assess Infection Risk. Msphere 2021, 6, e0050021.
- Brisse, S.; Fevre, C.; Passet, V.; Issenhuth-Jeanjean, S.; Tournebize, R.; Diancourt, L.; Grimont, P. Virulent Clones of Klebsiella pneumoniae: Identification and Evolutionary Scenario Based on Genomic and Phenotypic Characterization. PLoS ONE 2009, 4, e4982.
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269.
- Bouza, E.; Cercenado, E. Klebsiella and enterobacter: Antibiotic resistance and treatment implications. In Proceedings of the Seminars in Respiratory Infections, London, UK, 1 September 2002; Volume 17, pp. 215–230.
- Snitkin, E.S.; Zelazny, A.M.; Thomas, P.J.; Stock, F.; Henderson, D.K.; Palmore, T.N.; Segre, J.A.; NISC; Comparative Sequencing Program. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 2012, 4, 148ra116.
- Struve, C.; Roe, C.C.; Stegger, M.; Stahlhut, S.G.; Hansen, D.S.; Engelthaler, D.M.; Andersen, P.S.; Driebe, E.M.; Keim, P.; Krogfelt, K.A. Mapping the Evolution of Hypervirulent Klebsiella pneumoniae. mBio 2015, 6, e00630-15.
- Doorduijn, D.J.; Rooijakkers, S.H.; van Schaik, W.; Bardoel, B.W. Complement resistance mechanisms of Klebsiella pneumoniae. Immunobiology 2016, 221, 1102–1109.
- Conway, M.J.; Colpitts, T.M.; Fikrig, E. Role of the Vector in Arbovirus Transmission. Annu. Rev. Virol. 2014, 1, 71–88.
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433.
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.D.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101.
- Arnold, R.S.; Thom, K.A.; Sharma, S.; Phillips, M.; Johnson, J.K.; Morgan, D.J. Emergence of Klebsiella pneu-moniae carbapenemase (KPC)-producing bacteria. South. Med. J. 2011, 104, 40.
- Petrosillo, N.; Capone, A.; Di Bella, S.; Taglietti, F. Management of antibiotic resistance in the intensive care unit setting. Expert Rev. Anti-Infect. Ther. 2010, 8, 289–302.
- Centers for Disease Control and Prevention. Carbapenem-Resistant Enterobacteriaceae in Healthcare Settings; CDC: Atlanta, GA, USA, 2016.
- Garbati, M.A.; Al Godhair, A.I. The Growing Resistance of Klebsiella pneumonia; the Need to Expand Our Anti-biogram: Case Report and Review of the Literature. Afr. J. Infect. Dis. 2013, 7, 8–10.
- Lin, W.P.; Wang, J.T.; Chang, S.C.; Chang, F.Y.; Fung, C.P.; Chuang, Y.C.; Chen, Y.-S.; Shiau, Y.-R.; Tan, M.-C.; Lai, J.F.; et al. The antimicrobial susceptibility of klebsiella pneumoniae from community settings in Taiwan, a trend analysis. Sci. Rep. 2016, 6, 36280.
- Onori, R.; Gaiarsa, S.; Comandatore, F.; Pongolini, S.; Brisse, S.; Colombo, A.; Cassani, G.; Marone, P.; Grossi, P.; Bandi, C. Tracking nosocomial Klebsiella pneumoniae infections and outbreaks by whole-genome analysis: Small-scale Italian scenario within a single hospital. J. Clin. Microbiol. 2015, 53, 2861–2868.
- Quale, J. Global Spread of Carbapenemase-Producing Klebsiella pneumoniae-These pathogens arose in the mid-1990s and continue to spread, leaving few options for treating infected patients. Microbe 2008, 3, 516.
- Ripabelli, G.; Sammarco, M.; Salzo, A.; Scutellà, M.; Felice, V.; Tamburro, M. New Delhi metallo-β-lactamase (NDM-1)-producingKlebsiella pneumoniaeof sequence type ST11: First identification in a hospital of central Italy. Lett. Appl. Microbiol. 2020, 71, 652–659.
- Rahal, J.J. Antimicrobial Resistance among and Therapeutic Options against Gram-Negative Pathogens. Clin. Infect. Dis. 2009, 49, S4–S10.
- Girometti, N.; Lewis, R.E.; Giannella, M.; Ambretti, S.; Bartoletti, M.; Tedeschi, S.; Tumietto, F.; Cristini, F.; Trapani, F.; Viale, P.; et al. Klebsiella pneumoniae bloodstream infection: Epidemiology and impact of inappropriate empirical therapy. Medicine 2014, 93, 298–309.
- Aliabadi, A.A.; Rogak, S.N.; Bartlett, K.H.; Green, S.I. Preventing airborne disease transmission: Review of methods for ventilation design in health care facilities. Adv. Prev. Med. 2011, 2011, 124064.
- Codd, E.F. A relational model of data for large shared data banks. Commun. ACM 1970, 13, 377–387.
- Connolly, T.M.; Begg, C.E. Database Systems—A Practical Approach to Design Implementation and Management, 6th ed.; Pearson Education: Hudson, NY, USA, 2014; p. 64. ISBN 978-1292061184. Available online: http://www.cherrycreekeducation.com/bbk/b/Pearson_Database_Systems_A_Practical_Approach_to_Design_Implementation_and_Management_6th_Global_Edition_1292061189.pdf (accessed on 12 April 2023).
This entry is offline, you can click here to edit this entry!
