You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The whole variety of biochemical reactions, in which the nitric oxide (NO) molecule is produced, proceeds in plants by two major alternative mechanisms: (1) the oxidative or arginine-dependent and (2) the reductive or nitrite-dependent pathways, and both of them are characterized by a complex nature.
- nitric oxide
- NO production
- nitrate reductase
1. Mechanisms of Nitric Oxide Production in Plants
The whole variety of biochemical reactions, in which the NO molecule is produced, proceeds in plants by two major alternative mechanisms: (1) the oxidative or arginine-dependent and (2) the reductive or nitrite-dependent pathways (Figure 1), and both of them are characterized by a complex nature [1][2][3].
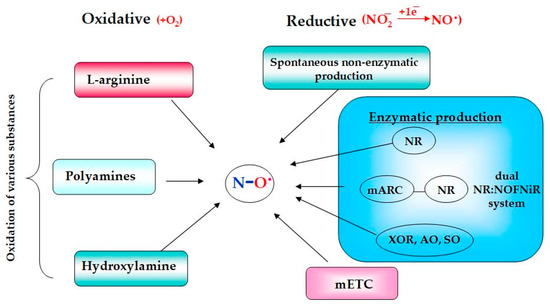
Figure 1. Schematic presentation of the main pathways of NO formation in plants.
2. Oxidative Mechanisms
Among the oxidative mechanisms of NO production, the most thoroughly studied are the oxidation of L-arginine and polyamines. Moreover, oxidative generation of NO can occur in plants from hydroxylamine [2][4][5]. The L-arginine-dependent pathway is mediated by the enzymatic activity of nitric oxide synthases (NOSs). During this two-step process, L-arginine interacts initially with molecular oxygen to form N-hydroxy-L-arginine that in turn is converted to citrulline with the release of gaseous NO (Figure 2). The guanidine group of arginine becomes the source of the nitrogen atom of the resulting NO molecule, while the oxygen atom comes from the oxygen molecule participating in the reaction [6][7][8].
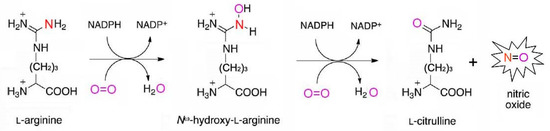
Figure 2. Schematic presentation of a two-step reaction of arginine-dependent NO formation.
The reaction has been well studied in animals, in which it is catalyzed by specific enzymes known as NO-synthases (NOSs, EC 1.14.13.39) [6][8]. Three isoforms of NO-synthases have been identified in mammals: NOS1—neuronal (nNOS); NOS2—inducible or macrophage (iNOS); NOS3—endothelial (eNOS), encoded by three different genes [6][8]. It should be noted that NOS1 and NOS3 are characterized by constitutive expression, while NOS2 is distinguished by inducible activity as indicated by the name of this isoform. Its activation occurs during organism infection and its main functions are connected with protective immune reactions. All isoforms of NO-synthases have a similar structure and operation, being in a homodimeric form. Each monomeric subunit contains two domains: (1) the reductase- or NAD(P)H-FAD-FMN-domain located in the C-terminal region; (2) the oxygenase- or Fe-Heme-domain located in the N-terminal region of the molecule, containing binding sites for heme tetrahydrobiopterin (THB4) and L-arginine. There is a calmodulin (CaM)-binding site between the reductase and oxygenase domains, playing an important role in the regulation of NOS enzymatic activity [6][8][9].
Numerous experimental data in which NO production was analyzed by the accumulation of citrulline, as well as the suppression of NO synthesis in plants by inhibitors of mammals’ NOS-activity, suggest the possibility of NO generation in plants in an arginine-dependent manner [3][4][7][10]. However, NOS-like enzymes similar to mammalian ones have not been identified yet in embryophytes, as evidenced by the large-scale screening of more than a thousand plant transcriptomes [8][11]. Among photosynthetic organisms, NOS-like sequences were found in 15 species of unicellular algae, including Ostreococcus tauri, in which the presence of a functionally active NOS enzyme with 45% homology with the NOS sequences of mammals was previously demonstrated [3][9][11][12][13]. Unicellular green algae of the Ostreococcus family are the smallest eukaryotic organisms living today on the planet that separated from the common ancestor with higher plants into an independent phylogenetic group in the early stages of evolution. There is an assumption that, in the course of evolutionary development during the transition to a terrestrial lifestyle, green plants have lost genes similar to mammalian NOS genes [8][13]. At the same time, in numerous experimental studies it was clearly demonstrated that the use of inhibitors of mammals’ NOS-activity led to the suppression of NO production and associated biological effects in various species of higher plants [4][10]. Based on these data the existence of certain polypeptides with redox-active domains has been hypothesized, that can be aggregated into a single enzymatic complex, catalyzing reactions of arginine-dependent NO formation in higher plants [7][10][13][14]. Thus, today the task has arisen to identify specific enzymes in higher plants that catalyze the reactions of arginine oxidation leading to production of L-citrulline and the release of NO [11][15][16][17].
Oxidative formation of NO in plants can occur from polyamines (PAs) with the involvement of PAs catabolism enzymes in particular copper amine oxidases (CuAO, EC 1.4.3.22). PAs are low-molecular nitrogen-containing compounds playing a certain role in plant growth and development as well as in stress adaptation [18]. The group of polyamines includes compounds such as spermine and spermidine, which can originate from arginine with the participation of arginase [2][7]. By using two mutant lines of Arabidopsis thaliana with over- or down-expression of the arginase gene, it was shown that NO production in these plants depends on the availability of arginine. Arginine-deficient plants were characterized by a reduced level of NO production, which was recovered after treatment with spermine [19]. Exogenous application of spermine or spermidine caused an increased production of NO in the root tips and in the vascular tissues of the leaves in A. thaliana seedlings treated with polyamines [2][20]. It is assumed that the generation of NO from PAs can be carried out with the participation of CuAO since the CuAO1 mutants of A. thaliana were characterized by impaired NO synthesis in response to treatment with polyamines [3][13][21]. There are 10 different genes of putative CuAOs that have been identified in A. thaliana. It was shown that, along with CuAO1, CuAO8 and polyamine oxidase (PAO, EC 1.5.3.13) can also participate in the oxidation of polyamines to NO [13][22][23].
Hydroxylamine (HA, NH2OH) can be another source of NO oxidative production in terrestrial plants. Evidence in favor of this assumption can be found in the data regarding enzymatic NO production from HA in bacterial and animal cells [2][24]. For example, HA-dependent NO formation in nitrifying bacteria may happen with the participation of hydroxylamine reductase [25]. It is also known that NO can be released from HA by interaction with superoxide anion [26]. Moreover, hydroxylamine can be a precursor of L-hydroxyarginine, which is known as an intermediate in the reaction of L-arginine conversion to citrulline with NO release [2][27]. The participation of HA in the production of NO by plants is evidenced by the data obtained on nia30 mutant lines of tobacco deficient in nitrate reductase with impaired nitrite-dependent NO production. It was shown by gas-phase chemiluminescence that HA addition to the nia30 suspension culture induced NO production by mutant tobacco cells [28][29]. The physiological significance of NO production from HA is not clear enough, since conclusive evidence of its functioning in plants has not yet been obtained. It is known that NH2OH is the first product of ammonium oxidation. In addition, HA formation may be mediated by S-nitrosoglutathione reductase (GSNOR) playing a key role in the maintaining of GSNO homeostasis, which functions as the main storage and transport form of NO in all living organisms including plants [2][30].
3. Reductive Nitrite-Dependent Mechanisms
The reductive pathways of NO generation are associated with reactions of reduction of nitrate/nitrite that occur or can proceed with the participation of enzymes able to catalyze the one-electron reduction of nitrite to NO. Non-enzymatic nitrite-dependent NO formation was detected in the apoplast of the barley aleurone layer taking place under low pH and high nitrate concentrations [4][31][32]. This reaction was described with the equation:
2NO2 + 2H+ ↔ 2HNO2 ↔ NO + NO2− + H2O ↔ 2NO + ½O2 + H2O
Non-enzymatic NO production is enhanced in the presence of reducing agents such as ascorbic acid or certain phenolic compounds. It has been suggested that NO produced in the apoplast of barley seeds is involved in the regulation of their germination [32].
Enzymatic reductive NO formation can occur in mitochondria with the participation of components of the mitochondrial electron transport chain (mETC), as well as in chloroplasts, peroxisomes, and in the cytoplasm of plant cells [15][33]. An important role in the reductive process of enzymatic NO production belongs to molybdenum-containing enzymes, including nitrate reductase (NR; EC 1.7.1.1). It is well known that the main functions of NR are associated with the process of nitrogen fixation, in which it catalyzes the reaction of the two-electron reduction of nitrate to nitrite. Moreover, under certain conditions, such as hypoxia and low pH values, it is able to catalyze the one-electron reduction of nitrite to NO [2][3][4].
3.1. NO Formation with Participation of Molybdenum Containing Enzymes
The family of plant molybdoenzymes is composed of five members: in addition to nitrate reductase, it includes amidoxime reductase or mitochondrial amidoxime reducing component (mARC), xanthine oxidoreductase (XOR, EC 1.17.3.2), aldehyde oxidase (AO, EC 1.2.3.1), and sulfide oxidase (SO, EC 1.8.3.1). These enzymes perform their own specific functions and at the same time have the potential ability to catalyze nitrite-derived NO formation. Therefore, they are designated in the literature as “non-dedicated” nitrite reductases [34][35].
The nitrite-reducing NO-forming activity of eukaryotic molybdoenzymes is determined by the presence of a specific molybdenum cofactor (Moco), the chemical nature of which was deciphered in 1992 by K. V. Rajagopalan and J. L. Johnson in their pioneering work [36][37][38]. Moco has a unique square-pyramidal structure (Figure 3), formed when the molybdenum atom interacts by thiol bonds with the tricyclic pterin called molybdopterin (MPT) also known in literature as pyranopterin or tetrahydropyranopterin. In general, the square-pyramidal geometry of Moco is stabilized by five coordination bounds between the Mo atom and: (1) one apical oxo-group; (2–3) two sulfur atoms of pyranopterin; (4) one labile –OH/OH2 group; (5) one terminal thiol group of cysteine residue or inorganic sulfur atom [34][37][38][39]. Molybdoenzymes have been classified into two families depending on the manner of Moco binding to the protein part of the enzyme molecule. The first is the SO family comprising three members (NR, SO, and mARC); and the second is the XO family including two other members (XOR and AO). In the case of the SO family Moco binds covalently through the terminal thiol group of the Cys residue, or through the terminal sulfur atom in the Moco structure of XO members [34][40].
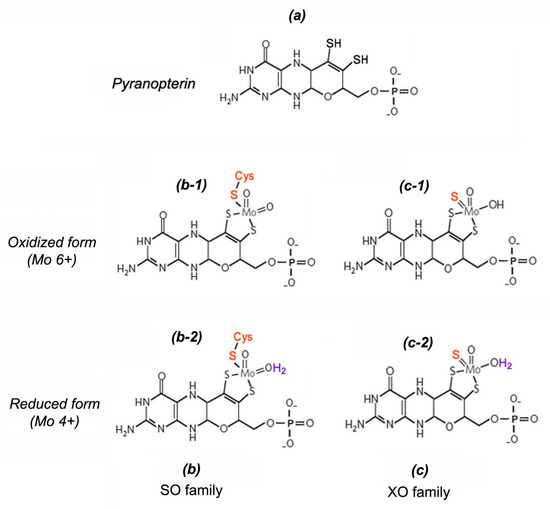
Figure 3. Structural organization of molybdenum cofactor (Moco): (a)—structure of pyranopterin-dithiolate heterocycle; (b)—Moco structure from molybdoenzymes of sulfite oxidase (SO) family in oxidized form (b-1) and in reduced form (b-2); (c)—Moco structure from molybdoenzymes of xanthine oxidase (XO) family in oxidized form (c-1) and in reduced form (c-2).
One of the reaction types catalyzed by molybdoenzymes is the transfer of an oxygen atom from the substrate to water (oxygen atom abstraction) taking place at the expense of the exchange of two electrons at which the oxidation state of molybdenum atom rotates between Mo6+ and Mo4+ [34][41]. This mechanism underlies the NR-dependent reaction of nitrate reduction to nitrite (Equation (1)).
ONO2− + 2e− + 2H+ → NO2 + H2O
NO2− + e− + H+ → NO + (1/2)H2O
A similar process takes place in the reaction of one-electron nitrite-reduction to NO catalyzed by molybdoenzymes in particular by the above-mentioned nitrate reductase (Equation (2)).
Role of Nitrate Reductase in the Process of NO Production
Assimilatory NR (Nitrate reductase; EC 1.7.1.1) is a ubiquitous enzyme in plants, fungi, and algae, responsible for the first step of nitrogen fixation and catalyzing the reduction of nitrate to nitrite [2][4][42]. It belongs to NAD(P)H-dependent molybdenum-containing enzymes of the SO family containing the Moco with a characteristic square-pyramidal structure [34]. In plants, the enzyme is found in cytosolic as well as in cytoplasmic membrane-bound forms [16]. As mentioned above the main function of NR is participation in the reaction of two-electron nitrate reduction to nitrite (NO3− → NO2−). Then nitrite is reduced to ammonium with the participation of another key enzyme of nitrogen metabolism, assimilatory nitrite reductase (NiR), which is necessary for subsequent synthesis of amino acids. At the same time, NR involvement in the one-electron reduction of nitrite was found, using NAD(P)H as the electron donor producing the NO molecule (Figure 4). The reaction of NR-dependent nitrite reduction to NO can be presented in an equation:
NAD(P)H + 3H2O + 2NO2− → NAD(P)+ + 5H2O+ 2NO
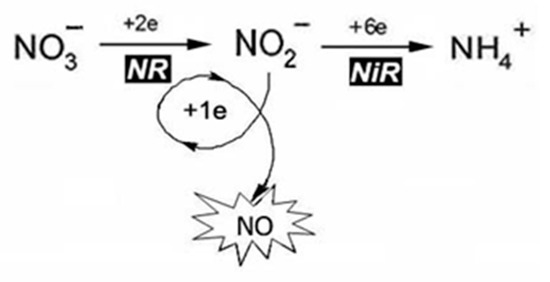
Figure 4. Schematic presentation of NO formation during reactions of nitrogen assimilation.
It is proposed to designate the nitrite-reducing capability of plant NR leading to NO generation as nitrite: NO-reductase activity (Ni-NR activity) [2][4][43].
At the present time the NR-dependent pathway is considered as one of the main mechanisms of NO production in plants. However, it should be stressed that the role of nitrate reductase in NO generation is not only its direct participation in one-electron nitrite reduction, but also in the production of nitrite itself, which is the main substrate for reductive mechanisms of NO biosynthesis in plants. In addition, NR can mediate electron transfer from NAD(P)H to another molybdoenzyme—mARC, which in turn directly catalyzes the reduction of nitrite to NO, as demonstrated in the model alga Chlamydomonas reinhardtii [4][44][45]. Meanwhile, NR is also involved in the utilization of NO and maintaining its homeostasis in plants, since this multifunctional enzyme is able to transfer electrons from NAD(P)H to hemoglobin THB1, which carry out a two-electron conversion of NO to nitrate [13][44].
A three-dimensional configuration of nitrate reductase, which is characterized by a multidomain structure, was proposed in the late 1990s [46]. At the same time new details of the molecular organization and functioning of NR have been revealed in the last two decades [42][47]. The enzyme is able to perform the catalytic activity being in homodimeric form (holo-NR), which is composed of monomers with Mm ~100 kDa and an approximate size of 900 amino acid units. Each monomer subunit harbors three prosthetic groups: (1) Moco-containing domain; (2) cytochrome-b5-containing Fe-heme- (Heme-) domain and (3) FAD-domain composed of FAD-cofactor interacting with the universal cellular reducing agent NAD(P)H (Figure 5). Two flexible regions Hinge1 and Hinge2 are located between the domains. The Hinge1 region which is located between the Moco and Heme domains is a variable amino acid fragment playing an important role in the regulation of NR catalytic activity by phosphorylation of a serine residue. The Hinge 2 region is located between the Heme and FAD domains. In the Moco domain there is a dimerization site that serves to assemble monomers and hold them together to make a homodimer form of enzyme [42][46].
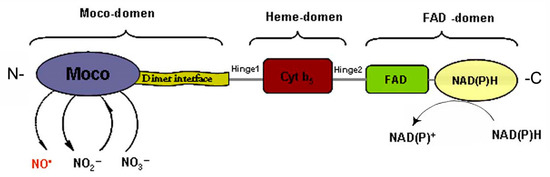
Figure 5. Multi-domain organization of nitrate reductase (NR). Structural FAD-, Heme- and Moco- domains are designated by curly braces at the top of the scheme. Functional sites of the domains are highlighted by different colors.
It is known that the potential difference of the redox centers determines the formation of electron transport chains in biological systems. The redox potential values for the three prosthetic groups of the homodimeric form NR (holo-NR) were evaluated using various approaches, in particular by voltammetric methods. It has been established that the redox potential values for the FAD-center is poised from 210 to 190, for the Fe-heme domain from 160 to 130, and for the Moco-center this magnitude is near to 0 mV. Thus, it becomes clear that this system determines the formation of a downward flow of electrons from NAD(P)H to Moco [48]. Furthermore, NR functions as a mini electron transport chain in which electrons are donated from NAD(P)H to FAD and migrated through the Heme domain to Moco where the molybdenum atom is reduced and its oxidation state cycles from +6 to +4. Then the electrons are used in the reaction of nitrate reduction, and in the course of NO generation they can be utilized for nitrite reduction [40][42][45][46].
The participation of NR in nitrite reduction with NO formation was first shown in leguminous plants [49]. Its role in NO production was also revealed later in other species, including wheat [50], A. thaliana [51][52][53], tomato [54], and maize [55]. The NO-forming NR capacity is not high and does not exceed 1% of the enzyme’s total activity and depends on various factors such as the ratio of the nitrate and nitrite ions, pH value, oxygen concentration, and post-translational modifications of the enzyme′s macromolecule. First of all, the NR-based NO production determined by nitrite contents can take place at relatively low nitrate and high nitrite concentrations because the affinity of enzyme to nitrite (Kmnitrite = 100 mM) is much higher than the normal cell nitrite concentrations (~10 nM∙g−1 FW) and exceeds the nitrate inhibition constant (Kinitrate = 50 mM) [2][56]. Second, NO-producing NR activity increases when the value of cellular pH decreases. It is noteworthy that with a decrease in pH the activity of plastidal nitrite reductase falls with concomitant nitrite accumulation, which promotes NR-inducible NO biosynthesis [2]. Moreover, the post-translational modifications of the enzyme’s molecule impact NR-dependent NO production. Phosphorylation of a conserved serine residue in one of the hinge regions of the enzyme monomer’s subunit causes NR interaction with 14-3-3 proteins leading to subsequent inactivation of the enzyme and its proteolytic degradation [2][47][57].
Different plant species are characterized by the existence of two or more NR isoforms differing by the specificity of functional activity, for example, primary interaction with NADH or NADPH, constitutive or inducible expression pattern. In A. thaliana two enzyme isoforms have been identified designated as NR1 and NR2 which are encoded by two different Nia1 and Nia2 genes. The fact that NR1 exhibits a higher Ni-NR activity, while NR2 is characterized by a preferential nitrate reductase activity, indicates that various isoforms of the enzyme differ in their specificity of functional activity in plant organisms [13]. Transgenic lines of A. thaliana with overexpression of the Nia1 and Nia2 genes and increased accumulation of the relevant proteins were distinguished by elevated levels of NO production in comparison with control plants. The significance of NR in the biosynthesis of NO has been proven using nia1 and nia2 mutant lines, as well as nia1nia2 double mutants [42].
It has been shown that NO produced with the NR participation is involved in the regulation of different developmental programs such as root morphogenesis [58], initiation of flowering [59], hormonal sensitivity, and stomatal movements [51][60]. In addition, NR-dependent NO plays an important role in the defensive reactions against pathogens as well as abiotic stress factors such as drought, salinity, HM ions, UV-radiation, and osmotic and temperature stress [42][61][62][63][64].
NO Formation by Dual System NR-mARC (NR:NOFNiR)
Until recently, NR was considered as the main enzymatic source of nitrite-dependent NO production in plant organisms [44]. However, the involvement of NR in the generation of NO molecules may be related to the enzyme’s diaphorase activity, which defines by the ability to shuttle electrons from the universal reducing agents NADH or NADPH to its reductase (FAD)- and then cytochrome (Heme)-domains. Then electrons are transferred to another molybdoenzyme, namely mARC, which directly catalyzes the nitrite reduction to NO due to the functioning of the Moco active site in the mARC structure [44][65]. Proteins of the mARC family have been described in prokaryotic and eukaryotic organisms and were first identified due to their ability to catalyze in vitro conversion of certain amidoximes to their active amino forms. Two human mARC isoforms have been identified which is both located on the outer mitochondrial membrane which determines the name of these proteins [39][66][67]. A. thaliana also contains two genes for amidoxime reductases ARC1 and ARC2, while the unicellular green algae Chlamydomonas reinhardtii has one ARC gene and the corresponding protein has cytoplasmic localization [44][68]. ARC proteins of animals and plants are characterized by an identical structure with a molecular weight of approximately 35 kDa performing their activity in monomeric form, unlike other molybdoenzymes functioning only when assembling to homodimers. The mARC structure has one prosthetic group, represented by Moco. Using animal systems, it was shown that the activity of mARC requires the participation of other protein partners, in particular, mitochondrial proteins such as cytochrome b5 reductase (Cyt b5 R) and cytochrome b5 (Cyt b5), and the active center of mARC facing into the cytoplasm. This three-component system is called the Amidoxime Reducing Complex (ARCO) through which electrons are transferred from NADH or NADPH over Cyt b5-R and Cyt b5 to the Moco site of mARC, where the substrate is reduced [39][44][65][67][68].
The substrate specificity of mARC has not been established conclusively. It was found that a wide range of hydroxylamines can be subjected to ARC-dependent reduction [39]. A broad range of hydroxylamines have been identified that can undergo ARC-dependent reduction, among which are the N-hydroxylated forms of nucleotide bases, in particular N-hydroxyaminopurine and N-hydroxycytosine. This fact may indicate the participation of mARC in the neutralization of mutagenic and harmful effects of abnormal bases [44][67]. Another substrate for mARC can be N-ω-hydroxy-L-arginine, which is considered to be an intermediate in NOS-dependent NO generation, and therefore mARC may act as a negative regulator of NOS activity [39].
It has been shown that human proteins hmARC1 and hmARC2 are involved in the production of NO occurring on the outer side of the mitochondrial membrane with the participation of the above-mentioned protein partners of mARC—Cyt b5 R and Cyt b5 [65][69]. It should be noted that the spatial combination of prosthetic groups in the mitochondrial ARCO system has an organization similar to the NR structure and resembles the alignment which can be observed in the intramolecular electron transport chain, in which the reductase (FAD)- and the Cyt b5 (Heme)- domains are also present [39]. Using various NR mutant lines of the alga Ch. Reinhardtii with deletions in the Moco or in the reductase and cytochrome domains of nitrate reductase, its role was clearly demonstrated in the electron donations to the Moco-center of ARC which are required for NO synthesis. It was shown that in algal cells both enzymes are localized in the cytoplasm, where NO production was detected. It is important to emphasize that ARC had a high specificity for nitrite in the presence of nitrate concentrations up to 1 mM that is such conditions when NR is unable to catalyze the reduction of nitrite to NO [44]. Taking into the account the critical role of this signaling molecule in the metabolism and vitality of photosynthetic organisms, it was concluded that the main function of plant ARCs is their participation in the process of NO production. For this reason, it was proposed to name plant ARC proteins as NOFNiR (NO Forming Nitrite Reductase), and the two-component NO-producing system formed by NR with ARC was denoted by the abbreviation NR:NOFNiR [4][44][45].
Possible Role of XOR, AO, SO in the Process of NO Formation
The ability to reduce nitrite to NO has been identified for molybdoenzymes such as xanthine oxidoreductase (XOR), aldehyde oxidase (AO), and sulfite oxidase (SO), so these enzymes, along with NR and mARC, were included in the group of so-called non-dedicated NO-forming nitrite reductases [34]. Protein molecules XOR and AO have a similar domain organization, and due to the structural similarity of their Moco domains, these enzymes were assigned to the XO family. They are homodimeric flavin-containing proteins with a molecular weight of about 290 kDa. Each monomeric unit contains two iron-sulfur centers [2Fe–2S], one Moco, and one FAD cofactor [35]. The XOR enzyme is ubiquitous in animals and plants and is characterized by a predominantly peroxisomal localization, although in animal tissues it is also detected in the cytoplasm and on the outer membrane of some cells [2][7][34]. It can function in the main enzyme form known as xanthine dehydrogenase (XDH) or in the form of xanthine oxidase (XO) which is formed from XDH, due to reversible oxidation of cysteine residues in its polypeptide chain, such as Cys535 or Cys992 in bovine XOR, or irreversible oxidation of other amino acid residues, such as Lys551 or Lys569 in the same protein [34]. In aerobic conditions both enzyme forms catalyze oxidation of hypoxanthine to xanthine followed by the formation of uric acid. XDH activity induces reduction of NADP to NADPH, while the activity of XO is associated with oxygen molecule reduction followed by superoxide generation, which spontaneously dismutates to hydrogen peroxide [2]. In different independent experiments using such methods as electron paramagnetic spectroscopy, chemiluminescence, or by using NO-selective electrodes, it was shown that XOR isolated from animal tissues is able in vitro to reduce the nitrite with NO formation under anaerobic conditions in the presence of NADH [34][70]. The involvement of XOR in NO production in plant organisms has been demonstrated using allopurinol which is an inhibitor of XOR activity. Its application repressed NO biosynthesis in the roots of white lupine, indicating a high possibility of XOR involvement in the formation of NO in plant organisms [4][71].
Aldehyde oxidases, as well as XORs, have been found in animal and plant cells and characterized by cytoplasmic localization. The specific functions of these enzymes are to catalyze the oxidation reactions of various aromatic and non-aromatic aldehydes with the formation of their corresponding carboxylic acids [67][72]. Four genes of aldehyde oxidases (AO1–AO4) have been identified in A. thaliana. The assembly of their expression products in various combinations leads to the formation of homo- and heterodimers with different substrate specificities. For example, the AO1 homodimer is able to oxidize indolyl-3-acetaldehyde to indolyl-3-acetic acid, which belongs to the hormonal substances of the auxin family. Another AO from Arabidopsis known as AOδ, active in an AO2/AO3 heterodimer form, is characterized by high specificity for abscisic aldehyde, which is a precursor of the ABA hormone. The involvement of plant AOs in the biosynthesis of phytohormones may indicate their role in various aspects of plant growth and development, including seed germination, vegetative growth, and adaptation to environmental stress factors [72]. The participation of these enzymes in NO production is evidenced by the data of the experiments in vitro with animal AOs, in which their ability to catalyze the nitrite reduction to NO in the presence of nitrite and such reducing substrates as aldehyde N-methylnicotinamide and NADH [34] has been shown, although AOs’ involvement in NO formation in plant organisms remains to be elucidated. Considering the close structural similarity of AO and XOR, it has been assumed that these molybdoenzymes can play a certain role in plant NO production being the additional enzymatic source of its biosynthesis in photosynthetic organisms [2][3][4].
NO biosynthesis in plants can occur with the involvement of sulfite oxidase (SO) since its nitrite reductase NO-synthesizing capacity has been detected in mammals [34][40][73]. The main functions of SO are associated with the process of sulfite detoxifying when its oxidation to sulfate occurs.
SO32− + H2O → SO42− + 2H+ + 2e−
This reaction is the final step in the oxidative degradation of sulfur-containing amino acids (cysteine and methionine). The SO structure of mammals or birds is characterized by a homodimeric structure and the presence of two prosthetic groups in each subunit: the N-terminal Heme-domain and the C-terminal molybdenum cofactor (unlike NR in which the Moco site is located in the N-terminus). It was revealed that SO in animal cells is localized in the mitochondrial intermembrane space, where the electrons that are released during the oxidation of sulfite are transferred through the Heme-domain to the universal electron acceptor cytochrome c. The SO Heme-domain can be hydrolyzed by partial proteolysis, resulting in a modified form of the enzyme with only one Moco redox-site. This incomplete form of SO is unable to transfer electrons to cytochrome c, but retains the ability to oxidize sulfite, which can be carried out in the presence of artificial electron acceptors [34]. The structure of plant sulfite oxidases was described after identification of the corresponding cDNA in Arabidopsis and comparative analysis of plant and animal SO sequences. Herewith the closest similarity reaching about 46% homology at the level of amino acid sequences was found between plant and chicken SO [74]. In addition, their Moco-containing domains were characterized by close structural similarity to that of NR [74][75]. It was found that plant SO also has a homodimeric organization and, like the truncated form of the animal enzyme, does not contain the Heme-domain. It transfers the electrons released during the oxidation of sulfite to molecular oxygen with the formation of superoxide and its subsequent dismutation to hydrogen peroxide. So, it is not surprising that in plant cells SO is localized in the peroxisomal matrix in which the processes of peroxide utilization take place [7][35][67].
It is notably that mammalian SO in the presence of sulfite is able to catalyze the one-electron reduction of nitrite to NO. The SO-catalyzed production of NO occurs at the molybdenum center, and sulfite acts as a reducing agent [34][73]. The reaction rate depends on the medium pH and increases approximately two-fold as the pH decreases from 7.4 to 6.5, although, even at these pH values, the nitrite-reducing activity of SO is significantly lower than that of XO and AO [34]. In addition, SO-catalyzed NO production increases as the oxygen content in the medium drops. Hypoxia constrains the intramolecular electron flow from the Moco of the SO and their movement to cytochrome c, which is accompanied by an increase in electron movement in nitrite and activation of its reduction with NO formation [34][73]. The data on the NO-producing activity of the mammal SO indicate the possibility of this molybdoenzyme involvement in NO generation in plant organisms, which needs to be confirmed experimentally [2][4][40].
3.2. Participation of Mitochondrial Electron Transport Chain (mETC) in NO Formation
There is evidence that the reactions of nitrite reduction with NO formation can be associated with the activity of mETC [2][4][76][77]. Mitochondria exhibit nitrite reductase activity and become an important source of NO in the cells of microorganisms, animal and plant tissues under conditions of hypoxia. It has been shown that, in the cells of some fungi, the formation of ATP in the absence of oxygen is based on the use of nitrite as a terminal electron acceptor [2][78][79]. Moreover, ATP production mediated by the reduction of nitrite to NO was revealed in some plant species under anoxia, in particular in the root tissues of barley and rice plants [80]. It was found that mETC enzyme components of complexes III and IV participate in the process of nitrite reduction with NO formation by the means of inhibitory analysis. The use of myxothiazole and cyanide, which are inhibitors of the activity of cytochrome c reductase (complex III) and cytochrome c oxidase (complex IV), respectively, led to the suppression of nitrite-dependent ATP generation associated with production of NO in the roots of barley and rice plants [2][80]. There is an opinion that the process of nitrite reduction to NO in mitochondria during anoxia has a protective effect on their structural organization contributing to maintaining of their activity and, in general, to increasing the viability of plant organisms under unfavorable conditions [2][4][33].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241411637
References
- Verma, N.; Tiwari, S.; Singh, V.P.; Prasad, S.M. Nitric oxide in plants: An ancient molecule with new tasks. Plant Growth Regul. 2020, 90, 1–13.
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168.
- Mamaeva, A.S.; Fomenkov, A.A.; Nosov, A.V.; Moshkov, I.E.; Novikova, G.V.; Mur, L.A.J.; Hall, M.A. Regulatory role of nitric oxide in plants. Russ. J. Plant Physiol. 2015, 62, 427–440.
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2018, 69, 3401–3411.
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133.
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A forty year journey: The generation and roles of NO in plants. Nitric Oxide 2019, 93, 53–70.
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide (NO) scaffolds the peroxisomal protein–protein interaction network in higher plants. Int. J. Mol. Sci. 2021, 22, 2444.
- Del Castello, F.; Nejamkin, A.; Cassia, R.; Correa-Aragunde, N.; Fernández, B.; Foresi, N.; Lombardo, C.; Ramirez, L.; Lamattina, L. The era of nitric oxide in plant biology: Twenty years tying up loose ends. Nitric Oxide 2019, 85, 17–27.
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Structure diversity of nitric oxide synthases (NOS): The emergence of new forms in photosynthetic organisms. Front. Plant Sci. 2013, 4, 232.
- Karpets, Y.V.; Kolupaev, Y.E. Functional interaction of nitric oxide with reactive oxygen species and calcium ions at development of plants adaptive responses. Bull. Kharkiv Natl. Agrar. Univ. Ser. Biol. 2017, 2, 6–31.
- Jeandroz, S.; Wipf, D.; Stuehr, D.J.; Lamattina, L.; Melkonian, M.; Tian, Z.; Zhu, Y.; Carpenter, E.J.; Wong, G.K.; Wendehenne, D. Occurrence, structure, and evolution of nitric oxide synthase-like proteins in the plant kingdom. Sci. Signal. 2016, 9, re2.
- León, J.; Costa-Broseta, Á. Present knowledge and controversies, deficiencies, and misconceptions on nitric oxide synthesis, sensing, and signaling in plants. Plant Cell Environ. 2020, 43, 1–15.
- Recalde, L.; Gómez Mansur, N.M.; Cabrera, A.V.; Matayoshi, C.L.; Gallego, S.M.; Groppa, M.D.; Benavides, M.P. Unraveling ties in the nitrogen network: Polyamines and nitric oxide emerging as essential players in signalling roadway. Ann. Appl. Biol. 2021, 178, 192–208.
- Corpas, F.J.; Barroso, J.B. Nitric oxide synthase-like activity in higher plants. Nitric Oxide 2017, 68, 5–6.
- Corpas, F.J.; Del Río, L.A.; Palma, J.M. Impact of nitric oxide (NO) on the ROS metabolism of peroxisomes. Plants 2019, 8, 37.
- Prochazkova, D.; Haisel, D.; Pavlikova, D. Nitric oxide biosynthesis in plants–the short overview. Plant Soil Environ. 2014, 60, 129–134.
- Kolbert, Z.; Lindermayr, C.; Loake, G.J. The role of nitric oxide in plant biology: Current insights and future perspectives. J. Exp. Bot. 2021, 72, 777–780.
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 2018, 255, 153–162.
- Flores, T.; Todd, C.D.; Tovar-Mendez, A.; Dhanoa, P.K.; Correa-Aragunde, N.; Hoyos, M.E.; Brownfield, D.M.; Mullen, R.T.; Lamattina, L.; Polacco, J.C. Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol. 2008, 147, 1936–1946.
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.; Scherer, G.F. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354.
- Wimalasekera, R.; Villar, C.; Begum, T.; Scherer, G.F. COPPER AMINE OXIDASE1 (CuAO1) of Arabidopsis thaliana contributes to abscisic acid-and polyamine-induced nitric oxide biosynthesis and abscisic acid signal transduction. Mol. Plant 2011, 4, 663–678.
- Groß, F.; Rudolf, E.E.; Thiele, B.; Durner, J.; Astier, J. Copper amine oxidase 8 regulates arginine-dependent nitric oxide production in Arabidopsis thaliana. J. Exp. Bot. 2017, 68, 2149–2162.
- Janků, M.; Tichá, T.; Luhová, L.; Petřivalský, M. Compartmentalization of reactive oxygen species and nitric oxide production in plant cells. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; Chapter 40, pp. 923–945.
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134.
- Caranto, J.D.; Lancaster, K.M. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc. Natl. Acad. Sci. USA 2017, 114, 8217–8222.
- Hooper, A.B.; Terry, K.R. Hydroxylamine oxidoreductase of Nitrosomonas: Production of nitric oxide from hydroxylamine. Biochim. Biophys. Acta-Enzym. 1979, 571, 12–20.
- DeMaster, E.G.; Raij, L.; Archer, S.L.; Weir, E.K. Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of L-arginine to nitric oxide. Biochem. Biophys. Res. Commun. 1989, 163, 527–533.
- Rümer, S.; Gupta, K.J.; Kaiser, W.M. Oxidation of hydroxylamines to NO by plant cells. Plant Signal. Behav. 2009, 4, 853–855.
- Rümer, S.; Gupta, K.J.; Kaiser, W.M. Plant cells oxidize hydroxylamines to NO. J. Exp. Bot. 2009, 60, 2065–2072.
- Hancock, J.T. Nitric oxide signaling in plants. Plants 2020, 9, 1550.
- Yamasaki, H. Nitrite-dependent nitric oxide production pathway: Implications for involvement of active nitrogen species in photoinhibition in vivo. Philos. Trans. R. Soc. Ser. B Biol. Sci. 2000, 355, 1477–1488.
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 2004, 16, 332–341.
- Gupta, K.J.; Kumari, A.; Florez-Sarasa, I.; Fernie, A.R.; Igamberdiev, A.U. Interaction of nitric oxide with the components of the plant mitochondrial electron transport chain. J. Exp. Bot. 2018, 69, 3413–3424.
- Maia, L.B.; Moura, J.J.G. Nitrite reduction by molybdoenzymes: A new class of nitric oxide-forming nitrite reductases. J. Biol. Inorg. Chem. 2015, 20, 403–433.
- Wang, J.; Keceli, G.; Cao, R.; Su, J.; Mi, Z. Molybdenum-containing nitrite reductases: Spectroscopic characterization and redox mechanism. Redox Rep. 2017, 22, 17–25.
- Rajagopalan, K.V.; Johnson, J.L. The pterin molybdenum cofactors. J. Biol. Chem. 1992, 267, 10199–10202.
- Mendel, R.R. Metabolism of Molybdenum. In Metallomics and the Cell Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; pp. 503–528.
- Nieter Burgmayer, S.J.; Kirk, M.L. The role of the pyranopterin dithiolene component of moco in molybdoenzyme catalysis. In Metallocofactors That Activate Small Molecules. Structure and Bonding; Ribbe, M., Ed.; Springer Nature: Basel, Switzerland, 2019; Volume 179, pp. 101–151.
- Bender, D.; Schwarz, G. Nitrite-dependent nitric oxide synthesis by molybdenum enzymes. FEBS Lett. 2018, 592, 2126–2139.
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174.
- Cordas, C.M.; Moura, J.J.G. Molybdenum and tungsten enzymes redox properties–A brief overview. Coord. Chem. Rev. 2019, 394, 53–64.
- Mohn, M.A.; Thaqi, B.; Fischer-Schrader, K. Isoform-specific NO synthesis by Arabidopsis thaliana nitrate reductase. Plants 2019, 8, 67.
- Pardo-Hernández, M.; López-Delacalle, M.; Rivero, R.M. ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 2020, 9, 1078.
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, Á.; Ocaña-Calahorro, F.; Mariscal, V.; Carreras, A.; Barroso, J.B.; Galván, A.; Fernández, E. A dual system formed by the ARC and NR molybdoenzymes mediates nitrite-dependent NO production in Chlamydomonas. Plant Cell Environ. 2016, 39, 2097–2107.
- Tejada-Jimenez, M.; Llamas, A.; Galván, A.; Fernández, E. Role of nitrate reductase in NO production in photosynthetic eukaryotes. Plants 2019, 8, 56.
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 277–303.
- Kaiser, W.M.; Planchet, E.; Rumer, S. Nitrate Reductase and Nitric Oxide. In Nitrogen Metabolism in Plants in the Post-Genomic Era; Wiley: Hoboken, NJ, USA, 2018; Volume 42.
- Campbell, W. Structure and function of eukaryotic NAD(P)H: Nitrate reductase. Cell. Mol. Life Sci. 2001, 58, 194–204.
- Dean, J.V.; Harper, J.E. The conversion of nitrite to nitrogen oxide(s) by the constitutive NAD(P)H-nitrate reductase enzyme from soybean. Plant Physiol. 1988, 88, 389–395.
- Rosales, E.P.; Iannone, M.F.; Groppa, M.D.; Benavides, M.P. Nitric oxide inhibits nitrate reductase activity in wheat leaves. Plant Physiol. Biochem. 2011, 49, 124–130.
- Hao, F.; Zhao, S.; Dong, H.; Zhang, H.; Sun, L.; Miao, C. Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 298–307.
- Chen, Z.H.; Wang, Y.; Wang, J.W.; Babla, M.; Zhao, C.; García-Mata, C.; Sani, E.; Differ, C.; Mak, M.; Hills, A.; et al. Nitrate reductase mutation alters potassium nutrition as well as nitric oxide-mediated control of guard cell ion channels in Arabidopsis. New Phytol. 2016, 209, 1456–1469.
- Zhao, C.; Cai, S.; Wang, Y.; Chen, Z. Loss of nitrate reductases NIA1 and NIA2 impairs stomatal closure by altering genes of core ABA signaling components in Arabidopsis. Plant Signal. Behav. 2016, 11, e1183088.
- Jin, C.W.; Du, S.T.; Zhang, Y.S.; Lin, X.Y.; Tang, C.X. Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomato (Solanum lycocarpum). Ann. Bot. 2009, 104, 9–17.
- Manoli, A.; Begheldo, M.; Genre, A.; Lanfranco, L.; Trevisan, S.; Quaggiotti, S. NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. J. Exp. Bot. 2014, 65, 185–200.
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 2002, 53, 103–110.
- Lea, U.S.; Ten Hoopen, F.; Provan, F.; Kaiser, W.M.; Meyer, C.; Lillo, C. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in high nitrite excretion and NO emission from leaf and root tissue. Planta 2004, 219, 59–65.
- Lombardo, M.C.; Lamattina, L. Nitric oxide is essential for vesicle formation and trafficking in Arabidopsis root hair growth. J. Exp. Bot. 2012, 63, 4875–4885.
- Seligman, K.; Saviani, E.E.; Oliveira, H.C.; Pinto-Maglio, C.A.F.; Salgado, I. Floral transition and nitric oxide emission during flower development in Arabidopsis thaliana is affected in nitrate reductase-deficient plants. Plant Cell Physiol. 2008, 49, 1112–1121.
- García-Mata, C.; Lamattina, L. Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol. 2002, 128, 790–792.
- Freschi, L. Nitric oxide and phytohormone interactions: Current status and perspectives. Front. Plant Sci. 2013, 4, 398.
- Hu, Y.; You, J.; Liang, X. Nitrate reductase-mediated nitric oxide production is involved in copper tolerance in shoots of hulless barley. Plant Cell Rep. 2015, 34, 367–379.
- Fu, Y.F.; Zhang, Z.W.; Yuan, S. Putative connections between nitrate reductase S-nitrosylation and NO synthesis under pathogen attacks and abiotic stresses. Front. Plant Sci. 2018, 9, 474.
- Zhao, G.; Zhao, Y.; Lou, W.; Su, J.; Wei, S.; Yang, X.; Wang, R.; Guan, R.; Pu, H.; Shen, W. Nitrate reductase-dependent nitric oxide is crucial for multi-walled carbon nanotube-induced plant tolerance against salinity. Nanoscale 2019, 11, 10511–10523.
- Sparacino-Watkins, C.E.; Tejero, J.; Sun, B.; Gauthier, M.C.; Thomas, J.; Ragireddy, V.; Merchant, B.A.; Wang, J.; Azarov, I.; Basu, P.; et al. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J. Biol. Chem. 2014, 289, 10345–10358.
- Havemeyer, A.; Bittner, F.; Wollers, S.; Mendel, R.; Kunze, T.; Clement, B. Identification of the missing component in the mitochondrial benzamidoxime prodrug-converting system as a novel molybdenum enzyme. J. Biol. Chem. 2006, 281, 34796–34802.
- Rana, M.S.; Bhantana, P.; Sun, X.-C.; Imran, M.; Shaaban, M.; Moussa, M.G.; Saleem, M.H.; Elyamine, A.M.; Binyamin, R.; Alam, M.; et al. Molybdenum as an essential element for crops: An overview. Biomed. J. Sci. Tech. Res. 2020, 24, 18535–18547.
- Tejada-Jiménez, M.; Chamizo-Ampudia, A.; Galván, A.; Fernández, E.; Llamas, Á. Molybdenum metabolism in plants. Metallomics 2013, 5, 1191–1203.
- Klein, J. Cellular Maturation of Mitochondrial Molybdoenzymes. Ph.D. Thesis, Universität zu Köln, Köln, Germany, 2012. Available online: http://kups.ub.uni-koeln.de/id/eprint/4944 (accessed on 6 November 2012).
- Godber, B.L.; Doel, J.J.; Sapkota, G.P.; Blake, D.R.; Stevens, C.R.; Eisenthal, R.; Harrison, R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 2000, 275, 7757–7763.
- Wang, B.L.; Tang, X.Y.; Cheng, L.Y.; Zhang, A.Z.; Zhang, W.H.; Zhang, F.S.; Liu, J.Q.; Cao, Y.; Allan, D.L.; Vance, C.P.; et al. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol. 2010, 187, 1112–1123.
- Kravchenko, A.P.; Toxobayeva, G.A.; Kausbekova, A.; Bersimbaev, R.I. TOR complex 1 impact on activity and genes expression of the aldehyde oxidase enzyme in Arabidopsis thaliana. News Natl. Acad. Sci. Repub. Kazakhstan-Ser. Biol. Med. 2017, 5, 57–65.
- Wang, J.; Krizowski, S.; Fischer-Schrader, K.; Niks, D.; Tejero, J.; Sparacino-Watkins, C.; Wang, L.; Ragireddy, V.; Frizzell, S.; Kelley, E.E.; et al. Sulfite oxidase catalyzes single-electron transfer at molybdenum domain to reduce nitrite to nitric oxide. Antioxid. Redox Signal. 2015, 23, 283–294.
- Eilers, T.; Schwarz, G.; Brinkmann, H.; Witt, C.; Richter, T.; Nieder, J.; Koch, B.; Hille, R.; Hänsch, R.; Mendel, R.R. Identification and biochemical characterization of Arabidopsis thaliana sulfite oxidase. A new player in plant sulfur metabolism. J. Biol. Chem. 2001, 276, 46989–46994.
- Schrader, N.; Fischer, K.; Theis, K.; Mendel, R.R.; Schwarz, G.; Kisker, C. The crystal structure of plant sulfite oxidase provides insights into sulfite oxidation in plants and animals. Structure 2003, 11, 1251–1263.
- Igamberdiev, A.U.; Ratcliffe, R.G.; Gupta, K.J. Plant mitochondria: Source and target for nitric oxide. Mitochondrion 2014, 19, 329–333.
- Ostroukhova, M.; Ermilova, E. New insights into NO generation and AOX1 upregulation in Chlamydomonas. Protistology 2019, 13, 19–25.
- Tielens, A.G.; Rotte, C.; van Hellemond, J.J.; Martin, W. Mitochondria as we don’t know them. Trends Biochem. Sci. 2002, 27, 564–572.
- Gupta, K.J.; Igamberdiev, A.U. The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion 2011, 11, 537–543.
- Stoimenova, M.; Igamberdiev, A.U.; Gupta, K.J.; Hill, R.D. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 2007, 226, 465–474.
This entry is offline, you can click here to edit this entry!
