MT (
N-acetyl-5-methoxy-tryptamine) is a naturally occurring compound in various species. In 1958, it was identified in the pineal gland of dairy cows [
1]. MT is an “old friend” but a “new compound” for plant biology. MT was first identified in higher plants as reported by Dubbels et al. [
2], Van Tassel et al. [
3], and Hattori et al. [
4]. MT has drawn a lot of study interest since it was discovered and found in plants in 1995. It has been detected and measured in more than 140 plant species in recent years [
5,
6]. It is a versatile compound that is extensively distributed in a variety of plant organs, including the roots, stems, leaves, fruits, and seeds. Different plant tissues contain significantly varied amounts of MT. Blask and his co-workers proposed the term “phytomelatonin” in 2004, referring to its plant-based source [
7]. Tryptophan, a type of indoleamine, is the starting molecule for MT like that of auxin and ought to be involved in the control of growth and development. It has physiological impacts on plants, which include stimulating seedling growth, formation of primary roots, lateral and adventitious roots, and modifying the branching and growth cycles of leaves and stems, and also resists against leaf senescence by enhancing photosynthesis, stimulating flowering and seed development [
8]. MT also takes part in several cellular processes in the name of antioxidant and free radical scavenging [
9]. Additionally, MT has been linked to improved seed sprouting, maturation, photosynthesis, biomass production, circadian rhythm, redox network, membrane integrity, root development, leaf senescence, osmoregulation, and resistance to environmental stresses like salt, drought, heat, oxidative stress, and heavy metals. MT levels in plants are noticeably higher when exposed to a various stressors, including salt, drought, temperatures, UV radiation, metal pollution, and pathogenic infections, implicating that MT plays a role in plant stress tolerance [
10]. It functions as an antioxidant and contributes to controlling ROS and nitrogen species (RNS) in plants because of its pleiotropic qualities. It is more efficient than glutathione and vitamin E at regulating a number of antioxidant enzymes, including glutathione reductase, catalase (CAT), peroxidase (POX), and superoxide dismutase (SOD). It boosts the mitochondria’s electron transport chain’s effectiveness, thereby reducing electron leakage. Because MT functions as a signaling molecule connected to defense systems against diverse biotic and abiotic threats, it is regarded as a master plant regulator that supports plant development and growth [
11]. The signaling molecules in MT biosynthesis in plants under stress are yet to be clearly identified [
12]. Employing MT as a bio-stimulator for the sustained production of crops without damaging the surrounding environment could, therefore, be of utmost relevance.
2. Biosynthesis of Melatonin in Plants
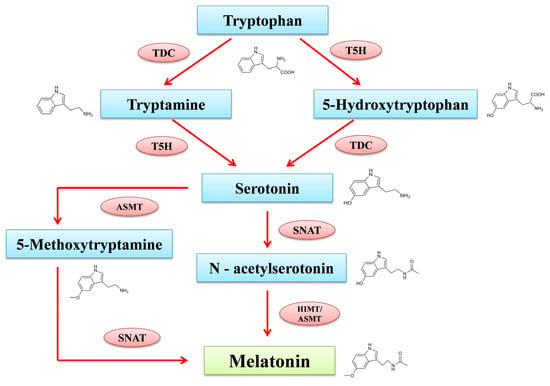
The MT production process in plants and animals is distinct. Many elements, including light, have a vital role in controlling its production in plants. MT is specifically expressed in chloroplasts and mitochondria in plants. Tryptophan acts as the precursor for the formation of phyto-melatonin, along with intermediates including tryptamine, serotonin,
N-acetyl serotonin, and 5-methoxy tryptamine (
Figure 1). According to the report from Tan and Reiter [
26], the intermediates of MT production are found in several sub-cellular compartments including the cytoplasm, mitochondria, endoplasmic reticulum and chloroplasts. Tryptophan decarboxylase (TDC) first decarboxylates tryptophan to produce tryptamine in the cytoplasm, tryptamine-5-hydroxylase (T5H), and then performs an enzymatic hydroxylation to produce serotonin in the endoplasmic reticulum.
N-acetyltransferase (SNAT) and acetyl serotonin methyl transferase (ASMT) convert serotonin through acetylation and methylation reactions into
N-acetyl serotonin in chloroplasts and 5-methoxytryptamine in the cytoplasm.
N-acetyl serotonin produced in chloroplast reacts with the ASMT in the cytoplasm and transforms into MT; meanwhile, 5-methoxytryptamine produced in cytoplasm moves into the chloroplast and reacts with SNAT to synthesize MT [
27]. Alternatively, an enzyme known as caffeic acid O-methyltransferase (COMT), which regulates several substrates, can also convert
N-acetyl serotonin into MT in a different route that has been studied through plants. COMT can also transform serotonin into 5-methoxytryptamine and produce MT through SNAT catalyzation [
28].
Figure 1. MT biosynthesis pathway in plant system. TDC: Tryptophan decarboxylase; T5H: tryptamine 5-hydroxylase; SNAT: serotonin-N-acetyltransferase; ASMT: N-aceylserotonin methyltransferase; COMT: caffeic acid O-methyltransferase.
3. Melatonin’s Role in Plant Growth and Physiology
3.1. Germination
In the life cycle of higher plants, seed germination is a complicated process governed by several coordinated metabolic, cellular, and molecular activities. It is also a crucial time for the establishment of crop populations. Germination involves a number of metabolic and physical processes. This stage, which is similarly susceptible to stress and critical for determining whether plants will survive under adverse conditions, is greatly influenced by the external environment.
3.2. Shoot and Root Growth
Due to the buildup of ABA, which further inactivates cell-wall-loosening enzymes under water stress in wheat, shifting the apoplastic pH from acidic to alkaline restricts the development of the plant’s shoots and roots [
46]. The process of cell elongation involves an indoleamine molecule [
47]. Pretreatment with MT results in a drop in intercellular pH to an acidic state and activates the enzymes responsible for loosening cell walls, which in turn triggers cell elongation like IAA [
48]. As a consequence, seed priming with MT enhanced seed germination and seedling development through synthesizing stress-related proteins and activating signaling pathways in rice under stressful conditions [
49]. Ahmad et al. [
50] stated that MT along with the application of nitrogen significantly improved the shoot fresh and dry biomass in maize seedlings. Exogenous MT enhances the accumulation of soluble sugars and the protein level, which regulates osmotic adjustment under stressful conditions in cotton [
51]. The application of MT stimulates the production of endogenous growth-inducing substances like metabolites, phytohormones, and increasing ROS and RNS scavenging systems in plants [
52] which might lead to the production of higher shoots and denser roots. The fact that MT also causes the auxin-related genes to become active suggests that the auxin signal pathway is necessary for MT-mediated root development [
53].
3.3. Gas Exchange
Photosynthesis is the most important physiological function found in all green plants that is severely affected by abiotic stresses [
61]. Abdulbaki et al. [
62] explained that abiotic stresses reduce the production of assimilatory powers (ATP and NADPH) and Rubisco activity by destroying the chloroplast grana structure and photosynthetic electron transport system. The reduced diffusion and concentration of intercellular CO
2 in the carboxylation site of rubisco also decreases the photosynthetic rate under stress [
63]. Chlorophyll is a key photosynthetic pigment found in all higher plants and plays a vital function in absorption of light energy. Fu et al. [
64] reported that the metabolite concentrations of chlorophyll a, chlorophyll b, and carotenoids were decreased under heat stress in wheat. The enhanced activity of chlorophyll-degrading enzymes like chlorophyllase, pheophytinase, and chlorophyll-degrading peroxidase catalyze the breakdown of chlorophyll molecules in response to stress [
65]. Wang et al. [
66] suggested that a direct link was observed between MT and the concentration of photosynthetic pigment in soybean. MT reduces the rate of chlorophyll degradation by lowering the transcript levels of pheophorbide-a-oxygenase (
PAO) which is involved in chlorophyll metabolism [
67]. The expressions of genes such as
Chlase,
PPH, and
Chl-PRX associated with degradation of chlorophyll biosynthesis were downregulated by MT in
Agrostis stolonifera [
68]. Shi et al. [
69] stated that MT increases the Bermuda grass photosynthetic pathway by protecting the chlorophyll molecule from degradation and enhances the expression of photosynthetic proteins like LHCa and PsaG during oxidative stress. MT also protects the chloroplast ultrastructure from oxidative damage and recovers photosynthetic accessory pigments like carotenoids, chlorophyll b, xanthophyll, and anthocyanin from stress [
70]. Liu et al. [
67] suggested that application of MT decreases the expression level and its relative mRNA abundance of genes involved in senescence (
SAG12) and the programmed cell death process. MT slows down the aging process of leaves by enhancing the ROS scavenging mechanism, which stabilizes the chloroplast structure and protects photosynthesis-related genes from deterioration [
71] in the tomato plant.
Stomata play a vital role in the regulation of photosynthesis, transpiration rate, and water status of the plant [
72]. MT regulates the opening of stomata through upregulation of the ABA catabolism process and simultaneously downregulates ABA anabolism that results in reduced accumulation of the endogenous ABA level. The decreased ABA level by MT reduces the production of H
2O
2 in guard cells of stomata that makes the stomata remain open and maintains the water status of the plant [
73]. Leaf water status and leaf temperature are positively regulated by transpiration rate. The increased transpiration rate by MT enables the plant to maintain a lower leaf temperature, thereby improving photosynthetic efficiency [
74]. The positive effect of MT on transpiration rate and stomatal conductance through the regulation of ABA level was also noticed in tomato [
75], rice [
76], and pepper [
77].
3.4. Antioxidant or ROS Scavenging
The crops are more vulnerable to the several abiotic stresses with changing climate during their growth phases. During stress conditions, plants convert 1–2% of the consumed oxygen into reactive oxygen species, specifically, hydroxyl radical (
•OH), hydrogen peroxide (H
2O
2), superoxide radical (O
2•−), and singlet oxygen (
1O
2). Stress enhances the production of ROS that results in cellular oxidative damage. The excessive generation and accumulation of ROS causes protein oxidation, lipid peroxidation, nucleic acid damage, enzyme inhibition, early leaf senescence, and necrosis [
80]. Plants produced various enzymatic, such as CAT, POX, APX, SOD, GPX, and GR, and non-enzymatic antioxidants, like vitamins, carotenoids, stilbenes, and flavonoids, to capture the excess ROS in the plant system and thereby protect the plants from oxidative stress. Currently, MT is an inevitable compound present in the plant system and functions as a powerful antioxidant using both direct and indirect mechanisms during abiotic stress conditions. MT scavenges free radicals produced under stressful circumstances by increasing the endogenous antioxidants such as ascorbic acid and glutathione [
58]. The expression level of genes related to antioxidant enzyme activity like SOD, CAT, APX, and GPX was also increased by MT in response to stress [
81]. Kaur et al. [
82] noticed that the Asada-Halliwell pathway, a crucial antioxidant enzymatic cycle, was regulated by MT in order to enhance the ROS scavenging mechanisms in stressed plants. Zhang et al. [
83] suggested that MT stimulates the activity of H
2O
2 scavenging enzymes such as CAT, POD, and APX as well as ABA-degrading enzymes. Furthermore, MT controls the AsA-GSH cycle, which is essential for ROS detoxification, and enzymes like APX, MDHAR, DHAR, and GR were involved in the regulation of this cycle [
84]. Rehman et al. [
85] explained that MT effectively scavenges ROS by increasing the activity of the antioxidant enzyme glutathione peroxidase (GPX), which scavenges lipid peroxides, hydroperoxides, and H
2O
2 under stress.
MT possesses amphiphilic characteristics that enable it to diffuse and distribute readily across lipid membranes and the cytoplasm. The MT-bound hydrophilic side of the lipid bilayer prevented lipid peroxidation by directly neutralizing the damaging chemicals produced under stressful circumstances [
86]. Lei et al. [
87] opined that application of MT to rapeseed minimizes the free radical formation and generation of ROS like H
2O
2 and O
2−. The integrity of the plant cell membrane was improved by MT through the increased activity of antioxidant enzymes like SOD, CAT, APX, and GPX [
88]. MT reduces the effects of oxidative stress by directly scavenging ROS through enhanced antioxidant enzyme activity that ultimately reduces the MDA level in plants [
89]. The increased antioxidant enzyme activity and defense system by the exogenous application of MT under stress conditions were also reported in wheat [
90], tomato [
91], cabbage [
92], and rice [
93]. The generation of superoxide anion radicals is inhibited by MT via limiting the level of O
2 flux under stress conditions when ADP levels are higher [
94]. MT functions through several methods as a mediator in many antioxidant pathways, such as the glutathione ascorbate cycle, peroxidases, superoxide dismutase, and CAT under abiotic stress responses in plants [
95]. Talaat and Todorova et al. [
96] also observed that the plants treated with MT have increased ascorbate (AsA) and reduced glutathione (GSH) content, thereby reducing the formation of H
2O
2 in plant cells. The increased non-enzymatic antioxidants like AsA and GSH production are thought to be crucial for maintaining the ROS balance in plants under stress. The positive role of MT on antioxidant enzyme activity was also reported by Ye et al. [
97] and Yan et al. [
98] in barley and tomato.
4. Melatonin’s Role in Secondary Metabolites’ Expression
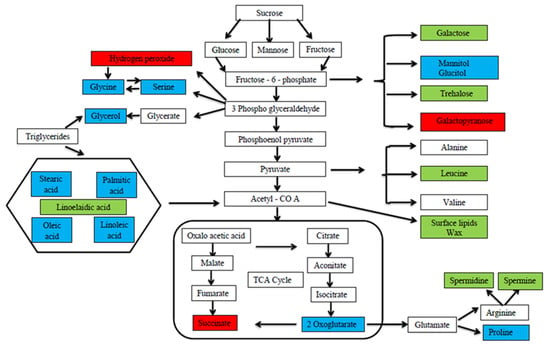
Abiotic stress downregulates the accumulation and concentration of plant metabolites, whereas foliar application of MT positively upregulates the metabolites in the plant system. At the cellular level, the concentration of several metabolites was altered by the exogenous application of MT that was both directly or indirectly involved in plant tolerance against drought stress in green gram, and the expressed metabolites were involved in the intermediates of different metabolic pathways [
99] (
Figure 2). Xie et al. [
100] reported that the metabolites involved in the carbon metabolic pathway which includes glycolysis, the oxidative pentose phosphate pathway and the tricarboxylic acid (TCA) cycle, were upregulated by MT and showed a direct link between the carbon metabolic pathway and MT in rice. Proline is one of the compatible solutes that accumulates in plant cells in response to cadmium stress and increases the osmotic adjustment in order to retain membrane integrity. In addition, the experiment found that exogenous application of MT could significantly improve the metabolite group such as amino acids, sugar, and sugar alcohols in tomato plant [
91] and the compounds were assigned as intermediates for plant metabolic pathways. Sheikhalipour et al. [
101] showed that increased proline concentration by MT also increases the stabilization of protein structures from denaturation under moisture stress. Saddhe et al. [
102] described that metabolites like proline and some sugars such as glucose, fructose, sucrose, and trehalose were involved in the regulation of osmotic adjustment under osmotic stress. MT increased the transcription level of various sucrose-related enzymes like sucrose synthase, invertase, phosphatase, and fructokinase and sucrose transporters in plant cells [
103]. Yang et al. [
104] explained the importance of MT between
MdFRK2 and plant growth and
MdFRK2 was found to be involved in the MT-mediated accumulation of sugars like glucose, fructose, and sucrose in apple leaves. Jiang et al. [
105] found that high levels of metabolite concentration related to amino acids were observed in MT treatment that results in enhanced physiological activities. The primary function of glycolysis in the plant metabolic pathway is to supply energy in the form of ATP and synthesize precursors essential for metabolism of fatty acids and amino acids [
106]. Zhang et al. [
107] stated that MT improves the metabolites engaged in carbohydrate and amino acid metabolism and upregulates the glycolysis pathway in plants. MT enhances plants’ tolerance to abiotic stresses through detoxification of ROS and osmotic adjustment by synthesizing and accumulating secondary metabolites such as phenols, ascorbic acid, and carbohydrates such as mannitol and ribose which play a major role in antioxidants and osmolytes [
108]. Foliar application of MT during drought stress expressed multifaceted metabolites in
Carya cathayensis which facilitates the upregulation of biosynthetic pathways such as ABC transporters, porphyrin and chlorophyll metabolism, carotenoid biosynthesis, carbon fixation and metabolism, sugar metabolism, and the phenylpropanoid pathway in MT-treated plants [
109]. For plants to fight against various environmental stresses, MT regulates the stress signaling pathways through the accumulation of various flavonoids, polyamines, and phenolic compounds in the plant system [
110].
Figure 2. Different metabolite expressions in control and MT-treated green gram plant under drought stress. In the figure, the blue color highlighted box shows the metabolite expressions in both control and MT-treated plants, the green color highlighted box shows the metabolite expression in MT-treated plants alone, and the red color highlighted box shows the metabolite expression in control plants [
99].
The GC-MS metabolomic study of the investigation showed that more than 50 compounds were expressed and regulated by MT treatment in cassava plants [
111]. These compounds include amino acids (glycine, arginine, and thymine), fatty acids (oleic acid, palmitic acid, streaic acid, linoleic acid, linolenic acid, and traumatic acid), antioxidants (coumarins, phenols, and flavonoids), aromatic compounds (piperidine), and digitoxin. Salt-stressed plants without MT treatment also expressed some compounds in minimum amounts such as gamolenic acid, gelsimine, burnamicine, oxalic acid, and melibiose, whereas traumatic acid, glycine, arginine, oleic acid, arginine, thymine, and phenols were some compounds found only in MT-treated plants and not in salt-stressed plants. MT application was responsible for the synthesis of spermidine, spermine, and putrescine bioactive compounds through activating precursors like arginine and ornithine [
89]. The various abiotic stress studies found that MT endorses the secondary metabolites like spermidine, spermine, and putrescine in
Cucumis sativus; flavones, flavanone, luteolin, and isoflavone in pigeon pea [
95,
112]; and rosmarinic acid, luteolin flavone, and apigenin flavone in
Dracocephalum kotschyi Boiss [
113].
5. Melatonin’s Role in Crop Yield and Quality
MT enhances the growth-related attributes as well as the photosynthetic pigments and thus maximizes the photoassimilate production and translocation efficiency from source to sink tissues and finally the yield [
114]. Khan et al. [
115] mentioned that in tomato plant, the number of fruits per plant, fruit yield, and quality characters (ascorbic acid, lycopene content, and β carotene) were increased in MT-treated plants. Hassan et al. [
116] reported that exogenous MT significantly improves the weight of the bunch, hands per bunches, total weight of hands, and finger length in banana. In addition, Hu et al. [
84] also stated that increased photosynthetic carbon metabolism and partitioning efficiency in the MT-treated plants enhanced the boll formation and seed yield in cotton. MT regulates a variety of physiological and biochemical processes in plants, thereby improving the net photosynthetic rate and productivity of the crop [
117]. Medina-Santamarina et al. [
118] explained that MT showed a positive effect on the improvement of sink strength that ultimately results in improved berry size, weight, and yield of pomegranate. MT enhances the seed filling rate, seed weight, and final yield of maize crop by regulating the hormonal balance [
50]. Jiang et al. [
105] also observed that MT delays the early leaf senescence process and improves the photosynthetic efficiency by minimizing the production of ROS, which shows a direct impact on the improvement of quality and yield of rice grains. Liu et al. [
67] reported that the number of fruits per plant, per fruit weight, and yield per plant were significantly improved in MT-treated cucumber plants. Application of MT showed a positive correlation between photosynthetic rate, antioxidant enzymes, and seed yield in soybean [
119] and maize [
120].
Ibrahim et al. [
126] observed an enhanced fruit quality in tomato due to MT application which improved the antioxidant enzymes, lycopene, ascorbic acid, and total soluble solids. Gurjar et al. [
127] found that exogenous MT increased the shelf life of fruits and vegetables. Medina-Santamarina et al. [
118] described that the quality parameters of pomegranate fruits like fruit size, color, total acidity, total soluble solids, fruit number per tree, and fruit yield were improved by the application of MT. Nasser et al. [
128] observed that the increase in transcriptome alterations during the ripening process in grape berries enhanced the quality of berries due to MT treatment. Under drought stress, foliar application of MT enhanced the yield and quality of
Moringa oleifera L. in terms of amino acid composition, glutamic acid, and nutrition such as nitrogen, phosphorus, potassium, calcium, and magnesium [
129]. In flax, total phenolic content, TSS, proline, and free amino acid contents of the seeds were increased by exogenous MT treatment [
130]. Farouk and Al-Amri [
131] reported that the application of MT in rosemary plants improved the essential oil content and yield under stress conditions. Foliar spray of MT in medicinal lemon verbena shrub (
Lippia citriodora) enhanced the yield and essential oil content by 52% and 32%, respectively, under stress conditions [
132].
6. Melatonin’s Role in Abiotic Stress Mitigation
Plants experience many adverse situations throughout their lifespan. In order to survive and reproduce successfully in adverse conditions such as drought, salinity, high temperature, flooding, and heavy metal stress, plants have evolved a variety of response mechanisms. MT is a universal compound participating in the nullification of the various abiotic stress responses as a pleiotropic signaling molecule. Furthermore, it is a proficient scavenger of RNS as well as ROS. Numerous research studies have been carried out to investigate the activities of MT in plants since its discovery, indicating its protective properties against abiotic stressors (Table 1).
Drought and high temperature stress reduce the permeability of water in the plants [
133]. Stomata play a vital role in regulation of photosynthesis, transpiration rate, and plant water status in response to abiotic stresses [
134]. Rao et al. [
135] opined that ABA acts as a key mediator for the closure of stomata under stress conditions, which ultimately affects a cascade of physiological and molecular processes. Wang et al. [
136] explained that exogenous MT ameliorates the oxidative stress and improves transpiration rate and stomatal conductance in sweet corn. The increase in transpiration rate and stomatal conductance might be due to the upregulation of the ABA catabolism process and the simultaneous downregulation of ABA anabolism that results in reduced accumulation of the endogenous ABA level; this fact was already reported by Hu et al. [
137]. The decreased ABA level reduces the production of H
2O
2 in guard cells of stomata that makes the stomata remain open and maintains the water status of the plant under stress [
29]. This might be the reason for the increased transpiration rate and stomatal conductance in green gram under water deficit and high temperature stress conditions. Jiang et al. [
138] reported that MT improves the stomatal conductance by regulating the ROS-mediated stomatal closure that results in a higher transpiration rate in response to stress. Leaf water status and leaf temperature are positively regulated by transpiration rate. The increased transpiration rate by MT enables the plant to maintain lower leaf temperatures, thereby improving photosynthetic efficiency [
139]. Supriya et al. [
140] found that an increased stomatal conductance in MT-treated plants regulates the canopy temperature by enhancing the water loss which ultimately results in lower water use efficiency under stress. At the single-leaf level, the water use efficiency is governed by stomatal conductance and transpiration rate [
136]. The response of water use efficiency is closely linked with physiological processes by regulating the concentration of CO
2 and H
2O in plant cells [
27]. MT maintains better water use efficiency under stress through the control of stomatal movements; therefore, it improves the net photosynthetic rate as reported by Li et al. [
141]. The positive effects of MT on transpiration rate and stomatal conductance through regulation of the ABA level were also noticed in tomato [
142], rice [
143], and barley [
144].