In EC, the expression of ErbB receptors is significantly different, compared with the premenopausal and postmenopausal endometrium, mainly because of the increased transcriptional activity of ErbB encoding genes in EC cells. Moreover, there are some differences in ErbB-2 receptor profile among EC subgroups that could be explained by the alterations in pathophysiology and clinical behavior of various EC histologic subtypes. The fact that ErbB-2 receptor expression is more common in aggressive EC histologic subtypes (papillary serous and clear cell) could indicate a future role of ErbB-targeted therapies in well-defined EC subgroups with overexpression of ErbB receptors.
- ErbB receptors
- EGF system
- physiology
- signaling pathways
1. Introduction
2. Physiology of ErbB Receptors
2.1. ErbB Receptors
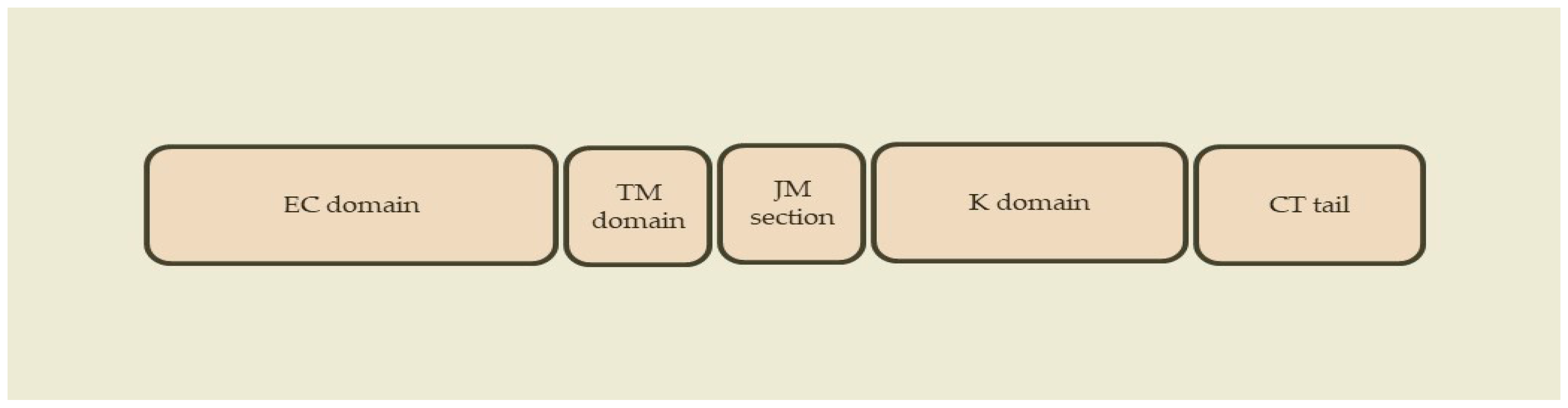
2.2. ErbB Ligands
2.3. Receptor Homodimerization and Heterodimerization
2.4. Intracellular Tyrosine Kinase Activation
3. ErbB Receptors in Endometrial Cancer
3.1. Profile of ErbB Receptors in Endometrial Cancer
3.2. Clinical Role in Endometrial Cancer
This entry is adapted from the peer-reviewed paper 10.3390/epigenomes7040024
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- WHO Globocan. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020; International Agency for Research on Cancer: Lyon, France, 2020.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
- Gitsch, G.; Hanzal, E.; Jensen, D.; Hacker, N.F. Endometrial cancer in premenopausal women 45 years and younger. Obstet. Gynecol. 1995, 85, 504–508.
- Duska, L.R.; Garrett, A.; Rueda, B.R.; Haas, J.; Chang, Y.; Fuller, A.F. Endometrial Cancer in Women 40 Years Old or Younger. Gynecol. Oncol. 2001, 83, 388–393.
- Erkanli, S.; Ayhan, A. Fertility-sparing therapy in young women with endometrial cancer: 2010 update. Int. J. Gynecol. Cancer 2010, 20, 1170–1187.
- Androutsopoulos, G. Current treatment options in patients with endometrial cancer. J. Community Med. Health Educ. 2012, 2, e113.
- Androutsopoulos, G.; Decavalas, G. Management of endometrial cancer. Int. J. Transl. Community Dis. 2013, 1, 101.
- Koufopoulos, N.; Carrer, D.; Koureas, N.; Sofopoulos, M.; Paraoulakis, I.; Androutsopoulos, G.; Arnogiannaki, N.; Zygouris, D.; Derdelis, G.; Terzakis, E. Pathological data on 19 cases of endometrioid carcinoma of the endometrium in women of reproductive age. Int. J. Gynecol. Cancer 2013, 23 (Suppl. 1), 322.
- Androutsopoulos, G.; Decavalas, G. Endometrial cancer: Current treatment strategies. World J. Oncol. Res. 2014, 1, 1–4.
- Androutsopoulos, G.; Michail, G.; Adonakis, G.; Decavalas, G. Current treatment approach of endometrial cancer. Int. J. Clin. Ther. Diagn. 2015, S1, 8–11.
- Androutsopoulos, G.; Adonakis, G.; Decavalas, G. Present and future in endometrial cancer treatment. Obstet. Gynecol. Int. J. 2015, 2, 00031.
- ACOG. ACOG practice bulletin No. 149: Endometrial cancer. Obstet. Gynecol. 2015, 125, 1006–1026.
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; Gonzalez-Martin, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41.
- Androutsopoulos, G.; Michail, G.; Decavalas, G. New insights in endometrial cancer treatment. Clin. Oncol. Endometrial Cancer 2016, 1, 1040.
- Androutsopoulos, G.; Decavalas, G. Standard and novel therapies in endometrial cancer. J. Gynecol. Women’s Health 2016, 1, 555564.
- Androutsopoulos, G.; Kotsopoulos, I.; Decavalas, G. Fertility preservation in young patients with endometrial cancer. World J. Oncol. Res. 2016, 3, 36–39.
- Sundar, S.; Balega, J.; Crosbie, E.; Drake, A.; Edmondson, R.; Fotopoulou, C.; Gallos, I.; Ganesan, R.; Gupta, J.; Johnson, N.; et al. BGCS uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 71–97.
- Androutsopoulos, G.; Kotsopoulos, I.; Korompelis, P.; Michail, G.; Adonakis, G.; Decavalas, G. Systematic lymphadenectomy or sentinel lymph node dissection in endometrial cancer: A clinical dilemma. Hell. J. Obst. Gynecol. 2017, 16, 14–19.
- Androutsopoulos, G.; Kotsopoulos, I.; Adonakis, G.; Decavalas, G. Conservative management of young patients with early stage endometrial cancer. J. Gynecol. Women’s Health 2017, 2, 555586.
- Androutsopoulos, G.; Adonakis, G.; Decavalas, G. ErbB targeted therapy in endometrial cancer. In Endometrial Cancer: Current Epidemiology, Detection and Management; Farghaly, S., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2014.
- Androutsopoulos, G.; Kotsopoulos, I.; Korompelis, P.; Michail, G.; Adonakis, G.; Decavalas, G. Conservative therapeutic approach in young patients with endometrial cancer: Is it really possible? Hell. J. Obst. Gynecol. 2017, 16, 7–23.
- Michail, G.D. Endometrial Cancer-Diagnosis. Int. J. Clin. Ther. Diagn. 2015, 1, 17–27.
- Weimer, J.; Hüttmann, M.; Nusilati, A.; Andreas, S.; Röseler, J.; Tribian, N.; Rogmans, C.; Stope, M.B.; Dahl, E.; Mustea, A.; et al. Fluorescence in situ hybridization test for detection of endometrial carcinoma cells by non-invasive vaginal swab. J. Cell. Mol. Med. 2023, 27, 379–391.
- Pouliakis, A.; Damaskou, V.; Margari, N.; Karakitsou, E.; Pergialiotis, V.; Valasoulis, G.; Michail, G.; Chrelias, C.; Chrelias, G.; Sioulas, V.; et al. Artificial Intelligence and Image Analysis for the Identification of Endometrial Malignancies: A Comparative Study. In Research Anthology on Medical Informatics in Breast and Cervical Cancer; I.R. Management Association, Ed.; IGI Global: Hershey, PA, USA, 2023; pp. 1–30.
- Pouliakis, A.; Damaskou, V.; Margari, N.; Karakitsou, E.; Pergialiotis, V.; Valasoulis, G.; Michail, G.; Chrelias, C.; Chrelias, G.; Sioulas, V.; et al. Artificial Intelligence and Image Analysis for the Identification of Endometrial Malignancies: A Comparative Study. In Quality Assurance in the Era of Individualized Medicine; Moumtzoglou, A.S., Ed.; IGI Global: Hershey, PA, USA, 2020; pp. 110–146.
- Pouliakis, A.; Margari, N.; Karakitsou, E.; Valasoulis, G.; Koufopoulos, N.; Koureas, N.; Alamanou, E.; Pergialiotis, V.; Damaskou, V.; Panayiotides, I.G. Artificial Intelligence via Competitive Learning and Image Analysis for Endometrial Malignancies: Discriminating Endometrial Cells and Lesions. Int. J. Reliab. Qual. E Healthc. 2019, 8, 38–54.
- Piedimonte, S.; Rosa, G.; Gerstl, B.; Sopocado, M.; Coronel, A.; Lleno, S.; Vicus, D. Evaluating the use of machine learning in endometrial cancer: A systematic review. Int. J. Gynecol. Cancer 2023, 33, 1383–1393.
- Purandare, N.C.; Trevisan, J.; Patel, I.I.; Gajjar, K.; Mitchell, A.L.; Theophilou, G.; Valasoulis, G.; Martin, M.; von Bünau, G.; Kyrgiou, M.; et al. Exploiting biospectroscopy as a novel screening tool for cervical cancer: Towards a framework to validate its accuracy in a routine clinical setting. Bioanalysis 2013, 5, 2697–2711.
- Theophilou, G.; Morais, C.L.M.; Halliwell, D.E.; Lima, K.M.G.; Drury, J.; Martin-Hirsch, P.L.; Stringfellow, H.F.; Hapangama, D.K.; Martin, F.L. Synchrotron- and focal plane array-based Fourier-transform infrared spectroscopy differentiates the basalis and functionalis epithelial endometrial regions and identifies putative stem cell regions of human endometrial glands. Anal. Bioanal. Chem. 2018, 410, 4541–4554.
- Jacobs, I.; Gentry-Maharaj, A.; Burnell, M.; Manchanda, R.; Singh, N.; Sharma, A.; Ryan, A.; Seif, M.W.; Amso, N.N.; Turner, G.; et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: A case-control study within the UKCTOCS cohort. Lancet Oncol. 2010, 12, 38–48.
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17.
- Doll, A.; Abal, M.; Rigau, M.; Monge, M.; Gonzalez, M.; Demajo, S.; Colás, E.; Llauradó, M.; Alazzouzi, H.; Planagumá, J.; et al. Novel molecular profiles of endometrial cancer-new light through old windows. J. Steroid. Biochem. Mol. Biol. 2008, 108, 221–229.
- Kandoth, C.; Schultz, N.; Cherniack, A.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.; Pashtan, I.; Shen, R.; Benz, C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73.
- Talhouk, A.; McConechy, M.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813.
- Le Gallo, M.; Bell, D. The emerging genomic landscape of endometrial cancer. Clin. Chem. 2014, 60, 98–110.
- Holbro, T.; Civenni, G.; Hynes, N.E. The ErbB receptors and their role in cancer progression. Exp. Cell Res. 2003, 284, 99–110.
- Marmor, M.D.; Skaria, K.B.; Yarden, Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int. J. Radiat. Oncol. 2004, 58, 903–913.
- Überall, I.; Kolář, Z.; Trojanec, R.; Berkovcová, J.; Hajdúch, M. The status and role of ErbB receptors in human cancer. Exp. Mol. Pathol. 2008, 84, 79–89.
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134.
- McDonell, L.M.; Kernohan, K.D.; Boycott, K.M.; Sawyer, S.L. Receptor tyrosine kinase mutations in developmental syndromes and cancer: Two sides of the same coin. Hum. Mol. Genet. 2015, 24, R60–R66.
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58.
- Casalini, P.; Iorio, M.V.; Galmozzi, E.; Ménard, S. Role of HER receptors family in development and differentiation. J. Cell. Physiol. 2004, 200, 343–350.
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584.
- Linggi, B.; Carpenter, G. ErbB receptors: New insights on mechanisms and biology. Trends Cell Biol. 2006, 16, 649–656.
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74.
- Hunter, T. The Croonian Lecture 1997. The phosphorylation of proteins on tyrosine: Its role in cell growth and disease. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 583–605.
- Mass, R.D. The HER receptor family: A rich target for therapeutic development. Int. J. Radiat. Oncol. 2004, 58, 932–940.
- Ogiso, H.; Ishitani, R.; Nureki, O.; Fukai, S.; Yamanaka, M.; Kim, J.H.; Saito, K.; Sakamoto, A.; Inoue, M.; Shirouzu, M.; et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002, 110, 775–787.
- Ferguson, K.M.; Berger, M.B.; Mendrola, J.M.; Cho, H.-S.; Leahy, D.J.; Lemmon, M.A. EGF Activates Its Receptor by Removing Interactions that Autoinhibit Ectodomain Dimerization. Mol. Cell 2003, 11, 507–517.
- Dawson, J.P.; Berger, M.; Lin, C.-C.; Schlessinger, J.; Lemmon, M.A.; Ferguson, K.M. Epidermal Growth Factor Receptor Dimerization and Activation Require Ligand-Induced Conformational Changes in the Dimer Interface. Mol. Cell. Biol. 2005, 25, 7734–7742.
- Özcan, F.; Klein, P.; Lemmon, M.A.; Lax, I.; Schlessinger, J. On the nature of low- and high-affinity EGF receptors on living cells. Proc. Natl. Acad. Sci. USA 2006, 103, 5735–5740.
- Olayioye, M.; Neve, R.; Lane, H.; Hynes, N. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167.
- Qian, X.; LeVea, C.; Freeman, J.; Dougall, W.; Greene, M. Heterodimerization of epidermal growth factor receptor and wild-type or kinase-deficient Neu: A mechanism of interreceptor kinase activation and transphosphorylation. Proc. Natl. Acad. Sci. USA 1994, 91, 1500–1504.
- Zhang, X.; Gureasko, J.; Shen, K.; Cole, P.A.; Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006, 125, 1137–1149.
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16, 1647–1655.
- Garrett, T.P.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Kofler, M.; Jorissen, R.N.; Nice, E.C.; Burgess, A.W.; et al. The Crystal Structure of a Truncated ErbB2 Ectodomain Reveals an Active Conformation, Poised to Interact with Other ErbB Receptors. Mol. Cell 2003, 11, 495–505.
- Citri, A.; Skaria, K.B.; Yarden, Y. The deaf and the dumb: The biology of ErbB-2 and ErbB-3. Exp. Cell Res. 2003, 284, 54–65.
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137.
- Zhou, S.; Shoelson, S.E.; Chaudhuri, M.; Gish, G.; Pawson, T.; Haser, W.G.; King, F.; Roberts, T.; Ratnofsky, S.; Lechleider, R.J.; et al. SH2 domains recognize specific phosphopeptide sequences. Cell 1993, 72, 767–778.
- Zhang, H.; Berezov, A.; Wang, Q.; Zhang, G.; Drebin, J.; Murali, R.; Greene, M.I. ErbB receptors: From oncogenes to targeted cancer therapies. J. Clin. Investig. 2007, 117, 2051–2058.
- Srinivasan, R.; Benton, E.; McCormick, F.; Thomas, H.; Gullick, W. Expression of the c-erbB-3/HER-3 and c-erbB-4/HER-4 growth factor receptors and their ligands, neuregulin-1 alpha, neuregulin-1 beta, and betacellulin, in normal endometrium and endometrial cancer. Clin. Cancer Res. 1999, 5, 2877–2883.
- Ejskjaer, K.; Sørensen, B.; Poulsen, S.; Mogensen, O.; Forman, A.; Nexø, E. Expression of the epidermal growth factor system in human endometrium during the menstrual cycle. Mol. Hum. Reprod. 2005, 11, 543–551.
- Ejskjaer, K.; Sorensen, B.S.; Poulsen, S.S.; Forman, A.; Nexo, E.; Mogensen, O. Expression of the epidermal growth factor system in endometrioid endometrial cancer. Gynecol. Oncol. 2007, 104, 158–167.
- Brys, M.; Semczuk, A.; Rechberger, T.; Krajewska, W.M. Expression of erbB-1 and erbB-2 genes in normal and pathological human endometrium. Oncol. Rep. 2007, 18, 261–265.
- Reinartz, J.; George, E.; Lindgren, B.; Niehans, G. Expression of p53, transforming growth factor alpha, epidermal growth factor receptor, and c-erbB-2 in endometrial carcinoma and correlation with survival and known predictors of survival. Hum. Pathol. 1994, 25, 1075–1083.
- Khalifa, M.A.; Mannel, R.S.; Haraway, S.D.; Walker, J.; Min, K.-W. Expression of EGFR, HER-2/neu, P53, and PCNA in Endometrioid, Serous Papillary, and Clear Cell Endometrial Adenocarcinomas. Gynecol. Oncol. 1994, 53, 84–92.
- Scambia, G.; Panici, P.B.; Ferrandina, G.; Battaglia, F.; Distefano, M.; D'Andrea, G.; De Vincenzo, R.; Maneschi, F.; Ranelletti, F.O.; Mancuso, S. Significance of epidermal growth factor receptor expression in primary human endometrial cancer. Int. J. Cancer 2007, 56, 26–30.
- Niikura, H.; Sasano, H.; Kaga, K.; Sato, S.; Yajima, A. Expression of epidermal growth factor family proteins and epidermal growth factor receptor in human endometrium. Hum. Pathol. 1996, 27, 282–289.
- Konecny, G.E.; Santos, L.; Winterhoff, B.; Hatmal, M.; Keeney, G.L.; Mariani, A.; Jones, M.; Neuper, C.; Thomas, B.; Muderspach, L.; et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br. J. Cancer 2008, 100, 89–95.
- Adonakis, G.; Androutsopoulos, G.; Koumoundourou, D.; Liava, A.; Ravazoula, P.; Kourounis, G. Expression of the epidermal growth factor system in endometrial cancer. Eur. J. Gynaecol. Oncol. 2008, 29, 450–454.
- Adonakis, G.; Androutsopoulos, G. The role of ErbB receptors in endometrial cancer. In Cancer of the Uterine Endometrium—Advances and Controversies; Saldivar, J.S., Ed.; IntechOpen: London, UK, 2012; pp. 23–38.
- Androutsopoulos, G.; Adonakis, G.; Gkermpesi, M.; Gkogkos, P.; Ravazoula, P.; Kourounis, G. Expression of the epidermal growth factor system in endometrial cancer after adjuvant tamoxifen treatment for breast cancer. Eur. J. Gynaecol. Oncol. 2006, 27, 490–494.
- Reyes, H.D.; Thiel, K.W.; Carlson, M.J.; Meng, X.; Yang, S.; Stephan, J.-M.; Leslie, K.K. Comprehensive profiling of EGFR/HER receptors for personalized treatment of gynecologic cancers. Mol. Diagn. Ther. 2014, 18, 137–151.
- Androutsopoulos, G.; Michail, G.; Adonakis, G.; Decavalas, G. Molecular mechanisms, expression and clinical role of ErbB receptors in endometrial cancer. Int. J. Clin. Ther. Diagn. 2015, S1, 28–32.
- Michail, G.; Styliara, I.; Panas, P.; Markatos, F.; Koumoundourou, D.; Ravazoula, P.; Adonakis, G.; Androutsopoulos, G. EP472 ErbB receptors profile in non-selected patients with endometrial cancer. Int. J. Gynecol. Cancer 2019, 29, A298–A299.
- Styliara, I.; Zarogianni, E.; Panas, P.; Michail, G.; Koumoundourou, D.; Ravazoula, P.; Adonakis, G.; Androutsopoulos, G. 299 EGF System receptors profiling in various histologic subgroups of endometrial cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, e85–e86.
- Androutsopoulos, G.; Adonakis, G.; Liava, A.; Ravazoula, P.; Decavalas, G. Expression and potential role of ErbB receptors in type II endometrial cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 204–208.
- Michail, G.; Panas, P.; Markatos, F.; Styliara, I.; Koumoundourou, D.; Ravazoula, P.; Adonakis, G.; Androutsopoulos, G. ErbB receptors profiling in selected patients with type II endometrial cancer. Int. J. Gynecol. Cancer 2019, 29 (Suppl. S4), A299.
- Zarogianni, E.; Panas, P.; Styliara, I.; Michail, G.; Koumoundourou, D.; Ravazoula, P.; Adonakis, G.; Androutsopoulos, G. EGF system receptors status in aggressive subtypes of endometrial cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, e86.
- Morrison, C.; Zanagnolo, V.; Ramirez, N.; Cohn, D.; Kelbick, N.; Copeland, L.; Maxwell, G.; Fowler, J. HER-2 is an independent prognostic factor in endometrial cancer: Association with outcome in a large cohort of surgically staged patients. J. Clin. Oncol. 2006, 24, 2376–2385.
- Engelsen, I.; Stefansson, I.; Beroukhim, R.; Sellers, W.; Meyerson, M.; Akslen, L.; Salvesen, H. HER-2/neu expression is associated with high tumor cell proliferation and aggressive phenotype in a population based patient series of endometrial carcinomas. Int. J. Oncol. 2008, 32, 307–316.
- Coronado, P.; Vidart, J.; Lopez-asenjo, J.; Fasero, M.; Furio-bacete, V.; Magrina, J.; Escudero, M. P53 overexpression predicts endometrial carcinoma recurrence better than HER-2/neu overexpression. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 98, 103–108.
- Halperin, R.; Zehavi, S.; Habler, L.; Hadas, E.; Bukovsky, I.; Schneider, D. Comparative immunohistochemical study of endometrioid and serous papillary carcinoma of endometrium. Eur. J. Gynaecol. Oncol. 2001, 22, 122–126.
- Santin, A.; Bellone, S.; Siegel, E.; Palmieri, M.; Thomas, M.; Cannon, M.; Kay, H.; Roman, J.; Burnett, A.; Pecorelli, S. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): A major prognostic indicator in uterine serous papillary cancer. Am. J. Obstet. Gynecol. 2005, 192, 813–818.
- Santin, A.; Bellone, S.; Van Stedum, S.; Bushen, W.; Palmieri, M.; Siegel, E.; De Las Casas, L.; Roman, J.; Burnett, A.; Pecorelli, S. Amplification of c-erbB2 oncogene: A major prognostic indicator in uterine serous papillary carcinoma. Cancer 2005, 104, 1391–1397.
- Slomovitz, B.; Broaddus, R.; Burke, T.; Sneige, N.; Soliman, P.; Wu, W.; Sun, C.; Munsell, M.; Gershenson, D.; Lu, K. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J. Clin. Oncol. 2004, 22, 3126–3132.
- Grushko, T.; Filiaci, V.; Mundt, A.; Ridderstrale, K.; Olopade, O.; Fleming, G. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 108, 3–9.
- Lukes, A.; Kohler, M.; Pieper, C.; Kerns, B.; Bentley, R.; Rodriguez, G.; Soper, J.; Clarke-Pearson, D.; Bast, R., Jr.; Berchuck, A. Multivariable analysis of DNA ploidy, p53, and HER-2/neu as prognostic factors in endometrial cancer. Cancer 1994, 73, 2380–2385.
- Odicino, F.; Bignotti, E.; Rossi, E.; Pasinetti, B.; Tassi, R.; Donzelli, C.; Falchetti, M.; Fontana, P.; Grigolato, P.; Pecorelli, S. HER-2/neu overexpression and amplification in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry, real-time reverse transcription-polymerase chain reaction, and fluorescence in situ hybridization. Int. J. Gynecol. Cancer 2008, 18, 14–21.
- Togami, S.; Sasajima, Y.; Oi, T.; Ishikawa, M.; Onda, T.; Ikeda, S.; Kato, T.; Tsuda, H.; Kasamatsu, T. Clinicopathological and prognostic impact of human epidermal growth factor receptor type 2 (HER2) and hormone receptor expression in uterine papillary serous carcinoma. Cancer Sci. 2012, 103, 926–932.
- Díaz-Montes, T.; Ji, H.; Smith Sehdev, A.; Zahurak, M.; Kurman, R.; Armstrong, D.; Bristow, R. Clinical significance of Her-2/neu overexpression in uterine serous carcinoma. Gynecol. Oncol. 2006, 100, 139–144.
- Androutsopoulos, G.; Styliara, I.; Zarogianni, E.; Michail, G.; Adonakis, G. Is it time to reconsider the clinical role of ErbB targeted therapy in endometrial cancer? In Endometrial Cancer; Farghaly, S., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2022; pp. 299–327.
