Nitrogen (N) is a crucial nutrient that plays a significant role in enhancing crop yield. Its availability, including both supply and deficiency, serves as a crucial signal for plant development. We discuss recent advances in understanding how epigenetic modifications, including DNA methylation, histone modification, and small RNA, participate in the regulation of N response and LN adaptation. Decoding the epigenome at various levels could accelerate the functional study of how plants respond to N availability. Understanding the epigenetic control of N signaling and adaptation can lead to new strategies to improve NUE and enhance crop productivity sustainably
1. Epigenetic Regulation of N Uptake
N metabolism in plants encompasses processes such as uptake, transportation, and assimilation, all of which are subject to precise and dynamic regulation by NMGs [
9,
10]. Notably, the expression of N transporters, such as NRT2.1, can be rapidly induced within just one hour in
Arabidopsis [
30]. Altering the expression levels of genes related to N transport, assimilation, and signaling using transgenic approaches can significantly enhance crop yield or NUE [
31,
32,
33,
34,
35]. Recent studies have highlighted the impact of chromatin regulation, including histone modification and chromatin structure, in modulating the activity of N transporters [
17,
23,
36].
In dicots, histone modification, such as tri-methylation of lysine 36 or lysine 27 on histone H3 (H3K36me3, H3K27me3) are implicated in the regulation of N uptake process. In
Arabidopsis, the H3K36me3 ‘writer’ SET DOMAIN GROUP8 (SDG8) plays a significant role in regulating genes related to energy metabolism, including NMGs and photosynthesis, through H3K36me3 modification [
21].
SDG8 is induced by N treatment in roots, particularly in the lateral root cap and pericycle [
37]. Recent studies have shown that
SDG8 mediates N-triggered gene-expression reprogramming, including
NRT2.1,
NRT1.5, and
GLUTAMINE SYNTHETASE 1;4 (
GLN1;4) [
38] (
Figure 1).
sdg8 mutants have shown lower nitrate acquisition than wild-type plants under high N conditions [
38]. In tomatoes (
Solanum lycopersicum), the homologs of
SDG8, namely
SlSDG33 and
SlSDG34, regulate
SlNRT1.1 in an N-dependent manner that is associated with the root response to N treatments [
36] (
Figure 1). Moreover, polycomb repressive complex 2 (PRC2), responsible for the addition of methyl groups to H3K27, directly targets and downregulates
AtNRT2.1 expression in
Arabidopsis [
6]. In high N conditions, HIGH NITROGEN INSENSITIVE 9/ interacts with SUPT6H, and CTD Assembly Factor 1 (HNI9/AtIWS1) recruits PRC2 to the
AtNRT2.1 locus to repress its transcription [
23] (
Figure 1). Moreover,
AtHNI9 control reactive oxygen species (ROS) homeostasis under conditions of excess N. The mutation of
AtHNI9 leads to elevated ROS levels and ROS-dependent upregulation of
AtNRT2.1, indicating the role of
HNI9 in balancing N uptake and ROS levels in response to N supply [
39].
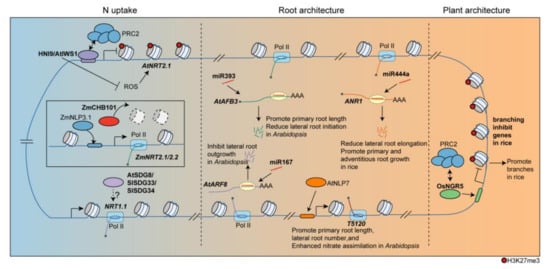
Figure 1. Epigenetic regulation of divergent processes in response to N. HNI9/AtIWS1 binds to the AtNRT2.1 locus and recruits PRC2 to suppress its expression. Conversely, ZmCHB101 regulates ZmNRT2.1 by reducing nucleosome densities. The homologs of SDG8 in tomatoes, SlSDG33 and SlSDG34, also regulate NRT1.1 under N supply. Epigenetic factors are involved in the regulation of N-dependent development processes as well. And miR444a reduces lateral root elongation by targeting ANR1 in rice. In Arabidopsis, miRNAs, such as miR167 and miR393, target transcripts involved in root development, particularly in response to N, thereby influencing root growth. Additionally, NLP7 modulates the expression of T5120, a long non-coding RNA. In rice, OsNGR5, which recruits PRC2 to inhibit branching-inhibition genes, influence plant architecture.
In monocots, N uptake was regulated by changes in chromatin rearrangement.
CHROMATIN REMODELING COMPLEX SUBUNIT B 101 (
CHB101) encodes a subunit of the ATP-dependent chromatin remodeling complex known as the SWITCH/SUCROSE NONFERMENTING (SWI/SNF) complex [
40,
41]. In maize,
ZmCHB101 has been found to govern the expression of nitrate transport genes, including
ZmNRT2.1 and
ZmNRT2.2, in response to nitrate supply [
17] (
Figure 1). The
ZmCHB101-RNAi lines showed significantly reduced nucleosome densities at the −1 and +1 nucleosome regions of
ZmNRT2.1 and
ZmNRT2.2 as compared to WT under nitrate treatment. These low nucleosome densities resulted in enhanced ZmNLP3.1 binding to the promoter regions of
ZmNRT2.1 and
ZmNRT2.2, further regulating their expression [
17] (
Figure 1).
2. Epigenetic Regulation of Root Architecture in Response to N
The N signal greatly influences the root system, which serves as the sensory organ for detecting external nitrogen. Nitrate, in particular, has been shown to modulate various aspects of root development, including primary root growth [
42,
43], lateral root initiation and elongation [
44,
45,
46,
47].
Recent discoveries highlight the regulatory role of microRNAs (miRNAs) on key TFs involved in root development in monocots. One such TF is ARABIDOPSIS NITRATE REGULATED 1 (ANR1), a member of the MIKC-type MADS (MCM1/AGAMOUS/DEFICIENS/SRFl) box family of TFs, which mediates the root response to external N [
48]. It stimulates lateral root growth, leading to an increase in lateral root number and length, as well as total shoot fresh weight [
49]. The conservation of ANR1 function has been observed in various species, including rice [
50] and chrysanthemum [
51]. Overexpression of miR444a in rice reduced lateral root elongation in a nitrate-dependent manner by targeting
ANR1 [
52] (
Figure 1). However, miR444a overexpression promoted primary and adventitious root growth and improved nitrate accumulation under high nitrate concentration conditions [
52].
In dicots, in particular
Arabidopsis, the presence of several ncRNAs has been observed in response to N. The miR393 also regulates primary root length and lateral root density in response to N availability in
Arabidopsis by targeting
AUXIN SIGNALING F-BOX 3 (
AFB3) [
53]. Similarly, miR167 inhibits lateral root outgrowth in response to N by targeting
AUXIN RESPONSE FACTOR 8 (
ARF8) in pericycle cells in
Arabidopsis [
37] (
Figure 1). Additionally,
T5120, a long non-coding RNA modulated by NLP7 and NRT1.1, promotes nitrate assimilation and root growth (primary root length and lateral root number) in
Arabidopsis [
22] (
Figure 1). Interestingly, the miRNA/target modules are responsive to downstream N metabolites, with miR393 and
ARF8 being induced by an N metabolite produced during N reduction, coordinating plant growth and development in response to both external and internal N availability [
53].
3. Epigenetic Regulation of Plant Architecture in Response to N
N supply exerts an epigenetic influence not only on root development but also on plant architecture, particularly for tillering in monocots. In rice, the application of N fertilizer induces changes in the genome-wide H3K27me3 pattern through NITROGEN-MEDIATED TILLER GROWTH RESPONSE 5 (NGR5). This mechanism facilitates the recruitment of PRC2, thus promoting the repression of branching-inhibitory genes (
Dwarf14 (
D14) and
SQUAMOSA-promoter binding protein-like 14 (
SPL14)) through H3K27me3 modification [
54] (
Figure 1). Interestingly, PRC2 has been reported to associate with the regulation of rice tillering with normal N condition. In particular, VIN3-LIKE 2 (OsVIL2) suppresses the expression of
TEOSINTE BRANCHED1 (
OsTB1), which is a branching-inhibitory gene, by recruiting PRC2 and promoting bud outgrowth [
55]. In this context, NRG5 acts as a missing link, connecting N signaling with the regulation of tillering through PRC2. Consequently, exploring the connection between N signaling and chromatin response to N can uncover crucial factors, like NGR5, involved in the regulation of NUE.
In conclusion, the integration of epigenetic regulations involving histone modification, chromatin remodeling, and miRNA regulation in a plant’s response to N underscores the profound influence of N on the epigenome, transcriptional processes, and subsequent divergence in growth and metabolism. Moreover, establishing the link between N signaling and chromatin response holds great significance. Considering the findings discussed above, exploring the role of epigenomic changes in plants adapted to different N conditions, such as LN, becomes particularly intriguing.
This entry is adapted from the peer-reviewed paper 10.3390/plants12142725