You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Estrogens, belonging to a group of steroid compounds, play an important role in both physiological and disease processes, mainly by interacting with estrogen receptors (ERs). Abnormal ER signaling may result in various cancers, including breast cancer (BC), one of the most often diagnosed cancers in women globally, and a second cause of female cancer-related death.
- estrogen receptor
- ER
- breast cancer
- ER signaling
1. Introduction
One of the most often diagnosed malignancies in women globally is breast cancer (BC), being now the second cause of death because of cancer [1][2]. The biological activity and treatment response of BC are influenced by a variety of histological and molecular abnormalities [3]. Despite improvements in the development of diagnostic methods and treatments, the incidence and mortality rate of breast cancer-bearing patients are rising internationally [4]. Age, family history, histological differentiation and grading, and the local and systemic advancement of the disease have all been studied to evaluate the patient risk and choose the best course of action [5][6]. The three main types of breast cancer are classified based on the hormone receptors’ status. The first group consists of tumors that have either tested positive for the estrogen receptor (ER) or the progesterone receptor (PR). The second group consists of tumors that have either tested positive for the human epidermal growth factor receptor 2 (HER2) with or without ER and PR positivity, whereas the third one is called triple-negative breast cancer (TNBC), since these types of tumors lack expression of all three receptors (ER, PR, HER2) [7]. Receptor status, among other variables, has been demonstrated as the one of most important factors in estimating the prognosis and therapeutic response [8]. Furthermore, breast cancer classification based on intrinsic molecular subtypes as a result of the microarray expression profiling has been distinguished [9][10]. These are termed luminal A (ER+PR+ tumors, expressing luminal genes such as ESR1, GATA3, XBP1, and FOXA1; characterized by the low expression of Ki-67), luminal B (ER+ with lower expression of luminal genes, e.g., PGR and FOX1 and a high expression of Ki-67, >20%), HER2-enriched (characterized by the HER2 positivity; however, not all clinically classified HER+ tumors are of these molecular subtype and intermediate expression of luminal genes), basal-like (increased expression of EGFR and basal cytokeratins with low expression of the luminal A-type genes), and claudin-low (ER-, PR-, and HER- tumors are also negative for claudin 3/4/7 and E-cadherin (reviewed in: [11][12][13]).
ERs are activated by estrogens and play important roles in the development of several cancers; in particular, breast [14], endometrial [15], and ovarian cancers [16]. Estrogens are a group of low molecular weight lipophilic molecules that occur in three forms: estrone (E1), estradiol (E2; the term estrogen is used in relation to E2, due to its predominant role in physiology), and estriol (E3) [17]; the fourth form produced during pregnancy, namely estetrol (E4), is a fetal estrogen with selective tissue actions [18]. These hormones contain in their structure a steroid skeleton made of four aromatic rings. One of them is the phenolic A ring, which is responsible for binding to the ER [19]. Estrogens, like other steroid hormones, are synthesized at the rough endoplasmic reticulum from its precursor—cholesterol, which is described in detail by Fuentes and Silvera (2019) [20]. Briefly, they are synthesized from androstenedione in the presence of oxygen and NADPH. The crucial enzyme involved in this process is aromatase (CYP19A1), an enzyme that participates in the final stage of E1 and E2 synthesis. The synthesis of estrogens takes place in the gonads (predominantly in the ovaries—granulosa cells), adrenal cortex, and adipose tissue, in smaller amounts also in other tissues, including breast and placenta [21], or fetal liver, in the case of E4 [18]. E1 and E2 can arise from testosterone in peripheral tissues (mainly adipose tissue) in the enzymatic reaction catalyzed via aromatase, which has a significant impact on the level of estrogen synthesis in postmenopausal women [22].
Estrogens, including E2 (the predominant circulating estrogen in humans) are transported in the blood along with specific proteins. They sequentially cross biological membranes by diffusing to the target sites, where they primarily act by attaching to specific nuclear ER. Receptor–ligand complexes can directly silence/activate gene expression or act indirectly by interacting with intracellular signaling molecules. The mechanism of action of estrogens is very diverse, and the nature of the response depends on both the genetic and physiological predisposition of the target cells. Estrogens are synthesized in both sexes; however, at different concentrations and with different functions [23]. These hormones play a significant role in the proliferation and growth of cells associated with reproduction and have a myriad of other cellular functions; for instance, carbohydrate and lipid metabolism, and the regulation of energy homeostasis [17][24]. Importantly, estrogens affect the cardiovascular [25] and central nervous system [26]. The effect of estrogens on the cardiovascular system may be protective, as shown by several studies, including large-scale clinical trials [27][28][29], but have also been associated with the risk of coronary heart disease [30]. Furthermore, estrogen-related malfunctions result in several autoimmune, metabolic, or degenerative pathologies and cancers, including the development of breast cancer [17].
The ER plays a key role in the development, progression, and invasion of ER-expressing BC [31]. ER-positive tumors have a more favorable prognosis compared to other BC types and are usually responsive to hormonal treatment. In the absence of ERα expression, BC exhibits more aggressive phenotypes [32].
2. Estrogen Receptors
The ER family includes the nuclear ER (nER) and G protein-coupled estrogen receptor 1 (GPER1) [33]. nER is characterized by conserved domain structures, such as the DNA-binding domain (DBD) and the ligand-binding domain (LBD) [34]. Two major nER isoforms, ERα and Erβ, are responsible for the regulation of the female reproductive system development, the preservation of bone mass, and the protection of the central nervous system, among other physiologically important processes [35]. The evolutionary origin of the estrogen-signaling system remains unclear; however, the research on invertebrates provided insight into the vertebrate pathway. Interestingly, the ER homologs have been identified in amphioxus [36][37], mollusks [38][39], and annelids [40]. Regarding the functional insights, the ERs from amphioxus and mollusks are not activated by estrogens [38][41][42], while in two annelid species, transcription is activated in response to the low doses of estrogens upon ER binding [40]. Based on the phylogenetic context, it was hypothesized the ER possibly originated in the bilateralian lineage [43]. In humans, the nERs are encoded by two different genes (ESR1 for ERα [44] and ESR2 for ERβ [45]) as a result of gene duplication in the early vertebrate lineage [46] that are located on different chromosomes—ESR1 is located on chromosome 6 and ESR2 on chromosome 14.
The nER is composed of six homologous A-F domains (Figure 1) representing the receptors’ structural regions and having unique functional characteristics. Domains A and B are located at the amino terminus (N-terminal domain) and contain the so-called activation of function domain 1 (AF-1), whose function is to activate the transcription of target genes [20]. Domain C possesses a zinc-finger motif and corresponds to the DBD domain, namely the DNA-binding domain. This domain is responsible for receptor dimerization and binding to the estrogen-dependent genes promoters’ sequences, called estrogen-response elements (ERE) [47]. The D domain is characterized by the presence of a nuclear localization signal (NLS), which, after the binding of a specific ligand, followed by the conformational change caused by this interaction, is exposed, and it is necessary for translocation to the nucleus. Domain D is the so-called hinge region (H), which is responsible for the functional synergy between fragments AF-1 and the second transcriptional activation domain—the AF-2 fragment located at the carboxyl terminus (C-terminus) [48]. The E domain is the ligand-binding domain (LBD), which contains the ligand-binding site (L). The F domain located at the end of the C-terminus probably acts as a modulator of transcriptional activity and is involved in the interaction with the coactivators [49][50].
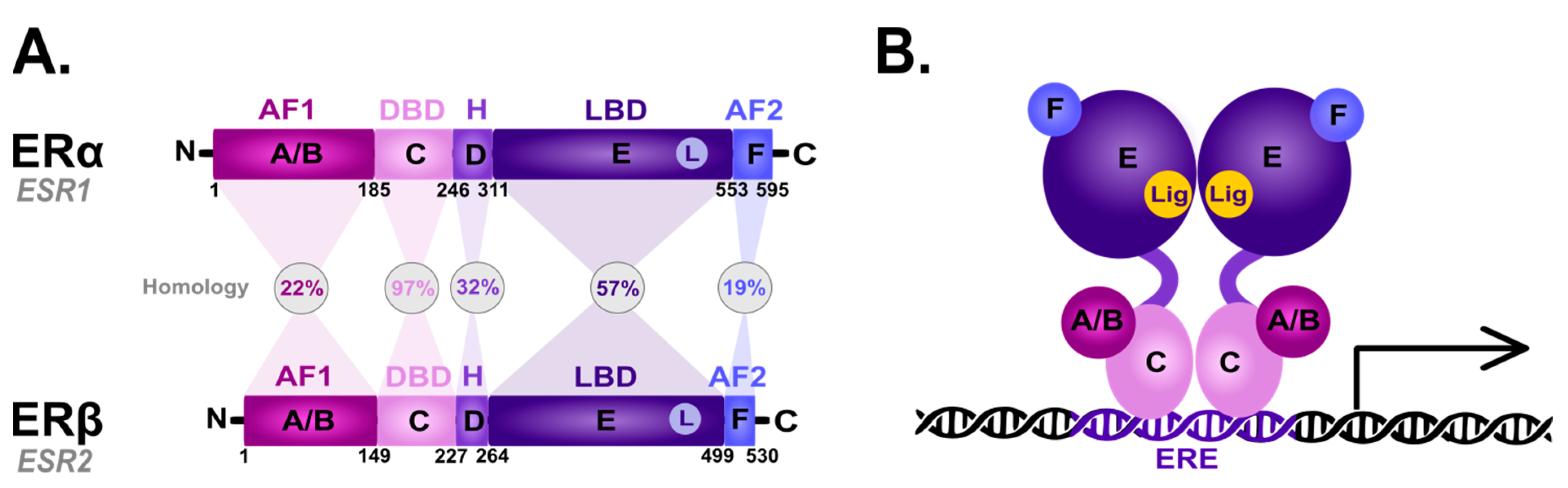
Figure 1. Scheme of the structural and functional regions of the estrogen receptor (ER). (A) Comparison of the domain topology of ERα and ERβ. The homology of the ERα and ERβ receptors was determined based on the amino acid sequence retrieved from the UniProt database (https://www.uniprot.org/; accessed on 12 March 2023; ESR1 ID: P03372, ESR2 ID: Q92731). AF1—the activation of function domain 1; DBD—the DNA-binding domain; H—hinge region; LBD—ligand-binding domain; AF2—the activation of function domain 2; A/B—the domains located at the N-terminus (N); C—the domain containing zinc-finger; D—the domain with nuclear localization signal; E/F—the domains located at the C-terminus (C). (B) Diagram of the estrogen receptor dimer binding to DNA in the estrogen-response elements (ERE). A-F as explained in the description of A; Lig—ligand.
ERα and ERβ show high homology in the LBD and DBDs, while they differ in the transcription-activating domain (AF-1) [20]. Due to alternative splicing, both receptor subtypes occur in isoforms [20][51][52][53][54]; five shorter isoforms for ERα, and three shorter isoforms and one longer isoform for ERβ [20]. They are also differentially expressed throughout the body [55][56]: ERα predominance is shown by the endometrial cells, ovary, hypothalamus and outgoing ducts’ testicles, while ERβ is expressed mainly in the kidney cells, brain, heart, bones, lungs, intestinal mucosa, prostate, and vascular endothelium. The deregulation of ERα expression and function is closely related to the carcinogenesis process in ovarian, uterine and breast cancer epithelial cells. On the other hand, ERβ inhibits ERα-mediated transcription and estradiol-induced cell proliferation, which is probably the reason why it is associated with benign forms of breast cancer [57][58][59]. The ERα/ERβ cellular ratio plays a key role in regulating E2 activity; for instance, in human T47D BC cells [60]. However, approximately 75% of breast tumors are ER-positive [61] and aberrations in the function are associated with ERα. Hence, ERα is one of the main clinical drug targets [62]. The primary function of both receptors is the downstream regulation of gene transcription upon E2 binding to control the cell proliferation and differentiation activated by the ER-dependent signal transduction [63].
GPER1 (also known as GPR30), is the second type of estrogen-dependent receptor and is a member of the transmembrane metabotropic receptors family, which was originally detected in breast cancer tissue [64]. The GPER1 coding gene is located on chromosome 7 [65]. It is created via a single polypeptide with an α-helical structure strongly folded and immersed in the cell membrane, through which the polypeptide chain passes seven times, forming a hydrophobic transmembrane domain [66]. The GPER1 is present in many cells and tissues. mRNA expression was confirmed, e.g., in the ovaries, prostate, thymus, bone marrow, skeletal muscles, liver, lungs, heart, kidney, pancreas, small intestine, and brain [67]. In response to the extracellular signal by its predominant ligand—E2, the GPER1 regulates many cellular processes via a rapid non-genomic dependent mechanism. Compared to normal tissues, GPER1 is detected with a higher expression in breast cancer cells [68].
3. Estrogen Signaling
3.1. Genomic Action of ER
The ER-dependent signaling can be classified as genomic and non-genomic with different activities and pathways involved, respectively (Figure 2). Genomic signaling (Figure 2; bottom panel) depends on the transcriptional activities via the gene expression, while non-genomic (Figure 2; top panel) depends on the activation of various signaling cascades, as reviewed in: [20][69].
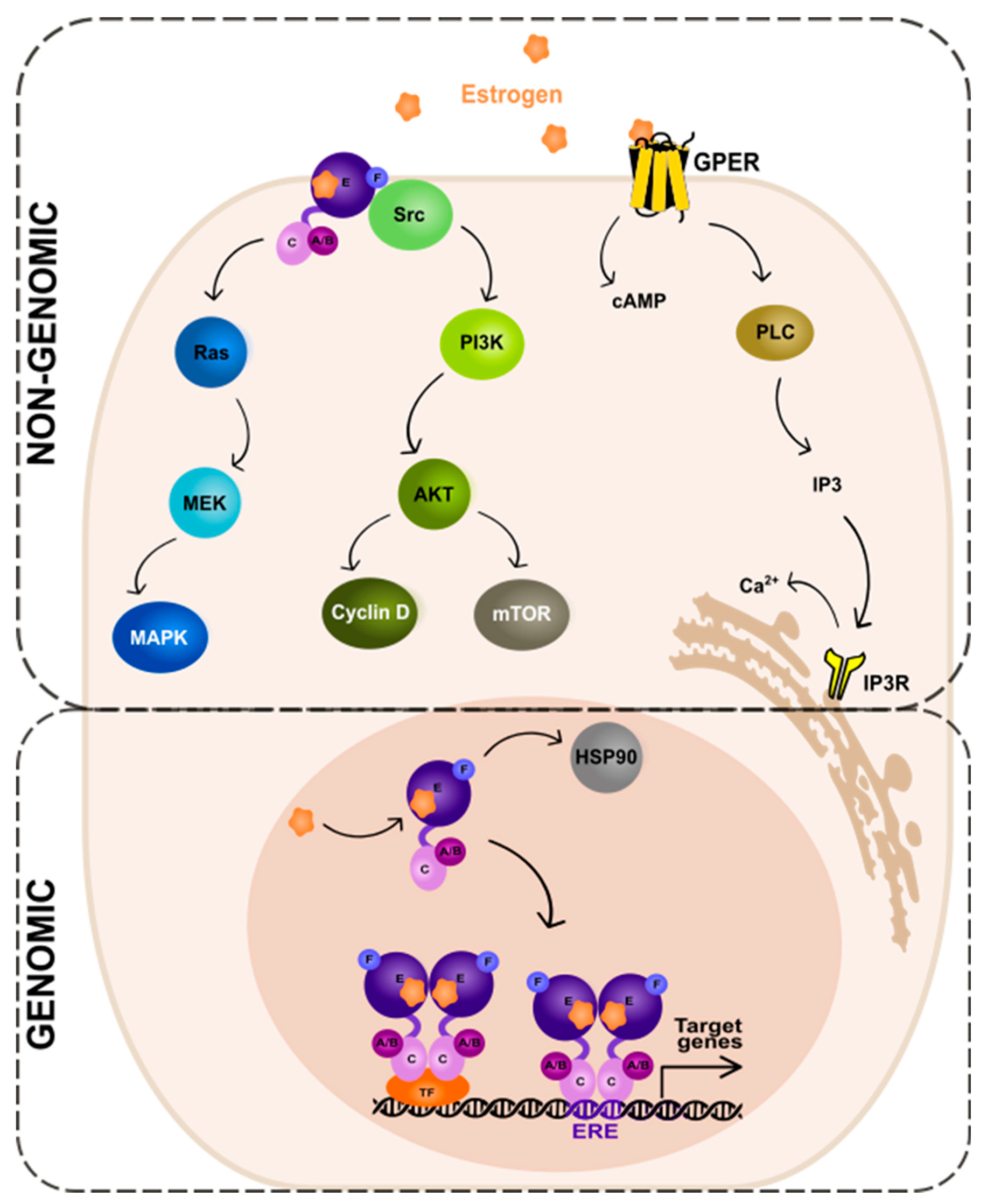
Figure 2. Genomic and non-genomic action of estrogen receptor (ER). Abbreviations: A/B—the domains of ER located at the N-terminus of estrogen receptor (N); C—the domain containing zinc-finger; E/F—the domains located at the C-terminus; GPER—G protein-coupled estrogen receptor 1; PI3K—phosphatidylinositide 3-kinase; AKT—serine/threonine kinase; mTOR—the mammalian target of rapamycin; cAMP—cyclic adenosine monophosphate; PLC—phospholipase C; IP3—inositol trisphosphate; IP3R—inositol trisphosphate receptor; HSP90—heat shock protein 90; ERE—estrogen-response element; TF—transcription factor.
In the genomic ER signaling, the complexes of estrogen and the estrogen receptor (ER) are translocated to the nucleus. There, they can indirectly bind to the DNA-binding transcription factors (TFs) via the TF response elements, using protein–protein interactions. By interactions with the coactivator proteins, ER can control the activation of TFs [70]. Nuclear ER can, for example, interact with specificity protein 1 (Sp1) and nuclear factor kappa B (NF-κB) via the so-called “non-classical” activity [71]. The target genes to be modified by the indirect action of ER do not contain the estrogen-response elements (EREs) in their promoters’ regions.
The expression of genes that contain EREs can be changed via the direct genomic action of ER. The receptor undergoes a ligand-specific conformational shift after ligand attachment to the ER, enabling the receptor to be released from the heat shock protein complex (HSP90) [72][73]. HSP90 is a molecular chaperone, which protects unbound ER from degradation [74]. Eckert and colleagues have shown nearly 40 years ago [75] that ERα without a ligand is a constantly degraded, short-lived protein (a half-life of 4–5 h). The ERα synthesis and turnover rates were determined in the MCF-7 breast cancer cells. For complete ER-mediated transcriptional activation, histone acetyltransferases (HATs) are necessary. HATs activities enable nucleosome repositioning, chromatin opening, and engagement with the general transcription machinery centered on RNA polymerase II. For example, the p300/CBP acetylates elements of the basal transcription machinery and interacts with other HATs, such as PCAF [76][77][78].
Importantly, there is functional crosstalk between the estrogen receptor and other steroid hormone receptors, such as the progesterone receptor (PR), glucocorticoid receptor (GR), and androgen receptor (AR) in breast cancer cells [79][80][81][82][83][84][85], as well as other cancer cell types, like endometrial [86][87]. These hormones have similar DNA-binding preferences and their genomic binding orchestrates the recruitment of other TFs and chromatin remodeling complexes [88][89][90]. Clearly, ER does not function on its own, and its action can be altered by other receptors. For instance, while co-expressed in BC cells, PR is not only an ERα-induced target gene but also an ERα-associated protein, which redirects ERα-associated chromatin binding events [81][84]. This, in turn, results in a unique gene expression in BC cells and is associated with patients’ outcome [81]; however, the mechanistic insight into ER modulation via PR for better BC management needs to be elucidated [84]. AR has also been shown to play a role in ER genomic binding in breast cancer [82] and its function and targeted therapies across BC subtypes have recently been reviewed in [91]. Additionally, in breast cancer cells, the liganded glucocorticoid receptor represses an ERα-regulated transcriptional program [92]. Tonsing-Carter and colleagues [93] have shown that GR modulation decreases ER-positive BC cells’ proliferation and suppresses ER (both wild-type and mutant) chromatin association.
3.2. Non-Genomic Action of ER
In the non-genomic ER signaling (Figure 2; top panel), estrogen binds to the receptor (mbER, i.e., the ER that is situated at the plasma membrane [94] or GPER1, the G-protein-coupled estrogen receptor 1 [95]). This mechanism starts outside of the nucleus and is unrelated to the transcription. The estrogen and ER complexes predominantly activate the kinase pathways. These include MAPK (mitogen-activated protein kinase) via the so-called Ras-Raf-MEK-MAPK pathway and PI3K (phosphatidylinositide 3-kinase)/AKT (serine/threonine kinase) via the PI3K-AKT-mammalian target of rapamycin (mTOR) pathway. The activation of the MAPK signaling pathway by estrogen has been studied in various cell types, including breast cancer [96], neuroblastoma [97], and endothelial [98] cells. Upon estrogen binding to the receptor, the small guanine nucleotide-binding protein—Ras (GTPase) is activated. Next, another protein kinase—Raf is activated, which then phosphorylates the MEK protein. This in turn leads to the phosphorylation and activation of MAPK. As a consequence, several TFs of the activating protein 1 family, e.g., c-Jun and c-Fos, are activated. These then regulate the transcription of the target genes [99][100][101].
An alternate pathway—the PI3K-AKT-mTOR, activated by mbER, relies on the direct contact of ER with different proteins; first, the tyrosine kinase Src, then the phosphatidylinositol 3-kinase (PI3K), and the AKT proteins that regulate the mTOR pathway. The AKT-dependent mechanisms of mTOR regulation is a key intracellular system that signals cellular growth and survival, and the hyperactivation of it is involved in the carcinogenesis of the ER-positive BC as well as the resistance to endocrine therapy [102].
The activation of receptors connected to G-proteins is another well-known non-genomic effect of sex hormones. GPER1 is a transmembrane receptor, which, once activated by estrogen or its derivatives, triggers the downstream signaling pathways that can affect a variety of physiological processes [95], such as cell proliferation, angiogenesis, and inflammation. The action of GPER1 generates cyclic adenosine monophosphate from the activation of the adenylate cyclase enzyme. Moreover, upon activation of a receptor by estrogen, the PLC (phospholipase C) enzyme is activated. The activated PLC cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into two secondary messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 diffuses into the cytoplasm and binds to the IP3 receptors on the endoplasmic reticulum, leading to the release of Ca2+ from the endoplasmic reticulum into the cytoplasm. This results in a rapid increase in intracellular Ca2+ concentration, which can trigger a variety of downstream signaling events. DAG, on the other hand, remains in the plasma membrane and activates protein kinase C (PKC), another downstream signaling molecule that can regulate various cellular processes [103].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15194689
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48.
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66.
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23.
- Rakha, E.A.; Reis-Filho, J.S.; Baehner, F.; Dabbs, D.J.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Breast Cancer Prognostic Classification in the Molecular Era: The Role of Histological Grade. Breast Cancer Res. 2010, 12, 207.
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. ESMO Guidelines Committee Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1674.
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the Treatment of Women with Early Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223.
- Cao, S.-S.; Lu, C.-T. Recent Perspectives of Breast Cancer Prognosis and Predictive Factors. Oncol. Lett. 2016, 12, 3674–3678.
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752.
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874.
- Dias, K.; Dvorkin-Gheva, A.; Hallett, R.M.; Wu, Y.; Hassell, J.; Pond, G.R.; Levine, M.; Whelan, T.; Bane, A.L. Claudin-Low Breast Cancer; Clinical & Pathological Characteristics. PLoS ONE 2017, 12, e0168669.
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer; Exon Publications: Brisbane, Australia, 2022.
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering Breast Cancer: From Biology to the Clinic. Cell 2023, 186, 1708–1728.
- Yue, W.; Wang, J.-P.; Li, Y.; Fan, P.; Liu, G.; Zhang, N.; Conaway, M.; Wang, H.; Korach, K.S.; Bocchinfuso, W.; et al. Effects of Estrogen on Breast Cancer Development: Role of Estrogen Receptor Independent Mechanisms. Int. J. Cancer 2010, 127, 1748–1757.
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63.
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen Signaling and Its Potential as a Target for Therapy in Ovarian Cancer. Cancers 2020, 12, 1647.
- Patel, S.; Homaei, A.; Raju, A.B.; Meher, B.R. Estrogen: The Necessary Evil for Human Health, and Ways to Tame It. Biomed. Pharmacother. 2018, 102, 403–411.
- Gallez, A.; Dias Da Silva, I.; Wuidar, V.; Foidart, J.-M.; Péqueux, C. Estetrol and Mammary Gland: Friends or Foes? J. Mammary Gland Biol. Neoplasia 2021, 26, 297–308.
- Baker, M.E. What Are the Physiological Estrogens? Steroids 2013, 78, 337–340.
- Fuentes, N.; Silveyra, P. Estrogen Receptor Signaling Mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170.
- Miller, W.L. Steroidogenesis: Unanswered Questions. Trends Endocrinol. Metab. 2017, 28, 771–793.
- Savolainen-Peltonen, H.; Vihma, V.; Leidenius, M.; Wang, F.; Turpeinen, U.; Hämäläinen, E.; Tikkanen, M.J.; Mikkola, T.S. Breast Adipose Tissue Estrogen Metabolism in Postmenopausal Women with or without Breast Cancer. J. Clin. Endocrinol. Metab. 2014, 99, E2661–E2667.
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in Male Physiology. Physiol. Rev. 2017, 97, 995–1043.
- Barros, R.P.A.; Gustafsson, J.-Å. Estrogen Receptors and the Metabolic Network. Cell Metab. 2011, 14, 289–299.
- Knowlton, A.A.; Lee, A.R. Estrogen and the Cardiovascular System. Pharmacol. Ther. 2012, 135, 54–70.
- Cersosimo, M.G.; Benarroch, E.E. Estrogen Actions in the Nervous System: Complexity and Clinical Implications. Neurology 2015, 85, 263–273.
- Bernelot Moens, S.J.; Schnitzler, G.R.; Nickerson, M.; Guo, H.; Ueda, K.; Lu, Q.; Aronovitz, M.J.; Nickerson, H.; Baur, W.E.; Hansen, U.; et al. Rapid Estrogen Receptor Signaling Is Essential for the Protective Effects of Estrogen against Vascular Injury. Circulation 2012, 126, 1993–2004.
- Schierbeck, L.L.; Rejnmark, L.; Tofteng, C.L.; Stilgren, L.; Eiken, P.; Mosekilde, L.; Køber, L.; Jensen, J.-E.B. Effect of Hormone Replacement Therapy on Cardiovascular Events in Recently Postmenopausal Women: Randomised Trial. BMJ 2012, 345, e6409.
- Hodis, H.N.; Mack, W.J.; Henderson, V.W.; Shoupe, D.; Budoff, M.J.; Hwang-Levine, J.; Li, Y.; Feng, M.; Dustin, L.; Kono, N.; et al. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N. Engl. J. Med. 2016, 374, 1221–1231.
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus Progestin and the Risk of Coronary Heart Disease. N. Engl. J. Med. 2003, 349, 523–534.
- Kurtanović, N.; Tomašević, N.; Matić, S.; Proia, E.; Sabatino, M.; Antonini, L.; Mladenović, M.; Ragno, R. Human Estrogen Receptor Alpha Antagonists, Part 3: 3-D Pharmacophore and 3-D QSAR Guided Brefeldin A Hit-to-Lead Optimization toward New Breast Cancer Suppressants. Molecules 2022, 27, 2823.
- Dunnwald, L.K.; Rossing, M.A.; Li, C.I. Hormone Receptor Status, Tumor Characteristics, and Prognosis: A Prospective Cohort of Breast Cancer Patients. Breast Cancer Res. 2007, 9, R6.
- Filardo, E.J.; Thomas, P. Minireview: G Protein-Coupled Estrogen Receptor-1, GPER-1: Its Mechanism of Action and Role in Female Reproductive Cancer, Renal and Vascular Physiology. Endocrinology 2012, 153, 2953–2962.
- Arao, Y.; Korach, K.S. The Physiological Role of Estrogen Receptor Functional Domains. Essays Biochem. 2021, 65, 867–875.
- Patel, H.K.; Bihani, T. Selective Estrogen Receptor Modulators (SERMs) and Selective Estrogen Receptor Degraders (SERDs) in Cancer Treatment. Pharmacol. Ther. 2018, 186, 1–24.
- Schubert, M.; Brunet, F.; Paris, M.; Bertrand, S.; Benoit, G.; Laudet, V. Nuclear Hormone Receptor Signaling in Amphioxus. Dev. Genes Evol. 2008, 218, 651–665.
- Holland, L.Z.; Albalat, R.; Azumi, K.; Benito-Gutiérrez, E.; Blow, M.J.; Bronner-Fraser, M.; Brunet, F.; Butts, T.; Candiani, S.; Dishaw, L.J.; et al. The Amphioxus Genome Illuminates Vertebrate Origins and Cephalochordate Biology. Genome Res. 2008, 18, 1100–1111.
- Keay, J.; Bridgham, J.T.; Thornton, J.W. The Octopus Vulgaris Estrogen Receptor Is a Constitutive Transcriptional Activator: Evolutionary and Functional Implications. Endocrinology 2006, 147, 3861–3869.
- Matsumoto, T.; Nakamura, A.M.; Mori, K.; Akiyama, I.; Hirose, H.; Takahashi, Y. Oyster Estrogen Receptor: cDNA Cloning and Immunolocalization. Gen. Comp. Endocrinol. 2007, 151, 195–201.
- Keay, J.; Thornton, J.W. Hormone-Activated Estrogen Receptors in Annelid Invertebrates: Implications for Evolution and Endocrine Disruption. Endocrinology 2009, 150, 1731–1738.
- Barnett, D.H. Identification and Characterization of Estrogen Receptor-Regulated Gene Expression Programs; University of Illinois: Urbana, IL, USA, 2010.
- Callard, G.V.; Tarrant, A.M.; Novillo, A.; Yacci, P.; Ciaccia, L.; Vajda, S.; Chuang, G.-Y.; Kozakov, D.; Greytak, S.R.; Sawyer, S.; et al. Evolutionary Origins of the Estrogen Signaling System: Insights from Amphioxus. J. Steroid Biochem. Mol. Biol. 2011, 127, 176–188.
- Thornton, J.W.; Need, E.; Crews, D. Resurrecting the Ancestral Steroid Receptor: Ancient Origin of Estrogen Signaling. Science 2003, 301, 1714–1717.
- Green, S.; Walter, P.; Kumar, V.; Krust, A.; Bornert, J.M.; Argos, P.; Chambon, P. Human Oestrogen Receptor cDNA: Sequence, Expression and Homology to v-Erb-A. Nature 1986, 320, 134–139.
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a Novel Receptor Expressed in Rat Prostate and Ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930.
- Thornton, J.W. Evolution of Vertebrate Steroid Receptors from an Ancestral Estrogen Receptor by Ligand Exploitation and Serial Genome Expansions. Proc. Natl. Acad. Sci. USA 2001, 98, 5671–5676.
- Mader, S.; Chambon, P.; White, J.H. Defining a Minimal Estrogen Receptor DNA Binding Domain. Nucleic Acids Res. 1993, 21, 1125–1132.
- Zwart, W.; de Leeuw, R.; Rondaij, M.; Neefjes, J.; Mancini, M.A.; Michalides, R. The Hinge Region of the Human Estrogen Receptor Determines Functional Synergy between AF-1 and AF-2 in the Quantitative Response to Estradiol and Tamoxifen. J. Cell Sci. 2010, 123, 1253–1261.
- Montano, M.M.; Müller, V.; Trobaugh, A.; Katzenellenbogen, B.S. The Carboxy-Terminal F Domain of the Human Estrogen Receptor: Role in the Transcriptional Activity of the Receptor and the Effectiveness of Antiestrogens as Estrogen Antagonists. Mol. Endocrinol. 1995, 9, 814–825.
- Koide, A.; Zhao, C.; Naganuma, M.; Abrams, J.; Deighton-Collins, S.; Skafar, D.F.; Koide, S. Identification of Regions within the F Domain of the Human Estrogen Receptor Alpha That Are Important for Modulating Transactivation and Protein-Protein Interactions. Mol. Endocrinol. 2007, 21, 829–842.
- Moore, J.T.; McKee, D.D.; Slentz-Kesler, K.; Moore, L.B.; Jones, S.A.; Horne, E.L.; Su, J.L.; Kliewer, S.A.; Lehmann, J.M.; Willson, T.M. Cloning and Characterization of Human Estrogen Receptor Beta Isoforms. Biochem. Biophys. Res. Commun. 1998, 247, 75–78.
- Flouriot, G.; Brand, H.; Denger, S.; Metivier, R.; Kos, M.; Reid, G.; Sonntag-Buck, V.; Gannon, F. Identification of a New Isoform of the Human Estrogen Receptor-Alpha (hER-Alpha) That Is Encoded by Distinct Transcripts and That Is Able to Repress hER-Alpha Activation Function 1. EMBO J. 2000, 19, 4688–4700.
- Wang, Z.; Zhang, X.; Shen, P.; Loggie, B.W.; Chang, Y.; Deuel, T.F. Identification, Cloning, and Expression of Human Estrogen Receptor-α36, a Novel Variant of Human Estrogen Receptor-α66. Biochem. Biophys. Res. Commun. 2005, 336, 1023–1027.
- Warner, M.; Fan, X.; Strom, A.; Wu, W.; Gustafsson, J.-Å. 25 Years of ERβ: A Personal Journey. J. Mol. Endocrinol. 2021, 68, R1–R9.
- Taylor, A.H.; Al-Azzawi, F. Immunolocalisation of Oestrogen Receptor Beta in Human Tissues. J. Mol. Endocrinol. 2000, 24, 145–155.
- Yu, K.; Huang, Z.-Y.; Xu, X.-L.; Li, J.; Fu, X.-W.; Deng, S.-L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724.
- Lindberg, M.K.; Movérare, S.; Skrtic, S.; Gao, H.; Dahlman-Wright, K.; Gustafsson, J.-A.; Ohlsson, C. Estrogen Receptor (ER)-Beta Reduces ERalpha-Regulated Gene Transcription, Supporting a “Ying Yang” Relationship between ERalpha and ERbeta in Mice. Mol. Endocrinol. 2003, 17, 203–208.
- Matthews, J.; Wihlén, B.; Tujague, M.; Wan, J.; Ström, A.; Gustafsson, J.-A. Estrogen Receptor (ER) β Modulates ERα-Mediated Transcriptional Activation by Altering the Recruitment of c-Fos and c-Jun to Estrogen-Responsive Promoters. Mol. Endocrinol. 2006, 20, 534–543.
- Lazennec, G.; Bresson, D.; Lucas, A.; Chauveau, C.; Vignon, F. ER Beta Inhibits Proliferation and Invasion of Breast Cancer Cells. Endocrinology 2001, 142, 4120–4130.
- Sotoca, A.M.C.; van den Berg, H.; Vervoort, J.; van der Saag, P.; Ström, A.; Gustafsson, J.-A.; Rietjens, I.; Murk, A.J. Influence of Cellular ERalpha/ERbeta Ratio on the ERalpha-Agonist Induced Proliferation of Human T47D Breast Cancer Cells. Toxicol. Sci. 2008, 105, 303–311.
- Heo, K.-S. Regulation of Post-Translational Modification in Breast Cancer Treatment. BMB Rep. 2019, 52, 113–118.
- Zhao, L.; Zhou, S.; Gustafsson, J.-Å. Nuclear Receptors: Recent Drug Discovery for Cancer Therapies. Endocr. Rev. 2019, 40, 1207–1249.
- Shanle, E.K.; Xu, W. Selectively Targeting Estrogen Receptors for Cancer Treatment. Adv. Drug Deliv. Rev. 2010, 62, 1265–1276.
- Carmeci, C.; Thompson, D.A.; Ring, H.Z.; Francke, U.; Weigel, R.J. Identification of a Gene (GPR30) with Homology to the G-Protein-Coupled Receptor Superfamily Associated with Estrogen Receptor Expression in Breast Cancer. Genomics 1997, 45, 607–617.
- Lafferty, A.R.; Torpy, D.J.; Stowasser, M.; Taymans, S.E.; Lin, J.P.; Huggard, P.; Gordon, R.D.; Stratakis, C.A. A Novel Genetic Locus for Low Renin Hypertension: Familial Hyperaldosteronism Type II Maps to Chromosome 7 (7p22). J. Med. Genet. 2000, 37, 831–835.
- Xu, S.; Yu, S.; Dong, D.; Lee, L.T.O. G Protein-Coupled Estrogen Receptor: A Potential Therapeutic Target in Cancer. Front. Endocrinol. 2019, 10, 725.
- Olde, B.; Leeb-Lundberg, L.M.F. GPR30/GPER1: Searching for a Role in Estrogen Physiology. Trends Endocrinol. Metab. 2009, 20, 409–416.
- Filardo, E.J.; Quinn, J.A.; Sabo, E. Association of the Membrane Estrogen Receptor, GPR30, with Breast Tumor Metastasis and Transactivation of the Epidermal Growth Factor Receptor. Steroids 2008, 73, 870–873.
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The Many Faces of Estrogen Signaling. Biochem. Med. 2014, 24, 329–342.
- Safe, S.; Kim, K. Non-Classical Genomic Estrogen Receptor (ER)/specificity Protein and ER/activating Protein-1 Signaling Pathways. J. Mol. Endocrinol. 2008, 41, 263–275.
- Björnström, L.; Sjöberg, M. Estrogen Receptor-Dependent Activation of AP-1 via Non-Genomic Signalling. Nucl. Recept. 2004, 2, 3.
- Powell, E.; Wang, Y.; Shapiro, D.J.; Xu, W. Differential Requirements of Hsp90 and DNA for the Formation of Estrogen Receptor Homodimers and Heterodimers. J. Biol. Chem. 2010, 285, 16125–16134.
- Le Romancer, M.; Poulard, C.; Cohen, P.; Sentis, S.; Renoir, J.-M.; Corbo, L. Cracking the Estrogen Receptor’s Posttranslational Code in Breast Tumors. Endocr. Rev. 2011, 32, 597–622.
- Dhamad, A.E.; Zhou, Z.; Zhou, J.; Du, Y. Systematic Proteomic Identification of the Heat Shock Proteins (Hsp) That Interact with Estrogen Receptor Alpha (ERα) and Biochemical Characterization of the ERα-Hsp70 Interaction. PLoS ONE 2016, 11, e0160312.
- Eckert, R.L.; Mullick, A.; Rorke, E.A.; Katzenellenbogen, B.S. Estrogen Receptor Synthesis and Turnover in MCF-7 Breast Cancer Cells Measured by a Density Shift Technique. Endocrinology 1984, 114, 629–637.
- Wilson, B.J.; Tremblay, A.M.; Deblois, G.; Sylvain-Drolet, G.; Giguère, V. An Acetylation Switch Modulates the Transcriptional Activity of Estrogen-Related Receptor Alpha. Mol. Endocrinol. 2010, 24, 1349–1358.
- Jin, Q.; Yu, L.-R.; Wang, L.; Zhang, Z.; Kasper, L.H.; Lee, J.-E.; Wang, C.; Brindle, P.K.; Dent, S.Y.R.; Ge, K. Distinct Roles of GCN5/PCAF-Mediated H3K9ac and CBP/p300-Mediated H3K18/27ac in Nuclear Receptor Transactivation. EMBO J. 2011, 30, 249–262.
- Murakami, S.; Nagari, A.; Kraus, W.L. Dynamic Assembly and Activation of Estrogen Receptor α Enhancers through Coregulator Switching. Genes Dev. 2017, 31, 1535–1548.
- Buxant, F.; Engohan-Aloghe, C.; Noël, J.-C. Estrogen Receptor, Progesterone Receptor, and Glucocorticoid Receptor Expression in Normal Breast Tissue, Breast in Situ Carcinoma, and Invasive Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 254–257.
- Pan, D.; Kocherginsky, M.; Conzen, S.D. Activation of the Glucocorticoid Receptor Is Associated with Poor Prognosis in Estrogen Receptor-Negative Breast Cancer. Cancer Res. 2011, 71, 6360–6370.
- Mohammed, H.; Russell, I.A.; Stark, R.; Rueda, O.M.; Hickey, T.E.; Tarulli, G.A.; Serandour, A.A.; Birrell, S.N.; Bruna, A.; Saadi, A.; et al. Progesterone Receptor Modulates ERα Action in Breast Cancer. Nature 2015, 523, 313–317.
- D’Amato, N.C.; Gordon, M.A.; Babbs, B.; Spoelstra, N.S.; Carson Butterfield, K.T.; Torkko, K.C.; Phan, V.T.; Barton, V.N.; Rogers, T.J.; Sartorius, C.A.; et al. Cooperative Dynamics of AR and ER Activity in Breast Cancer. Mol. Cancer Res. 2016, 14, 1054–1067.
- Hu, D.G.; Selth, L.A.; Tarulli, G.A.; Meech, R.; Wijayakumara, D.; Chanawong, A.; Russell, R.; Caldas, C.; Robinson, J.L.L.; Carroll, J.S.; et al. Androgen and Estrogen Receptors in Breast Cancer Coregulate Human UDP-Glucuronosyltransferases 2B15 and 2B17. Cancer Res. 2016, 76, 5881–5893.
- Singhal, H.; Greene, M.E.; Tarulli, G.; Zarnke, A.L.; Bourgo, R.J.; Laine, M.; Chang, Y.-F.; Ma, S.; Dembo, A.G.; Raj, G.V.; et al. Genomic Agonism and Phenotypic Antagonism between Estrogen and Progesterone Receptors in Breast Cancer. Sci. Adv. 2016, 2, e1501924.
- West, D.C.; Pan, D.; Tonsing-Carter, E.Y.; Hernandez, K.M.; Pierce, C.F.; Styke, S.C.; Bowie, K.R.; Garcia, T.I.; Kocherginsky, M.; Conzen, S.D. GR and ER Coactivation Alters the Expression of Differentiation Genes and Associates with Improved ER+ Breast Cancer Outcome. Mol. Cancer Res. 2016, 14, 707–719.
- Tangen, I.L.; Veneris, J.T.; Halle, M.K.; Werner, H.M.; Trovik, J.; Akslen, L.A.; Salvesen, H.B.; Conzen, S.D.; Fleming, G.F.; Krakstad, C. Expression of Glucocorticoid Receptor Is Associated with Aggressive Primary Endometrial Cancer and Increases from Primary to Metastatic Lesions. Gynecol. Oncol. 2017, 147, 672–677.
- Vahrenkamp, J.M.; Yang, C.-H.; Rodriguez, A.C.; Almomen, A.; Berrett, K.C.; Trujillo, A.N.; Guillen, K.P.; Welm, B.E.; Jarboe, E.A.; Janat-Amsbury, M.M.; et al. Clinical and Genomic Crosstalk between Glucocorticoid Receptor and Estrogen Receptor α In Endometrial Cancer. Cell Rep. 2018, 22, 2995–3005.
- Jeong, K.W.; Lee, Y.-H.; Stallcup, M.R. Recruitment of the SWI/SNF Chromatin Remodeling Complex to Steroid Hormone-Regulated Promoters by Nuclear Receptor Coactivator Flightless-I. J. Biol. Chem. 2009, 284, 29298–29309.
- Ballaré, C.; Castellano, G.; Gaveglia, L.; Althammer, S.; González-Vallinas, J.; Eyras, E.; Le Dily, F.; Zaurin, R.; Soronellas, D.; Vicent, G.P.; et al. Nucleosome-Driven Transcription Factor Binding and Gene Regulation. Mol. Cell 2013, 49, 67–79.
- Le Dily, F.; Vidal, E.; Cuartero, Y.; Quilez, J.; Nacht, A.S.; Vicent, G.P.; Carbonell-Caballero, J.; Sharma, P.; Villanueva-Cañas, J.L.; Ferrari, R.; et al. Hormone-Control Regions Mediate Steroid Receptor-Dependent Genome Organization. Genome Res. 2019, 29, 29–39.
- Kolyvas, E.A.; Caldas, C.; Kelly, K.; Ahmad, S.S. Androgen Receptor Function and Targeted Therapeutics across Breast Cancer Subtypes. Breast Cancer Res. 2022, 24, 79.
- Yang, F.; Ma, Q.; Liu, Z.; Li, W.; Tan, Y.; Jin, C.; Ma, W.; Hu, Y.; Shen, J.; Ohgi, K.A.; et al. Glucocorticoid Receptor:MegaTrans Switching Mediates the Repression of an ERα-Regulated Transcriptional Program. Mol. Cell 2017, 66, 321–331.e6.
- Tonsing-Carter, E.; Hernandez, K.M.; Kim, C.R.; Harkless, R.V.; Oh, A.; Bowie, K.R.; West-Szymanski, D.C.; Betancourt-Ponce, M.A.; Green, B.D.; Lastra, R.R.; et al. Glucocorticoid Receptor Modulation Decreases ER-Positive Breast Cancer Cell Proliferation and Suppresses Wild-Type and Mutant ER Chromatin Association. Breast Cancer Res. 2019, 21, 82.
- Levin, E.R. Plasma Membrane Estrogen Receptors. Trends Endocrinol. Metab. 2009, 20, 477–482.
- Pupo, M.; Maggiolini, M.; Musti, A.M. GPER Mediates Non-Genomic Effects of Estrogen. Methods Mol. Biol. 2016, 1366, 471–488.
- Migliaccio, A.; Di Domenico, M.; Castoria, G.; de Falco, A.; Bontempo, P.; Nola, E.; Auricchio, F. Tyrosine kinase/p21ras/MAP-Kinase Pathway Activation by Estradiol-Receptor Complex in MCF-7 Cells. EMBO J. 1996, 15, 1292–1300.
- Watters, J.J.; Campbell, J.S.; Cunningham, M.J.; Krebs, E.G.; Dorsa, D.M. Rapid Membrane Effects of Steroids in Neuroblastoma Cells: Effects of Estrogen on Mitogen Activated Protein Kinase Signalling Cascade and c-Fos Immediate Early Gene Transcription. Endocrinology 1997, 138, 4030–4033.
- Chen, Z.; Yuhanna, I.S.; Galcheva-Gargova, Z.; Karas, R.H.; Mendelsohn, M.E.; Shaul, P.W. Estrogen Receptor Alpha Mediates the Nongenomic Activation of Endothelial Nitric Oxide Synthase by Estrogen. J. Clin. Invest. 1999, 103, 401–406.
- Zivadinovic, D.; Watson, C.S. Membrane Estrogen Receptor-Alpha Levels Predict Estrogen-Induced ERK1/2 Activation in MCF-7 Cells. Breast Cancer Res. 2005, 7, R130–R144.
- Wang, Z.; Zhang, X.; Shen, P.; Loggie, B.W.; Chang, Y.; Deuel, T.F. A Variant of Estrogen Receptor-, hER-36: Transduction of Estrogen- and Antiestrogen-Dependent Membrane-Initiated Mitogenic Signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 9063–9068.
- Rocca, A.; Braga, L.; Volpe, M.C.; Maiocchi, S.; Generali, D. The Predictive and Prognostic Role of RAS-RAF-MEK-ERK Pathway Alterations in Breast Cancer: Revision of the Literature and Comparison with the Analysis of Cancer Genomic Datasets. Cancers 2022, 14, 5306.
- Ciruelos Gil, E.M. Targeting the PI3K/AKT/mTOR Pathway in Estrogen Receptor-Positive Breast Cancer. Cancer Treat. Rev. 2014, 40, 862–871.
- Saczko, J.; Michel, O.; Chwiłkowska, A.; Sawicka, E.; Mączyńska, J.; Kulbacka, J. Estrogen Receptors in Cell Membranes: Regulation and Signaling. In Transport Across Natural and Modified Biological Membranes and Its Implications in Physiology and Therapy; Kulbacka, J., Satkauskas, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 93–105. ISBN 9783319568959.
This entry is offline, you can click here to edit this entry!
