Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Orthopedics
Spinal muscular atrophy (SMA) is a rare neuromuscular disease, with an estimated incidence of about 1 in 10,000 live births. To date, three orphan drugs have been approved for the treatment of SMA: nusinersen, onasemnogene abeparvovec, and risdiplam.
- spinal muscular atrophy
- nusinersen
- onasemnogene abeparvovec
1. Preclinical Studies
The deeper knowledge of the molecular mechanisms underlying SMA’s onset and progression has represented a significant milestone for the development of specific therapeutic strategies (Figure 1).
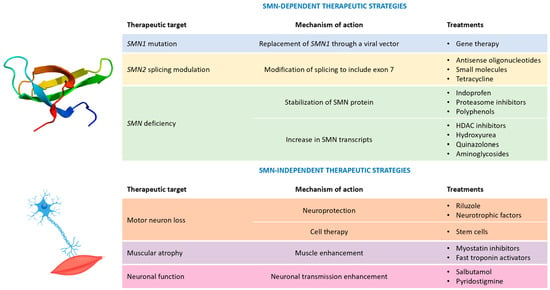
Figure 1. Possible therapeutic strategies for spinal muscular atrophy. Legend: HDAC = histone deacetylase; and SMN = survival motor neuron.
In this frame, animal testing provided a crucial help: several SMA animal models (Caenorhabditis elegans, zebrafish, and Drosophila) were indeed developed in the past. Nonetheless, only the setting up of a murine model able to accurately reproduce the human disease phenotype represented the actual cornerstone for preclinical studies [7]. Firstly, it must be considered that mice have only one SMN gene encoding for the SMN protein, instead of two as in humans. Moreover, homozygous mutations in the SMN gene induce early embryonic death in mice, whereas heterozygous SMN mice (SMN±) display a normal phenotype [8]. To overcome these issues, a novel transgenic murine model was developed, introducing the human SMN2 gene in the SMN-null mice. This strategy made the murine disease phenotype more similar to the human one, also considering that SMN2 manipulation allows for the introduction of specific patients’ mutations. This was also paralleled by the adoption of reliable and highly reproducible experimental protocols. The Translational Research in Europe for the Assessment and Treatment of Neuromuscular Diseases (TREAT-NMD) network [9] played a key role in this approach through the provision of internationally validated, standard operating procedures, useful to optimize protocols and accelerate the research process [10]. The timeline of the most important preclinical studies in the SMA field is summarized in Table 1.
Table 1. Timeline of preclinical studies of orphan drugs approved for spinal muscular atrophy treatment.
| Drug | Mechanism of Action | Timeline | Detected Issues |
|---|---|---|---|
| Nusinersen | Inclusion of exon 7 in SMN2 transcript |
|
|
| Onasemnogene abeparvovec | Replacement of SMN1 gene |
|
|
| Risdiplam | Inclusion of exon 7 in SMN2 transcript |
|
|
Abbreviations: ASO = antisense oligonucleotide; SMA = spinal muscular atrophy; and SMN = survival motor neuron.
1.1. Nusinersen
Nusinersen was the first SMA orphan drug approved in 2016 by the Food and Drug Administration (FDA) and, in 2017, by the EMA. Nusinersen belongs to the class of antisense oligonucleotides (ASOs) and is able to target a specific splicing silencer site (ISS-N1, intronic splice silencing) located within the SMN2 intron 7. As previously mentioned, small differences in the SMN2 gene sequence led to alternative splicing processes, inducing the skipping of exon 7 in the SMN2 transcript and the subsequent synthesis of a truncated, non-functional protein (SMNΔ7). Nusinersen prevents the binding of specific splicing repressors, hnRNPA1 and hnRNPA2, to ISS-N1, allowing the integration of exon 7 into the final transcript and the synthesis of a full-length SMN protein. Since 2006, systematic screenings have been conducted to find the most effective ASO. Several ASOs were tested in murine models and in patient-derived fibroblasts, shifting along the ISS-N1, one base at a time. Once the strongest sequence had been identified, the specific ASO was administered in SMA mice to evaluate the effects of the compound on in vivo and ex vivo disease readouts. These treatments markedly increased the SMN protein levels and ameliorated the disease phenotype, extending the median lifespan (severely reduced in the SMA mice) by 25-fold and leading to the significant recovery of motor functions (including α-motor neuron count, mean area of muscle fibers, heart weight, thickness of the interventricular septum, left ventricular wall, and integrity of neuromuscular junctions), which were all relevant effects from a clinical perspective [14]. Moreover, the pharmacokinetic analysis performed on murine models and non-human primates highlighted a good bioavailability of the compound at the central nervous system level if administered by intrathecal infusion. These promising data provided a solid base for the following successful clinical trials, ending with nusinersen’s approval for SMA treatment in children and adults. The main drawback of nusinersen use is represented by its inability to cross the blood–brain barrier (BBB) and the subsequent need for recurrent lumbar punctures for intrathecal administration. This considerably reduces therapeutic compliance, particularly for patients with severe scoliosis.
1.2. Onasemnogene Abeparvovec
The replacement of the SMN1 defective gene is an alternative therapeutic approach that has been recently explored. Onasemnogene abeparvovec was approved in 2020 as the first, and currently unique, gene therapy for SMA, and it was indicated for SMA patients aged <2 years with bi-allelic mutations in the SMN1 gene and three or fewer copies of the SMN2 gene or infantile-onset SMA. The small dimension of the SMN1 gene facilitated its delivery via a recombinant self-complementary adeno-associated viral vector serotype 9 (scAAV9). Preclinical studies in mice and non-human primates demonstrated the ability of the drug to cross the BBB after intravenous injection. Furthermore, this gene therapy significantly improved lifespan (median of 282 days for treated mice vs. 17.5 days for untreated SMAΔ7 mice) and ameliorated motor functions, particularly in early-treated animals [19,20]. Toxicology studies confirmed the safety and good tolerability of the treatment after intravenous injection, opening for the translatability of the drug in humans using this specific route of administration. In addition, it must be underlined that preclinical studies were performed on juvenile animals: the very encouraging obtained results supported again the importance of early administration. In fact, intravenous injection in mice within 24 h of birth showed greater efficacy compared to a later administration (within 5 or 10 days of birth), thus further reinforcing the relevance of neonatal screenings for early treatment.
1.3. Risdiplam
The success of nusinersen considerably boosted research for further therapies, in particular for those administered through a less invasive route. Innovative drug design techniques in combination with high-throughput screening methods permitted the identification of novel, small molecules that shared nusinersen’s mechanism of action, promoting the inclusion of exon 7. The first molecule of interest was an orally administered coumarin derivative, subsequently modified to overcome potential in vitro mutagenicity issues and the phototoxicity typically associated with this drug class. Further compound optimization ameliorated the selectivity and affinity to the target, allowing a reduction of the dose required to achieve the therapeutic goals while limiting the side effects. Finally, after these implementations, the pyrido-pyrimidinone derivative risdiplam was synthesized and successively approved by the FDA and EMA as the first oral drug for SMA treatment in 2020 and 2021, respectively [21]. Preclinical studies performed in mice and non-human primates confirmed the drug’s efficacy in restoring proper SMN protein levels and demonstrated a strong correlation between plasma and tissue concentration, overcoming the bioavailability issues of nusinersen [22]. In fact, several preclinical studies demonstrated that the SMN protein carries out a wide variety of actions at multiple levels and in different tissues, explaining the non-motor symptoms related to the pathology. Pharmacokinetic analyses documented the excellent distribution of risdiplam in several tissues, paving the way for an innovative therapy which combined an easier method of administration with the possibility to treat also peripheral symptoms. Real-world studies will help evaluate whether this systemic distribution could determine off-target effects, affecting the risk–benefit ratio of risdiplam.
2. Pivotal Clinical Trials
The characteristics of the pivotal clinical trials supporting the marketing approval of the three pharmacological therapies for the treatment of SMA are summarized in Table 2.
Table 2. Pivotal clinical trials leading to the marketing authorization of nusinersen, onasemnogene abeparvovec, and risdiplam.
| Study | Study Design | Status | Eligibility Criteria | Exposure | Outcome | Results | |
|---|---|---|---|---|---|---|---|
| Treatment Group | Control Group | ||||||
| Nusinersen | |||||||
| ENDEAR [23] | Phase III double-blind, controlled, randomized clinical trial. | Completed | Patients with homozygous deletions or mutations in the SMN1 gene and with two copies of the SMN2 gene and younger than 7 months at the screening date | 80 patients treated with nusinersen at a dose adjusted by cerebrospinal fluid volume, such that the patients 2 years old or older received a dose equivalent to 12 mg. | 41 patients treated with a sham procedure |
|
|
| Onasemnogene abeparvovec | |||||||
| STR1VE-US [24] | Phase III open-label, single-arm clinical trial | Completed | Patients with SMA type 1, with biallelic SMN1 mutations, with one or two copies of SMN2, and younger than 6 months of age at the date of onasemnogene abeparvovec administration | 22 patients treated with a single intravenous administration of onasemnogene abeparvovec, over approximately 60 min: 1.1 × 1014 vg/kg | 23 untreated children, aged less than 6 months and with SMA type 1 registered in the Pediatric Neuromuscular Clinical Research dataset (historical controls) |
|
|
| Risdiplam | |||||||
| SUNFISH—Part 2 [25] | Phase III double-blind, placebo-controlled, randomized clinical trial | Ongoing | Patients between 2 and 25 years of age, with type 2 or type 3 SMA | 120 patients treated with oral-administered risdiplam (5 mg/day for patients weighing ≥ 20 kg or 0.25 mg/kg/day for patients weighing < 20 kg) | 60 placebo-treated patients | Variation from the baseline in the 32-item Motor Function Measure’s total score for patients at the 12th month of treatment compared to the placebo group | At month 12, the mean change from baseline in the MFM32 score was 1.36 (95% CI: 0.61 to 2.11) in the treatment group and −0.19 (95% CI: −1.22 to 0.84), with a difference of 1.55 (95% CI: 0.30–2.81; p = 0.016) in favor of the treatment with risdiplam. |
| FIREFISH—Part 2 [26] | Phase II/III open-label, single-arm clinical trial | Completed | Patients aged 1 to 7 months with a type 1 SMA diagnosis and two copies of the SMN2 gene | 41 patients treated with risdiplam. The patients older than 5 months received a dose of 0.2 mg/kg/day; the patients younger than 5 months received a dose of 0.04 or 0.008 mg/kg/day, adjusted to 0.2 mg/kg/day within 1–3 months from starting treatment. | 16 historical controls derived from the NeuroNEXT study, and 24 historical controls | Proportion of patients able to sit without assistance for at least 5 s after 12 months of treatment, based on the 3rd edition of the Bayley Scales of Infant and Toddler Development | 12 out of the 41 patients (29%; 95% CI: 16 to 46) were able to sit without assistance for at least 5 s after 12 months of treatment; the percentage was significantly higher than in the historical controls (p < 0.001). |
Abbreviations: CI = confidence interval; MFM32 = 32-item Motor Function Measure; SMA = spinal muscular atrophy; SMN = spinal motor neuron; and vg = vector genomes.
The efficacy of nusinersen was proved in the ENDEAR study, a 13-month double-blind phase 3 clinical trial, which enrolled 121 patients diagnosed with infantile-onset SMA before 6 months of age and who were under 7 months of age at the time of receiving the first dose [23]. The patients were randomized, in a 2:1 ratio, to receive an intrathecal injection of nusinersen (treatment group) or a sham procedure (control group). The primary endpoints included the achievement of a motor milestone response defined according to the Hammersmith Infant Neurological Examination and the event-free survival (i.e., time to death or requiring permanent assisted ventilation). The results of the per protocol analysis showed that 51% of the patients in the treatment group and no patients in the control group achieved a motor milestone response and that the likelihood of event-free survival was higher in the treatment group than in the control group (hazard ratio for death or the use of permanent assisted ventilation, 0.53; p = 0.005). However, the ENDEAR trial was terminated early due to the results of the interim analysis and to the ethical consideration for the patients in the control group, and this led to the loss of data and a short time period for the assessment of the safety and the efficacy of nusinersen.
Concerning onasemnogene abeparvovec, its efficacy was demonstrated in the STR1VE-US study, an open-label phase 3 clinical trial including 22 symptomatic patients diagnosed with SMA type 1 who were younger than 6 months [24]. The enrolled patients were followed-up until the age of 18 months. The co-primary efficacy endpoints were independent sitting for ≥30 s at the study visit at 18 months of age and event-free (i.e., time to death or requiring permanent ventilation) survival at 14 months of age. Such endpoints were compared to 23 untreated infants aged ≤6 months with SMA type 1 from the Pediatric Neuromuscular Clinical Research (PNCR) dataset, a historical cohort of untreated infants with SMA type 1 [27]. The first co-primary endpoint was met by 13 (59%) of the 22 patients in the treatment group vs. 0 patients in the PNCR cohort, while the second was achieved by 20 (91%) patients in the treatment group vs. 6 (26%) in the PNCR cohort. The main limitations of the STR1VE-US trial were the lack of a randomized comparison to a control group, potentially leading to an overestimation of the treatment effect, the lack of masking for both intervention and outcome evaluation, the strict inclusion criteria, and the short follow-up period.
The marketing approval of risdiplam was based on two pivotal clinical trials: the FIREFISH trial in infantile-onset SMA [26] and the SUNFISH trial in late-onset SMA [25]. The FIREFISH study was an open-label, single-arm study including 41 infants aged between 1 and 7 months, with genetically confirmed Type 1 SMA. The primary endpoint was the ability to sit without support for at least 5 s after 12 months of treatment, a milestone that is never achieved in untreated patients affected by Type 1 SMA. After 12 months of treatment, 12 patients (29%) met this endpoint [26]. The internal validity of the FIREFISH trial was affected by the single-arm design, precluding a precise estimation of the magnitude of the benefit.
The SUNFISH study was a phase 3, randomized, double-blind, placebo-controlled clinical trial in patients aged between 2 and 25 years with confirmed type 2 or type 3 SMA. Patients were randomized, in a 2:1 ratio, to receive daily oral risdiplam (treatment group, N = 120) or daily oral placebo (control group, N = 60), and they were followed-up for 12 months. The primary endpoint was the change from the baseline in the 32-item Motor Function Measure (MFM32)’s total score at month 12. After one year, the least squares mean change from the baseline was 1.36 (95% confidence interval 0.61 to 2.11) in the risdiplam group and −0.19 (−1.22 to 0.84) in the placebo group, with a treatment difference of 1.55 (0.30 to 2.81, p = 0.016) in favor of risdiplam. However, the improvement in motor function was mainly detected in the younger patients (i.e., children aged between 2 and 5 years) rather than in the adolescent and adult patients, thus leaving unanswered questions for risdiplam use in adults with SMA when considering efficacy and long-term expectations [28].
This entry is adapted from the peer-reviewed paper 10.3390/brainsci13101446
This entry is offline, you can click here to edit this entry!
