Melatonin (MT), a naturally occurring compound, is found in various species worldwide. In 1958, it was first identified in the pineal gland of dairy cows. MT is an “old friend” but a “new compound” for plant biology. It brings experts and research minds from the broad field of plant sciences due to its considerable influence on plant systems. The MT production process in plants and animals is distinct, where it has been expressed explicitly in chloroplasts and mitochondria in plants. Tryptophan acts as the precursor for the formation of phyto-melatonin, along with intermediates including tryptamine, serotonin, N-acetyl serotonin, and 5-methoxy tryptamine. It plays a vital role in growth phases such as the seed germination and seedling growth of crop plants. MT significantly impacts the gas exchange, thereby improving physio-chemical functions in plant systems. During stress, the excessive generation and accumulation of reactive oxygen species (ROS) causes protein oxidation, lipid peroxidation, nucleic acid damage, and enzyme inhibition. Because it directly acts as an antioxidant compound, it awakens the plant antioxidant defense system during stress and reduces the production of ROS, which results in decreasing cellular oxidative damage. MT can enhance plant growth and development in response to various abiotic stresses such as drought, salinity, high temperature, flooding, and heavy metals by regulating the antioxidant mechanism of plants.
- melatonin
- indolamine
- abiotic stress
- antioxidant
- plant growth
1. Introduction
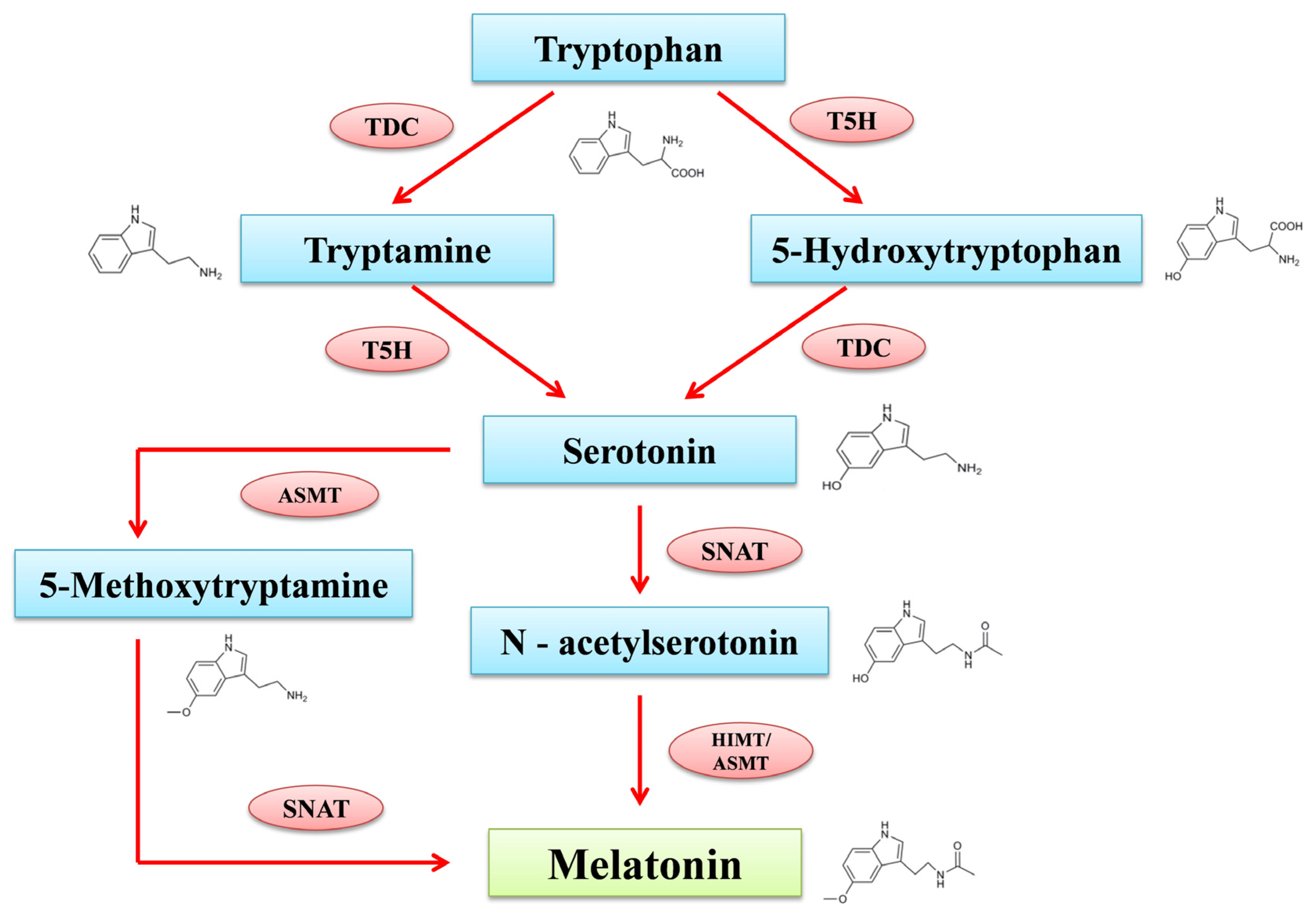
2. Biosynthesis of Melatonin in Plants
3. Melatonin’s Role in Plant Growth and Physiology
3.1. Germination
3.2. Shoot and Root Growth
3.3. Gas Exchange
3.4. Antioxidant or ROS Scavenging
4. Melatonin’s Role in Secondary Metabolites’ Expression
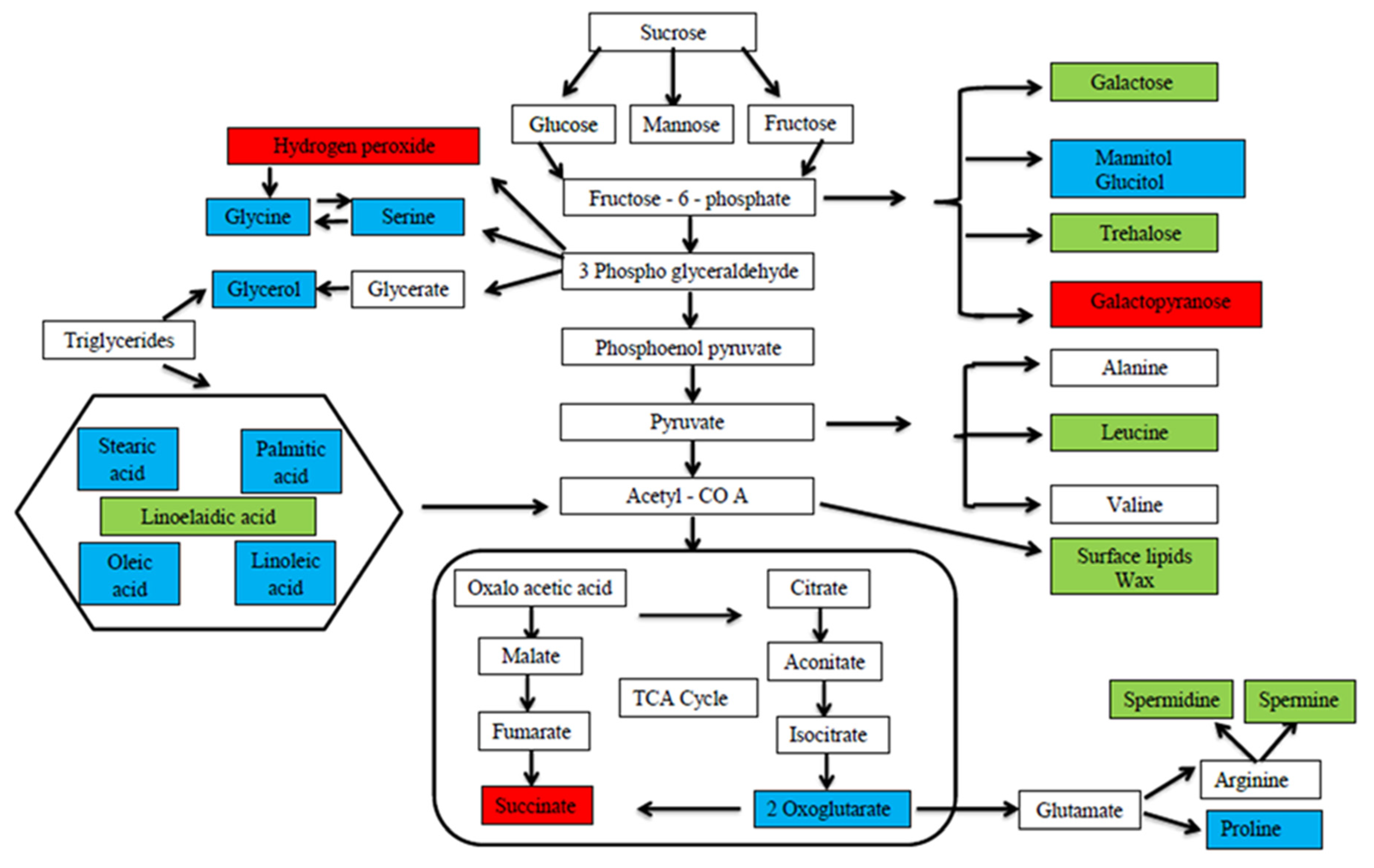
5. Melatonin’s Role in Crop Yield and Quality
6. Melatonin’s Role in Abiotic Stress Mitigation
This entry is adapted from the peer-reviewed paper 10.3390/agronomy13092405
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587.
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31.
- Van Tassel, D.L.; Roberts, N.; Lewy, A.; O’Neill, S.D. Melatonin in plant organs. J. Pineal Res. 2001, 31, 8–15.
- Hattori, A.; Herbert, D.C.; Vaughan, M.K.; Yaga, K.; Reiter, R. Melatonin inhibits luteinizing hormone releasing hormone (LHRH) induction of LH release from fetal rat pituitary cells. Neurosci. Lett. 1995, 184, 109–112.
- Zohar, R.; Izhaki, I.; Koplovich, A.; Ben-Shlomo, R. Phytomelatonin in the leaves and fruits of wild perennial plants. Phytochem. Lett. 2011, 4, 222–226.
- Murch, S.J.; Erland, L.A. A systematic review of melatonin in plants: An example of evolution of literature. Front. Plant Sci. 2021, 12, 683047.
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: Melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 2004, 25, 951–960.
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040.
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14.
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249.
- Zhou, Y.; Chen, M.; Guo, J.; Wang, Y.; Min, D.; Jiang, Q.; Ji, H.; Huang, C.; Wei, W.; Xu, H. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J. Exp. Bot. 2020, 71, 1842–1857.
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507.
- Khan, D.; Cai, N.; Zhu, W.; Li, L.; Guan, M.; Pu, X.; Chen, Q. The role of phytomelatonin receptor 1-mediated signaling in plant growth and stress response. Front. Plant Sci. 2023, 14, 1142753.
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605.
- Castañares, J.L.; Bouzo, C.A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant J. 2019, 5, 79–87.
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin improves drought resistance in maize seedlings by enhancing the antioxidant system and regulating abscisic acid metabolism to maintain stomatal opening under PEG-induced drought. J. Plant Biol. 2021, 64, 299–312.
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680.
- Hernández, I.G.; Gomez, F.J.V.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana acts as plant growth regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiol. Biochem. 2015, 94, 191–196.
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J. Plant Physiol. 2016, 193, 47–56.
- Zhang, H.; Qiu, Y.; Ji, Y.; Wu, X.; Xu, X.; Wu, P. Melatonin promotes seed germination via regulation of ABA signaling under low temperature stress in cucumber. J. Plant Growth Regul. 2023, 42, 2232–2245.
- Dradrach, A.; Iqbal, M.; Lewińska, K.; Jędroszka, N.; Rana, M.A.K.; Tanzeem-ul-Haq, H.S. Effects of soil application of chitosan and foliar melatonin on growth, photosynthesis, and heavy metals accumulation in wheat growing on wastewater polluted soil. Sustainability 2022, 14, 8293.
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576.
- Yin, X.; Bai, Y.L.; Gong, C.; Song, W.; Wu, Y.; Ye, T.; Feng, Y.Q. The phytomelatonin receptor PMTR1 regulates seed development and germination by modulating abscisic acid homeostasis in Arabidopsis thaliana. J. Pineal Res. 2022, 72, e12797.
- Jan, R.; Asif, S.; Asaf, S.; Du, X.-X.; Park, J.-R.; Nari, K.; Bhatta, D.; Lee, I.-j.; Kim, K.-M. Melatonin alleviates arsenic (As) toxicity in rice plants via modulating antioxidant defense system and secondary metabolites and reducing oxidative stress. Environ. Pollut. 2023, 318, 120868.
- Tan, D.-X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689.
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145.
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195.
- Tatar, Ö.; Brück, H.; Asch, F. Atmospheric and soil water deficit induced changes in chemical and hydraulic signals in wheat (Triticum aestivum L.). J. Agron. Crop Sci. 2023, 209, 242–250.
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827.
- Yang, L.; You, J.; Li, J.; Wang, Y.; Chan, Z. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611.
- Zeng, H.; Liu, M.; Wang, X.; Liu, L.; Wu, H.; Chen, X.; Wang, H.; Shen, Q.; Chen, G.; Wang, Y. Seed-soaking with melatonin for the improvement of seed germination, seedling growth, and the antioxidant defense system under flooding stress. Agronomy 2022, 12, 1918.
- Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Chi, Y.-X.; Zeeshan, M.; Nasar, J.; Zhou, X.-B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359.
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int. J. Mol. Sci. 2022, 23, 9456.
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators: Signalling under Stress Conditions; Springer: Berlin/Heidelberg, Germany, 2021; pp. 239–273.
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718.
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88.
- Abdulbaki, A.S.; Alsamadany, H.; Alzahrani, Y.; Olayinka, B.U. Rubisco and abiotic stresses in plants: Current assessment. Turk. J. Bot. 2022, 46, 541–552.
- Ullah, A.; Al-Rajhi, R.S.; Al-Sadi, A.M.; Farooq, M. Wheat genotypes with higher intercellular CO2 concentration, rate of photosynthesis, and antioxidant potential can better tolerate drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 2378–2391.
- Fu, J.; Krishna Jagadish, S.; Bowden, R.L. Effects of post-flowering heat stress on chlorophyll content and yield components of a spring wheat diversity panel. Crop Sci. 2022, 62, 1926–1936.
- Hundare, A.; Joshi, V.; Joshi, N. Salicylic acid attenuates salinity-induced growth inhibition in in vitro raised ginger (Zingiber officinale Roscoe) plantlets by regulating ionic balance and antioxidative system. Plant Stress 2022, 4, 100070.
- Wang, H.; Ren, C.; Cao, L.; Zhao, Q.; Jin, X.; Wang, M.; Zhang, M.; Yu, G.; Zhang, Y. Exogenous melatonin modulates physiological response to nitrogen and improves yield in nitrogen-deficient soybean (Glycine max L. Merr.). Front. Plant Sci. 2022, 13, 865758.
- Liu, K.; Jing, T.; Wang, Y.; Ai, X.; Bi, H. Melatonin delays leaf senescence and improves cucumber yield by modulating chlorophyll degradation and photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915.
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B.J.E.; Botany, E. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11.
- Shi, H.; Wang, X.; Tan, D.X.; Reiter, R.J.; Chan, Z. Comparative physiological and proteomic analyses reveal the actions of melatonin in the reduction of oxidative stress in Bermuda grass (Cynodon dactylon (L). Pers.). J. Pineal Res. 2015, 59, 120–131.
- Gholami, R.; Hoveizeh, N.F.; Zahedi, S.M.; Gholami, H.; Carillo, P. Melatonin alleviates the adverse effects of water stress in adult olive cultivars (Olea europea cv. Sevillana & Roughani) in field condition. Agric. Water Manag. 2022, 269, 107681.
- Yu, J.C.; Lu, J.Z.; Cui, X.Y.; Guo, L.; Wang, Z.J.; Liu, Y.D.; Wang, F.; Qi, M.F.; Liu, Y.F.; Li, T.L. Melatonin mediates reactive oxygen species homeostasis via SlCV to regulate leaf senescence in tomato plants. J. Pineal Res. 2022, 73, e12810.
- Guo, X.; Shi, Y.; Zhu, G.; Zhou, G. Melatonin Mitigated Salinity Stress on Alfalfa by Improving Antioxidant Defense and Osmoregulation. Agronomy 2023, 13, 1727.
- Kuppusamy, A.; Alagarswamy, S.; Karuppusami, K.M.; Maduraimuthu, D.; Natesan, S.; Ramalingam, K.; Muniyappan, U.; Subramanian, M.; Kanagarajan, S. Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants 2023, 12, 2535.
- Jahan, M.S.; Zhao, C.J.; Shi, L.B.; Liang, X.R.; Jabborova, D.; Nasar, J.; Zhou, X.B. Physiological mechanism of melatonin attenuating to osmotic stress tolerance in soybean seedlings. Front. Plant Sci. 2023, 14, 1193666.
- Jensen, N.B.; Ottosen, C.-O.; Zhou, R. Exogenous Melatonin Alters Stomatal Regulation in Tomato Seedlings Subjected to Combined Heat and Drought Stress through Mechanisms Distinct from ABA Signaling. Plants 2023, 12, 1156.
- Barman, D.; Ghimire, O.; Chinnusamy, V.; Kumar, R.; Arora, A. Amelioration of heat stress during reproductive stage in rice by melatonin. Indian J. Agric. Sci. 2019, 89, 1151–1156.
- Khosravi, S.; Haghighi, M.; Mottaghipisheh, J. Effects of melatonin foliar application on hot pepper growth and stress tolerance. Plant Stress 2023, 9, 100192.
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466.
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined application of arbuscular mycorrhizal fungi and exogenous melatonin alleviates drought stress and improves plant growth in tobacco seedlings. J. Plant Growth Regul. 2021, 40, 1074–1087.
- Gu, Q.; Xiao, Q.; Chen, Z.; Han, Y. Crosstalk between melatonin and reactive oxygen species in plant abiotic stress responses: An update. Int. J. Mol. Sci. 2022, 23, 5666.
- Kaur, P.; Singh, D.; Rashid, F.; Kumar, A.; Kaur, H.; Kaur, K.; Singh, A.; Bedi, N.; Bedi, P.M.S.; Singh, B. Role of Melatonin-A Signaling Molecule in Modulation of Antioxidant Defense System in Plants: Amelioration of Drought and Salinity Stress. In Environmental Stress Physiology of Plants and Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2021; Volume 124.
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2022, 41, 507–523.
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial effects of abscisic acid and melatonin in overcoming drought stress in cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054.
- Rehman, R.S.; Hussain, M.; Ali, M.; Zafar, S.A.; Pasha, A.N.; Bashir, H.; Ashraf, N.A.; Javed, A.; Shah, W.A. A Comprehensive Review on Melatonin Compound and Its Functions in Different Fungi and Plants. Int. J. Pathog. Res. 2022, 10, 9–21.
- Manafi, H.; Baninasab, B.; Gholami, M.; Talebi, M.; Khanizadeh, S. Exogenous melatonin alleviates heat-induced oxidative damage in strawberry (Fragaria × ananassa Duch. cv. Ventana) Plant. J. Plant Growth Regul. 2022, 41, 52–64.
- Lei, Y.; He, H.; Raza, A.; Liu, Z.; Xiaoyu, D.; Guijuan, W.; Yan, L.; Yong, C.; Xiling, Z. Exogenous melatonin confers cold tolerance in rapeseed (Brassica napus L.) seedlings by improving antioxidants and genes expression. Plant Signal. Behav. 2022, 17, 2129289.
- Roy, R.; Sultana, S.; Begum, N.; Fornara, D.; Barmon, M.; Zhang, R.; Sarker, T.; Rabbany, M.G. Exogenous melatonin reduces water deficit-induced oxidative stress and improves growth performance of Althaea rosea grown on coal mine spoils. Environ. Sci. Pollut. Res. 2022, 29, 61550–61560.
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield, and quality. J. Exp. Bot. 2022, 73, 5928–5946.
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809.
- Umapathi, M.; Kalarani, M.; Srinivasan, S.; Kalaiselvi, P. Alleviation of cadmium phytotoxicity through melatonin modulated physiological functions, antioxidants, and metabolites in tomato (Solanum lycopersicum L.). BioMetals 2022, 35, 1113–1132.
- Lee, J.; Lee, H.; Wi, S.; Yu, I.; Yeo, K.-H.; An, S.; Jang, Y.; Jang, S. Enhancement of growth and antioxidant enzyme activities on kimchi cabbage by melatonin foliar application under high temperature and drought stress conditions. Hortic. Sci. Technol. 2021, 39, 583–592.
- Yu, Y.; Deng, L.; Zhou, L.; Chen, G.; Wang, Y. Exogenous melatonin activates antioxidant systems to increase the ability of rice seeds to germinate under high temperature conditions. Plants 2022, 11, 886.
- Zhao, Q.; Chen, S.; Wang, G.; Du, Y.; Zhang, Z.; Yu, G.; Ren, C.; Zhang, Y.; Du, J. Exogenous melatonin enhances soybean (Glycine max (L.) Merr.) seedling tolerance to saline-alkali stress by regulating antioxidant response and DNA damage repair. Physiol. Plant. 2022, 174, e13731.
- Song, R.; Ritonga, F.N.; Yu, H.; Ding, C.; Zhao, X. Plant melatonin: Regulatory and protective role. Horticulturae 2022, 8, 810.
- Talaat, N.B.; Todorova, D. Antioxidant machinery and glyoxalase system regulation confers salt stress tolerance to wheat (Triticum aestivum L.) plants treated with melatonin and salicylic Acid. J. Soil Sci. Plant Nutr. 2022, 22, 3527–3540.
- Ye, F.; Jiang, M.; Zhang, P.; Liu, L.; Liu, S.; Zhao, C.; Li, X. Exogenous melatonin reprograms the rhizosphere microbial community to modulate the responses of barley to drought stress. Int. J. Mol. Sci. 2022, 23, 9665.
- Yan, R.; Li, S.; Cheng, Y.; Kebbeh, M.; Huan, C.; Zheng, X. Melatonin treatment maintains the quality of cherry tomato by regulating endogenous melatonin and ascorbate-glutathione cycle during room temperature. J. Food Biochem. 2022, 46, e14285.
- Udhayabharathi, M. Physiological and Metabolomic Studies of Drought Tolerance in Greengram (Vigna radiata L.) by Exogenous Melatonin. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2017.
- Xie, Z.; Wang, J.; Wang, W.; Wang, Y.; Xu, J.; Li, Z.; Zhao, X.; Fu, B. Integrated analysis of the transcriptome and metabolome revealed the molecular mechanisms underlying the enhanced salt tolerance of rice due to the application of exogenous melatonin. Front. Plant Sci. 2021, 11, 618680.
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Zareei, E.; Kulak, M.; Ali, S.; Nouraein, M.; Bahrami, M.K.; Gohari, G.; Fotopoulos, V. Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system and secondary metabolism-related genes. BMC Plant Biol. 2022, 22, 380.
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755.
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R. Melatonin and carbohydrate metabolism in plant cells. Plants 2021, 10, 1917.
- Yang, J.; Zhang, C.; Wang, Z.; Sun, S.; Zhan, R.; Zhao, Y.; Ma, B.; Ma, F.; Li, M. Melatonin-mediated sugar accumulation and growth inhibition in apple plants involves down-regulation of fructokinase 2 expression and activity. Front. Plant Sci. 2019, 10, 150.
- Jiang, Y.; Huang, S.; Ma, L.; Kong, L.; Pan, S.; Tang, X.; Tian, H.; Duan, M.; Mo, Z. Effect of exogenous melatonin application on the grain yield and antioxidant capacity in aromatic rice under combined lead–cadmium stress. Antioxidants 2022, 11, 776.
- Peixoto, B.; Baena-González, E. Management of plant central metabolism by SnRK1 protein kinases. J. Exp. Bot. 2022, 73, 7068–7082.
- Zhang, G.; Yan, Y.; Zeng, X.; Wang, Y.; Zhang, Y. Quantitative proteomics analysis reveals proteins associated with high melatonin content in barley seeds under NaCl-induced salt stress. J. Agric. Food Chem. 2022, 70, 8492–8510.
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913.
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675.
- Pan, Y.; Xu, X.; Li, L.; Sun, Q.; Wang, Q.; Huang, H.; Tong, Z.; Zhang, J. Melatonin-mediated development and abiotic stress tolerance in plants. Front. Plant Sci. 2023, 14, 1100827.
- Bhavithra, S. Mitigation of Salt Stress in Cassava (Manihot esculenta Crantz) by Exogenous Melatonin. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2021.
- Shah, A.A.; Ahmed, S.; Ali, A.; Yasin, N.A. 2-Hydroxymelatonin mitigates cadmium stress in cucumis sativus seedlings: Modulation of antioxidant enzymes and polyamines. Chemosphere 2020, 243, 125308.
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M.; Talebi, M.; Ghanati, F. Melatonin and calcium modulate the production of rosmarinic acid, luteolin, and apigenin in Dracocephalum kotschyi under salinity stress. Phytochemistry 2020, 177, 112422.
- Behera, B.; Kancheti, M.; Raza, M.B.; Shiv, A.; Mangal, V.; Rathod, G.; Altaf, M.A.; Kumar, A.; Aftab, T.; Kumar, R. Mechanistic insight on boron-mediated toxicity in plant vis-a-vis its mitigation strategies: A review. Int. J. Phytoremediation 2023, 25, 9–26.
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Melatonin influences stomatal behavior, root morphology, cell viability, photosynthetic responses, fruit yield, and fruit quality of tomato plants exposed to salt stress. J. Plant Growth Regul. 2023, 42, 2408–2432.
- Hassan, I.F.; Gaballah, M.S.; Ogbaga, C.C.; Murad, S.A.; Brysiewicz, A.; Bakr, B.M.; Mira, A.; Alam-Eldein, S.M. Does melatonin improve the yield attributes of field-droughted banana under Egyptian semi-arid conditions? J. Water Land Dev. 2022, 52, 221–231.
- Hu, C.-h.; Zheng, Y.; Tong, C.-l.; Zhang, D.-j. Effects of exogenous melatonin on plant growth, root hormones and photosynthetic characteristics of trifoliate orange subjected to salt stress. Plant Growth Regul. 2022, 97, 551–558.
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.M.; García-Pastor, M.E.; Zapata, P.J.; Valero, D.; Guillén, F. Melatonin treatment of pomegranate trees increases crop yield and quality parameters at harvest and during storage. Agronomy 2021, 11, 861.
- Oliveira-Spolaor, B.; Chiari-Bertoli, S.; Silva-Sukert, D.; Sala, H.R.; Picoli de Oliveira, B.F.; de Freitas, Í.R.; Lima-Moro, A. Exogenous melatonin induces tolerance to drought stress damage in seedlings and soybean plants. Chil. J. Agric. Res. 2022, 82, 515–526.
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.-B. Melatonin application alleviates stress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: A meta-analysis. Antioxidants 2022, 11, 512.
- Ibrahim, M.F.; Elbar, O.H.A.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; El-Gawad, H.G.A. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 2020, 9, 1276.
- Gurjar, P.; Killadi, B.; Pareek, P.K.; Hada, T. Application of melatonin in maintaining post harvest quality of fruits and vegetables: A review. Agric. Rev. 2022, 43, 193–198.
- Nasser, M.A.; El-Mogy, M.M.; Samaan, M.S.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.E.; Mahmoud, S.F.; Al-Harbi, N.A.; Al-Qahtani, S.M. Postharvest exogenous melatonin treatment of table grape berry enhances quality and maintains bioactive compounds during refrigerated storage. Horticulturae 2022, 8, 860.
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18.
- Sadak, M.S.; Bakry, B.A. Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919.
- Farouk, S.; Al-Amri, S. Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification. Ind. Crops Prod. 2019, 142, 111823.
- Hosseini, M.S.; Samsampour, D.; Zahedi, S.M.; Zamanian, K.; Rahman, M.M.; Mostofa, M.G.; Tran, L.S.P. Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol. Plant. 2021, 172, 1363–1375.
- Prasad, V.R.; Govindaraj, M.; Djanaguiraman, M.; Djalovic, I.; Shailani, A.; Rawat, N.; Singla-Pareek, S.L.; Pareek, A.; Prasad, P.V. Drought and high temperature stress in sorghum: Physiological, genetic, and molecular insights and breeding approaches. Int. J. Mol. Sci. 2021, 22, 9826.
- Peng, P.; Li, R.; Chen, Z.-H.; Wang, Y. Stomata at the crossroad of molecular interaction between biotic and abiotic stress responses in plants. Front. Plant Sci. 2022, 13, 1031891.
- Rao, S.; Tian, Y.; Zhang, C.; Qin, Y.; Liu, M.; Niu, S.; Li, Y.; Chen, J. The JASMONATE ZIM-domain–OPEN STOMATA1 cascade integrates jasmonic acid and abscisic acid signaling to regulate drought tolerance by mediating stomatal closure in poplar. J. Exp. Bot. 2023, 74, 443–457.
- Wang, D.; Wang, J.; Shi, S.; Huang, L.; Zhu, M.; Li, F. Exogenous melatonin ameliorates salinity-induced oxidative stress and improves photosynthetic capacity in sweet corn seedlings. Photosynthetica 2021, 59, 815769.
- Hu, W.; Zhang, J.; Wu, Z.; Loka, D.A.; Zhao, W.; Chen, B.; Wang, Y.; Meng, Y.; Zhou, Z.; Gao, L. Effects of single and combined exogenous application of abscisic acid and melatonin on cotton carbohydrate metabolism and yield under drought stress. Ind. Crops Prod. 2022, 176, 114302.
- Li, H.; Guo, Y.; Lan, Z.; Zhang, Z.; Ahammed, G.J.; Chang, J.; Zhang, Y.; Wei, C.; Zhang, X. Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021, 303, 110761.
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331.
- Teng, Z.; Zheng, W.; Jiang, S.; Hong, S.-B.; Zhu, Z.; Zang, Y. Role of melatonin in promoting plant growth by regulating carbon assimilation and ATP accumulation. Plant Sci. 2022, 319, 111276.
- Supriya, L.; Durgeshwar, P.; Muthamilarasan, M.; Padmaja, G. Melatonin mediated differential regulation of drought tolerance in sensitive and tolerant varieties of upland cotton (Gossypium hirsutum L.). Front. Plant Sci. 2022, 13, 821353.
- Li, Y.; Zhang, L.; Yu, Y.; Zeng, H.; Deng, L.; Zhu, L.; Chen, G.; Wang, Y. Melatonin-induced resilience strategies against the damaging impacts of drought stress in rice. Agronomy 2022, 12, 813.
- Hu, E.; Liu, M.; Zhou, R.; Jiang, F.; Sun, M.; Wen, J.; Zhu, Z.; Wu, Z. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci. Hortic. 2021, 285, 110176.
- Li, R.; Yang, R.; Zheng, W.; Wu, L.; Zhang, C.; Zhang, H. Melatonin promotes SGT1-involved signals to ameliorate drought stress adaption in rice. Int. J. Mol. Sci. 2022, 23, 599.
- Yang, X.; Chen, J.; Ma, Y.; Huang, M.; Qiu, T.; Bian, H.; Han, N.; Wang, J. Function, mechanism, and application of plant melatonin: An update with a focus on the cereal crop, barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634.
