Understanding the mechanisms triggering the initiation of retinal regeneration in amphibians may advance the quest for prevention and treatment options for degenerating human retina diseases. Natural retinal regeneration in amphibians requires two cell sources, namely retinal pigment epithelium (RPE) and ciliary marginal zone. The disruption of RPE interaction with photoreceptors through surgery or injury triggers local and systemic responses for retinal protection. In mammals, disease-induced damage to the retina results in the shutdown of the function, cellular or oxidative stress, pronounced immune response, cell death and retinal degeneration. In contrast to retinal pathology in mammals, regenerative responses in amphibians have taxon-specific features ensuring efficient regeneration. These include rapid hemostasis, the recruitment of cells and factors of endogenous defense systems, activities of the immature immune system, high cell viability, and the efficiency of the extracellular matrix, cytoskeleton, and cell surface remodeling. These reactions are controlled by specific signaling pathways, transcription factors, and the epigenome, which are insufficiently studied.
- amphibia
- eye
- retina
- regeneration
- cell sources
- injury-induced
- regenerative responses
1. Introduction
2. Source Cells and Means of Retinal Regeneration
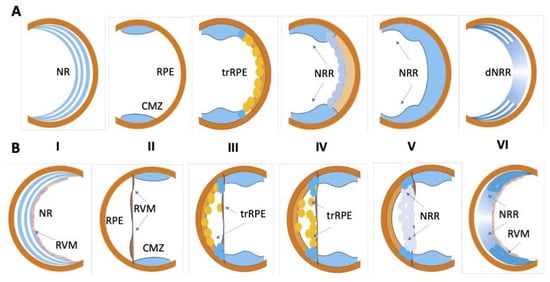
3. Retinal Damage, Methods, and Consequences
4. Early Events That Occur after Separation of Neural Retina and Retinal Pigment Epithelium
4.1. Cell Stress and Cell Death after Retinal Damage in Amphibians
4.2. Disturbance of Retinal Cell Contacts, Rearrangement of Cytoskeleton and ECM
4.3. Role of Immune System in NR Regeneration in Amphibians
4.4. Role of Blood Factors and Cells in Initiation of NR Regeneration in Amphibia
4.5. Participants of Molecular Regulatory Networks at the Stage of Initiation of Retinal Regeneration in Amphibians
5. Conclusions
The success of retinal regeneration as well as other tissues in amphibians is determined by a set of interactions between cells that are sources of regeneration, on the one hand, and cells and factors of the “primitive” immune system, on the other. In these interactions, macrophages that are characterized by morpho-functional heterogeneity play a special, multilateral role; however, differing between Anura and Urodela. The significance of this role is noted at different stages of NR regeneration; during the initiation of retinal regeneration in Urodela, it is detected while RPE cells are freed of the initial features, with changes in the cell surface and in the formation of the pro-regenerative environment. A feature of the initiation of NR regeneration in amphibians is also the rapid coagulation hemostasis provided by platelet recruitment and by the involvement of blood factors (complement, thrombin, and tissue factor) that were found in Urodela during lens regeneration, which is a different regeneration system but close to the retinal one. The role of cells of the immune and circulatory systems and the regulatory factors produced by them in the NR regeneration in amphibians are among the most important fields for further research. In general, the main feature of NR regeneration triggers in amphibians is that they are not only a multidimensional response to damage but they also create a permissive environment allowing the source cells to activate and rebuild the retina de novo. This circumstance is a key to finding approaches to triggering the retinal regeneration in mammals and humans.
This entry is adapted from the peer-reviewed paper 10.3390/life13101981
References
- Stocum, D.L. Regenerative Biology and Medicine, 1st ed.; Academic Press: Burlington, UK, 2006; ISBN 9780080493022.
- Carlson, B.M. Principles of Regenerative Biology; Elsevier/Academic Press: New York, NY, USA, 2007; ISBN 9780123694393.
- Tanaka, E.M.; Reddien, P.W. The cellular basis for animal regeneration. Dev. Cell. 2011, 21, 172–185.
- Joven, A.; Elewa, A.; Simon, A. Model Systems for Regeneration: Salamanders. Development 2019, 146, dev167700.
- Phipps, L.S.; Marshall, L.; Dorey, K.; Amaya, E. Model systems for regeneration: Xenopus. Development 2020, 147, dev180844.
- Slater, P.G.; Palacios, M.; Larraín, J. Xenopus, a Model to Study Wound Healing and Regeneration: Experimental Approaches. Cold Spring Harb. Protoc. 2021, 2021.
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809.
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911.
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618.
- Morescalchi, F.; Duse, S.; Gambicorti, E.; Romano, M.R.; Costagliola, C.; Semeraro, F. Proliferative Vitreoretinopathy after Eye Injuries: An Overexpression of Growth Factors and Cytokines Leading to a Retinal Keloid. Mediat. Inflamm. 2013, 2013, 269787.
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240.
- Markitantova, Y.V.; Simirskii, V.N. Inherited Eye Diseases with Retinal Manifestations through the Eyes of Homeobox Genes. Int. J. Mol. Sci. 2020, 21, 1602.
- Fernandes, A.-R.; Zielinska, A.; Sanchez Lopez, E.; dos Santos, T.; Garcia, M.L.; Silva, A.M.; Karczewski, J.; Souto, E.B. Exudative versus Nonexudative Age-Related Macular Degeneration: Physiopathology and Treatment Options. Int. J. Mol. Sci. 2022, 23, 2592.
- Del Rio-Tsonis, K.; Tsonis, P.A. Eye regeneration at the molecular age. Dev. Dyn. 2003, 226, 211–224.
- Tsonis, P.A.; del Rio-Tsonis, K. Lens and retina regeneration: Transdifferentiation, stem cells and clinical applications. Exp. Eye Res. 2004, 78, 161–172.
- Filoni, S. Retina and lens regeneration in anuran amphibians. Semin. Cell Dev. Biol. 2009, 20, 528–534.
- Henry, J.J.; Tsonis, P.A. Molecular and cellular aspects of amphibian lens regeneration. Prog. Retin. Eye Res. 2010, 29, 543–555.
- Mitashov, V.I. Mechanisms of retina regeneration in urodeles. Int. J. Dev. Biol. 1996, 40, 833–844.
- Mitashov, V. Retinal regeneration in amphibians. Int. J. Dev. Biol. 1997, 41, 893–905.
- Yoshii, C.; Ueda, Y.; Okamoto, M.; Araki, M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev. Biol. 2007, 303, 45–56.
- Vergara, M.N.; Del Rio-Tsonis, K. Retinal regeneration in the Xenopus laevis tadpole: A new model system. Mol. Vis. 2009, 15, 1000–1013.
- Chiba, C. The retinal pigment epithelium: An important player of retinal disorders and regeneration. Exp. Eye Res. 2014, 123, 107–114.
- Ail, D.; Perron, M. Retinal degeneration and regeneration—Lessons from fishes and amphibians. Curr. Pathobiol. Rep. 2017, 5, 67–78.
- Grigoryan, E.N. Pigment Epithelia of the Eye: Cell-Type Conversion in Regeneration and Disease. Life 2022, 12, 382.
- Stone, L.S. The role of retinal pigment cells in regenerating neural retina of adult salamander eye. J. Exp. Zool. 1950, 113, 9–31.
- Levine, R.J. Regeneration of the retina in the adult newt, Triturus cristatus, following surgical division of the eye by a limbal incision. Exp. Zool. 1975, 192, 363–380.
- Chiba, C.; Mitashov, V.I. Cellular and molecular events in the adult newt retinal regeneration. In Strategies for Retinal Tissue Repair and Regeneration in Vertebrates: From Fish to Human; Chiba, C., Ed.; Research Signpost: Kerala, India, 2007; pp. 15–33.
- Beddaoui, M.; Coupland, S.G.; Tsilfidis, C. Recovery of function following regeneration of the damaged retina in the adult newt, Notophthalmus viridescens. Doc. Ophthalmol. 2012, 125, 91–100.
- Grigoryan, E.N.; Markitantova, Y.V. Cellular and Molecular Preconditions for Retinal Pigment Epithelium (RPE) Natural Reprogramming during Retinal Regeneration in Urodela. Biomedicines 2016, 4, 28.
- Grigoryan, E.N.; Markitantova, Y.V. Molecular Strategies for Transdifferentiation of Retinal Pigment Epithelial Cells in Amphibians and Mammals In Vivo. Russ. J. Dev. Biol. 2021, 52, 220–243.
- Yasumuro, H.; Sakurai, K.; Toyama, F.; Maruo, F.; Chiba, C. Implications of a Multi-Step Trigger of Retinal Regeneration in the Adult Newt. Biomedicines 2017, 5, 25.
- Islam, M.R.; Nakamura, K.; Casco-Robles, M.M.; Kunahong, A.; Inami, W.; Toyama, F.; Maruo, F.; Chiba, C. The newt reprograms mature RPE cells into a unique multipotent state for retinal regeneration. Sci. Rep. 2014, 4, 6043.
- Markitantova, Y.V.; Makar’ev, E.O.; Smirnova, Y.A.; Zinov’eva, R.D.; Mitashov, V.I. Analysis of the expression pattern of regulatory genes Pax6, Prox1, and Six3 during regeneration of eye structures in the newt. Biol. Bull. 2004, 31, 428–436.
- Markitantova, Y.V.; Avdonin, P.P.; Grigoryan, E.N.; Zinovieva, R.D. Identification of the pitx1 embryogenesis regulatory gene in a regenerating newt retina. Dokl. Biol. Sci. 2010, 435, 421–424.
- Sakami, S.; Hisatomi, O.; Sakakibara, S.; Liu, J. Down regulation of Otx2 in the dedifferentiated RPE cells of regenerating newt retina. Dev. Brain Res. 2005, 155, 49–59.
- Avdonin, P.P.; Markitantova, Y.V.; Zinov’eva, R.D.; Mitashov, V.I. Expression of regulatory genes Pax6, Otx2, Six3, and FGF2 during newt retina regeneration. Biol. Bull. 2008, 35, 355–361.
- Avdonin, P.P.; Grigoryan, E.N.; Markitantova, Y.V. Transcriptional factor Pitx2: Localization during triton retina regeneration. Biol. Bull. 2010, 37, 231–235.
- Inami, W.; Islam, M.R.; Nakamura, K.; Yoshikawa, T.; Yasumuro, H.; Casco-Robles, M.M.; Toyama, F.; Maruo, F.; Chiba, C. Expression of two classes of pax6 transcripts in reprogramming retinal pigment epithelium cells of the adult newt. Zool. Sci. 2016, 33, 21–30.
- Mitashov, V.I.; Panova, I.G.; Koussoulakos, S. Transdifferentiation potencies of ciliary and pigment epithelium cells of lower vertebrates and mammals. Russ. J. Dev. Biol. 2004, 35, 395–403.
- Grigorian, E.N.; Ivanova, I.P.; Poplinskaia, V.A. The discovery of new internal sources of neural retinal regeneration after its detachment in newts: Morphological and quantitative research. Izv. Akad. Nauk. Ser. Biol. 1996, 3, 319–332.
- Grigoryan, E. Alternative intrinsic cell sources for neural retina regeneration in adult urodelean amphibians. In Strategies for Retinal Tissue Repair and Regeneration in Vertebrates: From Fish to Human; Chiba, C., Ed.; Research Signpost: Kerala, India, 2007; pp. 35–62.
- Araki, M. Regeneration of the amphibian retina: Role of tissue interaction and related signaling molecules on RPE transdifferentiation. Dev. Growth Differ. 2007, 49, 109–120.
- Miyake, A.; Araki, M. Retinal stem/progenitor cells in the ciliary marginal zone complete retinal regeneration: A study of retinal regeneration in a novel animal model. Dev. Neurobiol. 2014, 74, 739–756.
- Tseng, A.S. Seeing the future: Using Xenopus to understand eye regeneration. Genesis 2017, 55, e23003.
- Sakaguchi, D.S.; Janick, L.M.; Reh, T.A. Basic fibroblast growth factor (FGF-2) induced transdifferentiation of retinal pigment epithelium: Generation of neurons and glia. Dev. Dyn. 1997, 209, 387–398.
- Andreazzoli, M.; Gestri, G.; Angeloni, D.; Menna, E.; Barsacchi, G. Role of Xrx1 in Xenopus eye and anterior brain development. Development. 1999, 126, 2451–2460.
- Martinez-De Luna, R.I.; Kelly, L.E.; El-Hodiri, H.M. The Retinal Homeobox (Rx) gene is necessary for retinal regeneration. Dev. Biol. 2011, 353, 10–18.
- Grigoryan, E.N.; Anton, H.J.; Poplinskaya, V.A.; Aleinikova, K.S.; Domaratskaya, E.I.; Novikova, Y.P.; Almeida, E. Signs of Müller cell gliotic response found in the retina of newts exposed to real and simulated microgravity. Adv. Space Res. 2012, 49, 1465–1471.
- Grigorian, E.N.; Poplinskaia, V.A. Discovery of internal sources of the neural retinal regeneration after its detachment in Pleurodeles: II. The radioautography study. Izv. Akad. Nauk. Ser. Biol. 1999, 5, 583–591.
- Novikova, Y.P.; Poplinskaia, V.A.; Aleinikova, K.S.; Grigorian, E.N. A study of the localization and accumulation of S-phase cells in the retina of newt Pleurodeles waltl after experimental pigment epithelial detachment. Ontogenez 2008, 39, 143–150.
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424.
- Grigoryan, E.N. Impact of Microgravity and Other Spaceflight Factors on Retina of Vertebrates and Humans In Vivo and In Vitro. Life 2023, 13, 1263.
- Choi, R.Y.; Engbretson, G.A.; Solessio, E.C.; Jones, G.A.; Coughli, A.; Aleksic, I.; Zuber, M.E. Cone degeneration following rod ablation in a reversible model of retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2011, 52, 364–373.
- Hasegawa, M. Restitution of the eye after removal of the retina and lens in the newt Triturus pyrrhogaster. Embryologia 1958, 4, 1–32.
- Keefe, J.R. An analysis of urodelian retinal regeneration. I. Studies of the cellular source of the retinal regeneration in Notophthalmus viridescens utilizing 3H-thymidine and colchicine. J. Exp. Zool. 1973, 184, 185–206.
- Keefe, J.R. An analysis of urodelian retinal regeneration: II. Ultrastructural features of retinal regeneration in Notophthalmus viridescens. J. Exp. Zool. 1973, 184, 207–232.
- Grigorian, E.N.; Anton, H.J. The characteristics of eye regeneration in newts after complete retinal detachment induced by a change in the effect of gravitation in an experiment using a clinostat. Izv Akad Nauk Ser. Biol. 1994, 3, 342–352.
- Cebulla, C.M.; Zelinka, C.P.; Scott, M.A.; Lubow, M.; Bingham, A.; Rasiah, S.; Mahmoud, A.M.; Fischer, A.J. A chick model of retinal detachment: Cone rich and novel. PLoS ONE 2012, 7, e44257.
- Grigorian, E.N.; Poplinskaia, V.A. Changes in the relative number of bipolar-like cells in the retina of Pleurodeles waltl as a function of age and as a result of light exposure. Ontogenez 2002, 33, 111–117.
- Grigoryan, E.N. Cytological Basis of Eye Tissue Transdifferentiation in Vertebrates. Ph.D. Thesis, Institute of Developmental Biology, RAS, Moscow, Russia, 1998.
- Hellsten, U.; Harland, R.M.; Gilchrist, M.J.; Hendrix, D.; Jurka, J.; Kapitonov, V.; Ovcharenko, I.; Putnam, N.H.; Shu, S.; Taher, L. The genome of the Western clawed frog Xenopus tropicalis. Science 2010, 328, 633–636.
- Session, A.M.; Uno, Y.; Kwon, T.; Chapman, J.A.; Toyoda, A.; Takahashi, S.; Fukui, A.; Hikosaka, A.; Suzuki, A.; Kondo, M.; et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 2016, 538, 336–343.
- Amin, N.M.; Tandon, P.; Osborne Nishimura, E.; Conlon, F.L. RNA-seq in the tetraploid Xenopus laevis enables genome-wide insight in a classic developmental biology model organism. Methods 2014, 66, 398–409.
- Lee-Liu, D.; Sun, L.; Dovichi, N.J.; Larraín, J. Quantitative proteomics after spinal cord injury (SCI) in a regenerative and a nonregenerative stage in the frog Xenopus laevis. Mol. Cell Proteom. 2018, 17, 592–606.
- Chesneau, A.; Bronchain, O.; Perron, M. Conditional Chemogenetic Ablation of Photoreceptor Cells in Xenopus retina. Methods Mol. Biol. 2018, 1865, 133–146.
- Martinez-De Luna, R.I.; Zuber, M.E. Rod-Specific Ablation Using the Nitroreductase/Metronidazole System to Investigate Regeneration in Xenopus. Cold Spring Harb. Protoc. 2018, 12.
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990.
- Markitantova, Y.V.; Simirskii, V.N. Endogenous and Exogenous Regulation of Redox Homeostasis in Retinal Pigment Epithelium Cells: An Updated Antioxidant Perspective. Int. J. Mol. Sci. 2023, 24, 10776.
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. J. Biol. Inorg. Chem. 2011, 16, 977–989.
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. Landmark Ed. 2021, 26, 50–96.
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436.
- Markitantova, Y.V.; Simirskii, V.N. Conservatism and Variability of the Antioxidant Defense System in the Retinal Pigment Epithelium of Vertebrates. J. Evol. Biochem. Physiol. 2023, 59, 655–675.
- Rattner, A.; Toulabi, L.; Williams, J.; Yu, H.; Nathans, J. The genomic response of the retinal pigment epithelium to light damage and retinal detachment. J. Neurosci. 2008, 28, 9880–9889.
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated Autophagy in the RPE Is Associated with Increased Susceptibility to Oxidative Stress and AMD. Autophagy 2014, 10, 1989–2005.
- Markitantova, Y.V.; Simirskii, V.N. Role of the Redox System in Initiation of a Regenerative Response of Neural Eye Tissues in Vertebrates. Russ. J. Dev. Biol. 2020, 51, 16–30.
- Wang, X.; Nookala, S.; Narayanan, C.; Giorgianni, F.; Beranova-Giorgianni, S.; McCollum, G.; Gerling, I.; Penn, J.S.; Jablonski, M.M. Proteomic analysis of the retina: Removal of RPE alters outer segment assembly and retinal protein expression. Glia 2009, 57, 380–392.
- Dhirachaikulpanich, D.; Lagger, C.; Chatsirisupachai, K.; de Magalhães, J.P.; Paraoan, L. Intercellular communication analysis of the human retinal pigment epithelial and choroidal cells predicts pathways associated with aging, cellular senescence and age-related macular degeneration. Front. Aging Neurosci. 2022, 14, 1016293.
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393.
- Galluzzi, L.; Bravo-San Pedro, J.M.; Kepp, O.; Kroemer, J. Regulated cell death and adaptive stress responses. Cell. Mol. Life Sci. 2016, 73, 2405–2410.
- Wan, J.; Goldman, D. Retina regeneration in zebrafish. Curr. Opin. Genet. Dev. 2016, 40, 41–47.
- Carbonell, M.B.; Zapata Cardona, J.; Delgado, J.P. Post-amputation reactive oxygen species production is necessary for axolotl limb regeneration. Front. Cell Dev. Biol. 2022, 10, 921520.
- Hameed, L.S.; Berg, D.A.; Belnoue, L.; Jensen, L.D.; Cao, Y.; Simon, A. Environmental changes in oxygen tension reveal ROS-dependent neurogenesis and regeneration in the adult newt brain. eLife 2015, 4, e08422.
- Beasley, T.C.; Tytell, M.; Sweatt, A.J. Heat shock protein 70 in the retina of Xenopus laevis, in vivo and in vitro: Effect of metabolic stress. Cell Tissue Res. 1997, 290, 525–538.
- Avdonin, P.P.; Markitantova, Y.V.; Poplinskaya, V.A.; Grigoryan, E.N. Determination of mRNA-transcripts and heat shock proteins HSP70 and HSP90 in retina of the adult Spanish Ribbed Newt Pleurodeles waltl. Izv. Akad. Nauk. Ser. Biol. 2013, 4, 389–397.
- Grigoryan, E.N. Shared triggering mechanisms of retinal regeneration in lower vertebrates and retinal rescue in higher ones. In Tissue Regeneration—From Basic Biology to Clinical Application; Davies, J., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 145–164.
- Kaarniranta, K.; Salminen, A.; Eskelinen, E.L.; Kopitz, J. Heat shock proteins as gatekeepers of proteolytic pathways—Implications for age-related macular degeneration (AMD). Ageing Res. Rev. 2009, 8, 128–139.
- Li, F.; Huang, Q.; Chen, J.; Peng, Y.; Roop, D.; Bedford, J.S.; Li, C.-Y. Apoptotic cells activate the “Phoenix Rising” pathway to promote wound healing and tissue regeneration Sci. Signal. 2010, 3, ra13.
- Pellettieri, J.; Fitzgerald, P.; Watanabe, S.; Mancuso, J.; Green, D.R.; Sánchez Alvarado, A. Cell death and tissue remodeling in planarian regeneration. Dev. Biol. 2010, 338, 76–85.
- Ryoo, H.D.; Bergmann, A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797.
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089.
- Klemm, J.; Stinchfield, M.J.; Harris, R.E. Necrosis induced apoptosis promotes regeneration in Drosophila wing imaginal discs. Genetics 2021, 219, iyab144.
- Vecino, E.; Hernandez, M.; Garcia, M. Cell death in the developing vertebrate retina. Int. J. Dev. Biol. 2004, 48, 965–974.
- Valenciano, A.I.; Boya, P.; De La Rosa, E.J. Early neural cell death: Numbers and cues from the developing neuroretina. Int. J. Dev. Biol. 2009, 53, 1515–1528.
- Vecino, E.; Acera, A. Development and programed cell death in the mammalian eye. Int. J. Dev. Biol. 2015, 59, 63–71.
- Kaneko, Y.; Matsumoto, G.; Hanyu, Y. The occurrence of apoptosis during retinal regeneration in adult newts. Brain Res. Dev. Brain Res. 1999, 117, 225–228.
- Hanus, J.; Anderson, C.; Wang, S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res. Rev. 2015, 24 Pt B, 286–298.
- Zhang, M.; Jiang, N.; Chu, Y.; Postnikova, O.; Varghese, R.; Horvath, A.; Cheema, A.K.; Golestaneh, N. Dysregulated metabolic pathways in age-related macular degeneration. Sci. Rep. 2020, 10, 2464.
- Nagai, H.; Noguchi, T.; Takeda, K.; Ichijo, H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J. Biochem. Mol. Biol. 2007, 40, 1–6.
- Zacks, D.N.; Han, Y.; Zeng, Y.; Swaroop, A. Activation of signaling pathways and stress-response genes in an experimental model of retinal detachment. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1691–1695.
- Totsuka, K.; Ueta, T.; Uchida, T.; Roggia, M.F.; Nakagawa, S.; Vavvas, D.G.; Honjo, M.; Aihara, M. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 2019, 181, 316–324.
- Intartaglia, D.; Giamundo, G.; Conte, I. Autophagy in the retinal pigment epithelium: A new vision and future challenges. FEBS J. 2022, 289, 7199–7212.
- Remé, C.; Grimm, C.; Hafezi, F.; Iseli, H.; Wenzel, A. Why study rod cell death in retinal degenerations and how? Doc. Ophthalmol. 2003, 106, 25–29.
- Uebersax, E.D.; Grindstaff, R.D.; Defoe, D.M. Survival of the retinal pigment epithelium in vitro: Comparison of freshly isolated and subcultured cells. Exp. Eye Res. 2000, 70, 381–390.
- Defoe, D.M.; Easterling, K.C. Reattachment of retinas to cultured pigment epithelial monolayers from Xenopus laevis. Invest. Ophthalmol. Vis. Sci. 1994, 35, 2466–2476.
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171.
- Brock, C.K.; Wallin, S.T.; Ruiz, O.E.; Samms, K.M.; Mandal, A.; Sumner, E.A.; Eisenhoffer, G.T. Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nat. Commun. 2019, 10, 1044.
- Avalos, P.N.; Forsthoefel, D.J. An Emerging Frontier in Intercellular Communication: Extracellular Vesicles in Regeneration. Front. Cell Dev. Biol. 2022, 10, 849905.
- Middleton, R.C.; Rogers, R.G.; De Couto, G.; Tseliou, E.; Luther, K.; Holewinski, R.; Soetkamp, D.; Van Eyk, J.E.; Antes, T.J.; Marbán, E. Newt cells secrete extracellular vesicles with therapeutic bioactivity in mammalian cardiomyocytes. J. Extracell. Vesicles 2018, 7, 1456888.
- Danilchik, M.; Tumarkin, T. Exosomal trafficking in Xenopus development. Genesis 2017, 55, e23011.
- Jo, S.-H.; Kim, C.; Park, S.-H. Novel Marine Organism-Derived Extracellular Vesicles for Control of Anti-inflammation. Tissue Eng. Regen. Med. 2021, 18, 71–79.
- Fuhrmann, S.; Zou, C.J.; Levine, E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150.
- Rinaldi, C.; Donato, L.; Alibrandi, S.; Scimone, C.; D’Angelo, R.; Sidoti, A. Oxidative Stress and the Neurovascular Unit. Life 2021, 11, 767.
- Grigoryan, E.N. The complete neural retina’s detachment induces the changes of cytokeratin expression in retinal pigmented epithelium cells in newts. Izv. Akad. Nauk, Ser. Biol. 1995, 4, 412–421.
- Grigoryan, E.N.; Anton, H.J. Analysis of keratin expression in retinal pigment epithelium cells during their transdifferentiation in newts. Ontogenez 1995, 26, 310–323.
- Grigoryan, E.N.; Anton, H.J. An appearance and distribution of neurofilament proteins (NF-200) in trans- differentiating retinal pigment cells and other eye cells during the process of neural retina regeneration in newts. Ontogenez 1993, 24, 39–52.
- Dvoriantchikova, D.; Seemungal, R.J.; Ivanov, D. The epigenetic basis for the impaired ability of adult murine retinal pigment epithelium cells to regenerate retinal tissue. Sci. Rep. 2019, 9, 3860.
- Bonilha, V.L. Retinal pigment epithelium (RPE) cytoskeleton in vivo and in vitro. Exp. Eye Res. 2014, 126, 38–45.
- Hasegawa, A.; Hisatomi, O.; Yamamoto, S.; Ono, E.; Tokunaga, F. Stathmin expression during newt retina regeneration. Exp. Eye Res. 2007, 85, 518–527.
- Rubin, C.I.; Atweh, G.F. The role of stathmin in the regulation of the cell cycle. Cell Biochem. 2004, 93, 242–250.
- Nikolaev, A.A. Epigenetic features of pigment epithelium reprogramming during retinal regeneration after photo-induced detachment in Pleurodeles waltl newt. Bachelor’s Thesis, Moscow State University, Moscow, Russia, 2018.
- Nakamura, K.; Islam, M.R.; Takayanagi, M.; Yasumuro, H.; Inami, W.; Kunahong, A.; Casco-Robles, R.M.; Toyama, F.; Chiba, C. A Transcriptome for the Study of Early Processes of Retinal Regeneration in the Adult Newt, Cynops pyrrhogaster. PLoS ONE 2014, 9, e109831.
- Hausman, R.E. Ocular extracellular matrices in development. Prog. Retin. Eye Res. 2007, 26, 162–188.
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801.
- Grigoryan, E.N.; Dol’nikova, A.E.; Belkin, V.M. Fibronectin distribution during the transdifferentiation and proliferation of eye cells after retinal detachment and removal of the crystalline lens in newts. Russ. J. Dev. Biol. 1990, 21, 403–408.
- Ortiz, J.R.; Vigny, M.; Courtois, Y.; Jeanny, J.-C. Immunocytochemical study of extracellular matrix components during lens and neural retina regeneration in the adult newt. Exp. Eye Res. 1992, 54, 861–870.
- Mitashov, V.I.; Arsanto, J.P.; Markitantova, Y.V.; Thouveny, Y. Remodelling processes during neural retina regeneration in adult urodeles: An immunohistochemical survey. Int. J. Dev. Biol. 1995, 39, 993–1003.
- Nabeshima, A.; Nishibayashi, C.; Ueda, Y.; Ogino, H.; Araki, M. Loss of cell-extracellular matrix interaction triggers retinal regeneration accompanied by Rax and Pax6 activation. Genesis 2013, 51, 410–419.
- Reh, T.A.; Nagy, T. A possible role for the vascular membrane in retinal regeneration in Rana catesbienna tadpoles. Dev. Biol. 1987, 122, 471–482.
- Nagy, T.; Reh, T.A. Inhibition of retinal regeneration in larval Rana by an antibody directed against a laminin–heparan sulfate proteoglycan. Dev. Brain Res. 1994, 81, 131–134.
- Naitoh, H.; Suganuma, Y.; Ueda, Y.; Sato, T.; Hiramuki, Y.; Fujisawa-Sehara, A.; Taketani, S.; Araki, M. Upregulation of matrix metalloproteinase triggers transdifferentiation of retinal pigmented epithelial cells in Xenopus laevis: A Link between inflammatory response and regeneration. Dev. Neurobiol. 2017, 77, 1086–1100.
- Gadani, S.P.; Walsh, J.T.; Lukens, J.R. Dealing with danger in the CNS: The response of the immune system to injury. Neuron 2015, 87, 47–62.
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527.
- Aurora, A.B.; Olson, E.N. Immune modulation of stem cells and regeneration. Cell Stem Cell 2014, 15, 14–25.
- Favier, A.-L.; Nikovics, K. Molecular and Cellular Mechanisms of Inflammation and Tissue Regeneration. Biomedicines 2023, 11, 1416.
- Alibardi, L. Organ regeneration evolved in fish and amphibians in relation to metamorphosis: Speculations on a post-embryonic developmental process lost in amniotes after the water to land transition. Ann. Anat. Anat. Anzeiger 2019, 222, 114–119.
- Mescher, A.L.; Neff, A.W.; King, M.W. Inflammation and immunity in organ regeneration. Dev. Comp. Immunol. 2017, 66, 98–110.
- Mastellos, D.C.; DeAngelis, R.A.; Lambris, J.D. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin. Immunol. 2013, 25, 29–38.
- Bolanos Castro, L.A.; Walters, H.E.; Vazquez, R.O.G.; Yun, M.H. Immunity in salamander regeneration: Where are we standing and where are we headed? Dev. Dynam. 2021, 250, 753–767.
- Mescher, A.L.; Neff, A.W. Regenerative capacity and the developing immune system. Adv. Biochem. Eng. Biotechnol. 2005, 93, 39–66.
- Mescher, A.L.; Neff, A.W. Limb Regeneration in Amphibians: Immunological Considerations. Sci. World J. 2006, 6, 1–11.
- Banda, C.H.; Shiraishi, M.; Mitsui, K.; Okada, Y.; Danno, D.; Ishiura, R.; Maemura, K.; Chiba, C.; Mizoguchi, A.; Imanaka-Yoshida, K.; et al. Structural and functional analysis of the newt lymphatic system. Sci. Rep. 2023, 13, 6902.
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Reactive oxygen species (ros)—A family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur. Cells Mater. 2012, 24, 249–265.
- Yoo, S.K.; Huttenlocher, A. Innate Immunity: Wounds Burst H2O2 Signals to Leukocytes. Curr. Biol. 2009, 19, R553–R555.
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999.
- Godwin, J.W.; Debuque, R.; Salimova, E.; Rosenthal, N.A. Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen. Med. 2017, 2, 22.
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420.
- Pough, F.H. The advantages of ectothermy for tetrapods. Am. Nat. 1980, 115, 92–1126.
- Nespolo, R.F.; Figueroa, J.; Solano-Iguaran, J.J. Studying the evolutionary significance of thermal adaptation in ectotherms: The diversification of amphibians’ energetics. J. Therm. Biol. 2017, 68, 5–13.
- Mitashov, V.I.; Starostin, V.I.; Sludskaia, A.I.; Parshina, E.F. 3H-thymidine incorporation into the macrophages in the process of eye regeneration in adult tritons. Ontogenez 1979, 10, 365–371.
- Keefe, J.R. An analysis of urodelean retinal regeneration. III. Degradation of extruded melanin granules in Notophthalmus viridescens. J. Exp. Zool. 1973, 184, 233–237.
- Flajnik, M.F.; Miler, K.; Du Pasquier, L. Evolution of the immune system. In Fundamental Immunology, 5th ed.; Paul, W.E., Ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2003; pp. 519–570.
- Pollet, N. Expression of immune genes during metamorphosis of Xenopus: A survey. Front. Biosci. 2010, 15, 348–358.
- Godwin, J.W.; Rosenthal, N. Scar-free wound healing and regeneration in amphibians: Immunological influences on regenerative success. Differentiation 2014, 87, 66–75.
- Rodgers, A.K.; Smith, J.J.; Voss, S.R. Identification of immune and non-immune cells in regenerating axolotl limbs by single-cell sequencing. Exp. Cell Res. 2020, 394, 112149.
- Nurden, A.T. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front. Biosci. 2018, 23, 726–751.
- Ferdous, F.; Scott, T.R. A comparative examination of thrombocyte/platelet immunity. Immunol. Lett. 2015, 163, 32–39.
- Imokawa, Y.; Brockes, J.P. Selective activation of thrombin is a critical determinant for vertebrate lens regeneration. Curr. Biol. 2003, 13, 877–881.
- Imokawa, Y.; Simon, A.; Brockes, J.P. A critical role for thrombin in vertebrate lens regeneration. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 765–776.
- Godwin, J.W.; Liem, K.F.; Brockes, J.P. Tissue factor expression in newt iris coincides with thrombin activation and lens regeneration. Mech. Dev. 2010, 127, 321–328.
- Yoshikawa, T.; Mizuno, A.; Yasumuro, H.; Inami, W.; Vergara, M.N.; del Rio-Tsonis, K.; Chiba, C. MEK-ERK and heparin-susceptible signaling pathways are involved in cell-cycle entry of the wound edge retinal pigment epithelium cells in the adult newt. Pigment. Cell Melanoma Res. 2012, 25, 66–82.
- Mizuno, A.; Yasumuro, H.; Yoshikawa, T.; Inami, W.; Chiba, C. MEK-ERK signaling in adult newt retinal pigment epithelium cells is strengthened immediately after surgical induction of retinal regeneration. Neurosci. Lett. 2012, 523, 39–44.
- Susaki, K.; Chiba, C. MEK mediates in vitro neural transdifferentiation of the adult newt retinal pigment epithelium cells: Is FGF2 an induction factor? Pigment. Cell Res. 2007, 20, 364–379.
- Ikegami, Y.; Mitsuda, S.; Araki, M. Neural cell differentiation from retinal pigment epithelial cells of the newt: An organ culture model for the urodele retinal regeneration. J. Neurobiol. 2002, 50, 209–220.
- Mitusda, S.; Yoshii, C.; Ikegami, Y.; Araki, M. Tissue interaction between the retinal pigment epithelium and the choroid triggers retinal regeneration of the newt Cynops pyrrhogaster. Dev. Biol. 2005, 280, 122–132.
- Qin, Z.; Kidd, A.R., 3rd; Thomas, J.L.; Poss, K.D.; Hyde, D.R.; Raymond, P.A.; Thummel, R. FGF signaling regulates rod photoreceptor cell maintenance and regeneration in zebrafish. Exp. Eye Res. 2011, 93, 726–734.
- Forouzanfar, F.; Shojapour, M.; Aghili, Z.S.; Asgharzade, S. Growth Factors as Tools in Photoreceptor Cell Regeneration and Vision Recovery. Curr. Drug Targets 2020, 21, 573–581.
- Park, C.M.; Hollenberg, M.J. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev. Biol. 1989, 134, 201–205.
- Spence, J.R.; Madhavan, M.; Aycinena, J.C.; Del Rio-Tsonis, K. Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6. Mol. Vis. 2007, 13, 57–65.
